Abstract
Debate persists regarding the relative role of cognitive versus emotional processes in driving successful performance on the widely used Iowa Gambling Task (IGT). From the time of its initial development, patterns of IGT performance were commonly interpreted as primarily reflecting implicit, emotion-based processes. Surprisingly, little research has tried to directly compare the extent to which measures tapping relevant cognitive versus emotional competencies predict IGT performance in the same study. The current investigation attempts to address this question by comparing patterns of associations between IGT performance, cognitive intelligence (Wechsler Abbreviated Scale of Intelligence; WASI) and three commonly employed measures of emotional intelligence (EI; Mayer–Salovey–Caruso Emotional Intelligence Test, MSCEIT; Bar-On Emotional Quotient Inventory, EQ-i; Self-Rated Emotional Intelligence Scale, SREIS). Results indicated that IGT performance was more strongly associated with cognitive, than emotional, intelligence. To the extent that the IGT indeed mimics “real-world” decision-making, our findings, coupled with the results of existing research, may highlight the role of deliberate, cognitive capacities over implicit, emotional processes in contributing to at least some domains of decision-making relevant to everyday life.
Keywords: Iowa Gambling Task, Emotional intelligence, Intelligence quotient, Decision-making
1. Introduction
The relative role of emotional versus cognitive processes in driving judgment and decision-making in everyday life remains a topic of substantial interest in the empirical literature (see Kahneman, 2011; Vastfjall & Slovic, 2013). The Iowa Gambling Task (IGT) is among the most extensively used neuropsychological paradigms designed to assess “real-world” decision-making (Bechara, 2004; Toplak, Sorge, Benoit, West, & Stanovich, 2010; for a detailed description of the IGT see Bechara, Damasio, Damasio, & Anderson, 1994; Bechara, Damasio, & Damasio, 2000). In its most common form, the IGT is presented as a simple card game with the explicit goal of winning as much money as possible by selecting cards, one at a time, from any of four decks. With each card selection, the participant wins or loses varying amounts of money. As the game progresses, the participant has the opportunity to learn from experience that some of the decks produce relatively large wins but even larger losses (i.e., “bad decks”), while other decks have modest wins but even smaller losses (i.e., “good decks”). Consistently selecting from the bad decks will ultimately lead to total loss, while selecting consistently from the good decks will lead to long term gain. Early work with the IGT showed that healthy participants begin the game by selecting randomly among the decks, but they soon appear to learn the contingencies as the game proceeds, progressively avoiding the bad decks in favor of the good ones. Critically, during the early phases of the game, healthy individuals also begin to show increased skin conductance responses when considering “bad” deck selections, even before they claim any conscious awareness of the contingencies of the task. This increase in skin conductance has been suggested as evidence that participants have begun to learn the deck values at a pre-conscious, emotional level, before they have formed an explicit cognitive understanding of the task (Bechara et al., 2000, 1994). However, the extent to which successful IGT performance is driven more by implicit, emotion-based versus explicit, cognitive processes remains a matter of significant debate (Demaree, Burns, & DeDonno, 2010; Maia & McClelland, 2004).
The IGT is believed to mimic real-life decision-making in that it incorporates the experience of rewards and losses, as well as factoring uncertainty of outcomes and risk (Bechara, Damasio, Tranel, & Damasio, 1997). Whereas overt decision-making is thought to rely on explicit knowledge and reasoning, patterns of IGT performance initially reported in the literature indicated that participants are able to decide advantageously without declarative knowledge of the best strategy (e.g., Bechara et al., 1997). Such findings have been used to bolster the argument that successful IGT performance may be driven more by implicit, emotion-based processes (i.e., “hot” decision-making), rather than primarily through explicit insight of the most favorable strategy derived from data-oriented cost/benefit analyses (i.e., “cold” decision-making; Dunn, Dalgleish, & Lawrence, 2006).
The somatic marker hypothesis (SMH; Damasio, Tranel, & Damasio, 1991; Damasio, 1994, 1996, 2004) provides an explanatory framework for understanding how emotion-based decision-making processes may be driving successful IGT performance. More specifically, the SMH posits that, through prior experience with stimuli or situations, individuals acquire emotion-based biasing signals generated from the physiological systems of the body (“somatic markers”), and that these signals are re-activated when considering analogous response options in the future. These somatic markers may be experienced as visceral “hunches” or “gut feelings” that can bias decision-making (Damasio, 2004). These markers are proposed to help direct attention toward or away from particular response options and thus facilitate more streamlined and efficient decision-making. Interestingly, individuals with damage to a specific region of the brain, the ventromedial prefrontal cortex (VMPFC), appear to be impaired in this process, and tend to exhibit relatively poor performance on the IGT, despite otherwise preserved intellectual capacities (Bechara et al., 1994). It is important to note that although VMPFC lesion patients tend to have generally intact cognitive abilities, they often show profound deficits in social–emotional domains, including deficits in emotion expression, affective experience and regulation, and frequently show a pattern of maladaptive decision-making in their everyday lives (Damasio, 1994). The term “myopia for the future” has been applied to these VMPFC lesion patients as they often display a relatively heightened preference for immediate reward, while neglecting longer-term consequences. It is also critical to point out that, while these VMPFC lesion patients exhibit relatively normal skin conductance responses (SCRs) after a win or loss, they fail to show the anticipatory SCRs exhibited by healthy controls when contemplating a high-risk choice on the IGT (Bechara, Tranel, Damasio, & Damasio, 1996). This pattern of findings has formed the crux of the SMH, suggesting that the VMPFC may be a key brain region involved in integrating physiological responses with cognitive data to form a feeling or hunch that biases decision selection (Bechara et al., 1996). Other regions proposed to underlie the “somatic marker circuitry” (SMC) include the amygdala, insula, anterior cingulate, basal ganglia and somatosensory cortex (Bechara & Damasio, 2005; Dunn et al., 2006). Indeed, one hypothetical model posits that emotional intelligence capacities rely heavily upon the SMC (Bar-On, Tranel, Denburg, & Bechara, 2003). Additionally, a recent review found IGT performance to be generally uncorre-lated with traditional “cold cognition” types of executive function tasks (Toplak et al., 2010), suggesting that emotional, rather than cognitive or executive, abilities may primarily drive performance on the IGT.
However, the role of emotion in biasing decision-making on the IGT has not been universally observed. For example, Maia and McClelland (2004) reported results contradicting the notion that IGT participants decide advantageously without declarative knowledge of the best strategy (Bechara et al., 1997). Specifically, the study showed that when participants performed advantageously in the IGT, they tended to be consciously aware of the “goodness” and “badness” of relative decks. Paralleling these findings, Guillaume et al. (2009) showed that better performance on the IGT was associated with explicit knowledge of the underlying contingencies. Moreover, conscious knowledge was not associated with anticipatory SCRs in that study, suggesting that explicit awareness and somatic cues may have two distinct influences on decision-making (Guillaume et al., 2009). Despite the large body of research examining the influence of emotion or cognitive ability separately on IGT performance, there is a surprising paucity of research that aims to disentangle the relative contributions of cognitive versus emotional processes within the same study. To our knowledge, only one study (Demaree et al., 2010) has directly compared the influences of cognitive intelligence (IQ) versus emotional intelligence (EI) on IGT performance in a healthy sample. Interestingly, findings from the latter study showed IQ to be a better predictor of IGT performance than EI, suggesting that the IGT may, in fact, tap cognitive processes to a greater extent than emotional ones (at least EI).
However, the conclusions of the Demaree et al. (2010) study are limited by several factors. First, the authors used a single, self-report measure of emotional intelligence, the Schutte Emotional Intelligence Scale (SEIS; Schutte et al., 1998), which implicitly assumes that patients can reliably access and accurately report on their EI abilities. As noted by the authors, a self-report measure of emotional intelligence may not be sensitive to the fact that participants might rely, at least in part, on implicit, rather than on explicit, knowledge of emotional cues to make their decisions on the IGT, thereby likely limiting the validity of the self-report SEIS. In an attempt to address this limitation, in the current study we employed concurrent EI measures utilizing self-report methodologies (i.e., Bar-On Emotional Quotient Inventory; EQ-i; Bar-On, 2002; Self-Rated Emotional Intelligence Scale; SREIS; Brackett, Rivers, Shiffman, Lerner, & Salovey, 2006), as well as the most commonly used performance-based measure of EI (Mayer–Salovey–Caruso Emotional Intelligence Test; MSCEIT; Mayer, Salovey, Caruso, & Sitarenios, 2003). Second, to assess cognitive ability, the Demaree et al. study relied on the Mill Hill Vocabulary Scale rather than on a “gold standard” measure of IQ (i.e., Wechsler or Stanford–Binet intelligence scales). Thus, in the current study, we use the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1997). Third, Demaree et al. used a convenience sample of undergraduate students, while our sample consisted of a more diverse group of volunteers with a broader age and education range.
In the present study, we aimed to assess the unique contributions of EI and IQ in predicting IGT performance. We used a Wechsler scale to measure IQ and several commonly-employed EI measures (both self-report and performance-based instruments) to assess the relative predictive value of these constructs. We hypothesized that IQ would be more strongly associated with IGT performance relative to our measures of EI, supporting the notion that the IGT may rely more on cognitive than emotional intelligence.
2. Method
2.1. Participants
Sixty-five healthy volunteers were recruited from the greater Boston area (32 females; ages 18–45, M = 30.15, SD = 8.01). Some convergent and divergent validity data related to cognitive and emotional intelligence from these participants have been reported elsewhere (Webb et al., 2013), but the associations with the IGT are novel and have not been previously reported. Participants classified themselves as 69.2% White, 15.4% African-American, 9.2% Asian, 3.1% Other, and 3.1% “more than 1 race.” Additionally, 4.6% of the sample identified as Hispanic. The primary language of all participants was English. The mean number of years of education was 14.9 (range 11–20). Participants were screened by a trained clinical research assistant for history of DSM-IV Axis I psychopathology, substance abuse, and serious medical or neurological conditions, based on a series of questions adapted from the Structured Clinical Interview for DSM-IV-TR (SCID-I; First, Spitzer, Gibbon, & Williams, 2001). All participants provided written informed consent and received compensation. The study protocol was approved by the McLean Hospital Institutional Review Board and the US Army Human Research Protection Office.
2.2. Measures
2.2.1. Iowa Gambling Task
The Iowa Gambling Task (IGT; Bechara et al., 1997) is a widely used decision-making paradigm that involves the learning of a punishment–reward contingency. Presented as a computerized game, participants start with a play loan of $2000 and choose cards from four identically appearing decks (A′, B′, C′, or D′) in an effort to win as much money as possible. All card selections yield a monetary gain, and some card selections are also associated with a loss that immediately follows the win. Decks A′ and B′ are disadvantageous, high-risk (“bad”) decks, meaning that these cards reveal large rewards but even larger losses (expected value < 0). Decks C′ and D′ are advantageous, low-risk (“good”) decks, in which cards represent more modest rewards but also much smaller losses (expected value > 0). There are 100 trials and participants are free to switch between decks as often as they like. Participants received standard instructions (Bechara, Tranel, Damasio, & Damasio, 1994) emphasizing that some decks are worse than others and it is possible to win the game by avoiding the bad decks.
2.2.2. Emotional intelligence
Three indices of EI were administered to the participants. The Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT; Mayer et al., 2003) is a computerized, performance-based measure of emotional intelligence. The MSCEIT consists of 141 items designed to assess the following four “branches” of EI: (1) perceiving emotions (Perceiving), (2) using emotions to facilitate thought (Facilitating), (3) understanding emotions (Understanding), and (4) managing emotions (Managing). The measure yields a Total EI score and two Area scores, Experiential EI and Strategic EI. Experiential EI reflects the ability to perceive emotions in oneself, other persons, and various stimuli (e.g., music and art), and to utilize emotional information to facilitate thought. Strategic EI reflects the ability to understand emotions and their development in oneself and others, and to manage them effectively. Mayer, Salovey and Caruso (2002) reported good reliability values for total MSCEIT scores, including internal consistency (split-half reliability = .91) and test–retest reliability (.86). For additional information on the psychometric properties of the MSCEIT, see Mayer et al. (2002) and Mayer et al. (2003).
The Bar-On Emotional Quotient Inventory (EQ-i; Bar-On, 2002) is a computerized, 133-item self-report assessment of trait EI (i.e., self-perceived EI). The EQ-i yields a Total Emotional Quotient (EQ) and five composite scores (i.e., Interpersonal, Intrapersonal, Adaptability, Stress Management, General Mood). Bar-On (2004) reported that the EQ-i demonstrated good reliability (internal consistency and test–retest reliability). For detailed information on the psychometric properties of the EQ-I, see Bar-On (2004).
The Self-Rated Emotional Intelligence Scale (SREIS; Brackett et al., 2006) is a 19-item self-report questionnaire that taps into similar EI capacities assessed by the MSCEIT. Like the MSCEIT, the SREIS measures the perception, management, use, and understanding of emotions in oneself and others, but uses a self-report format that is similar to that of the EQ-i. Items such as “By looking at people’s facial expressions, I recognize the emotions they are experiencing” are rated on a 5-point Likert scale ranging from 1 (“very inaccurate”) to 5 (“very accurate”). In a series of studies, Brackett et al. (2006) reported the following Cronbach’s alphas for the SREIS: .84, .77, and .66 for Studies 1, 2, and 3, respectively.
2.2.3. Cognitive intelligence
The Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1997) was used to assess IQ. The WASI is one of the most widely used intelligence scales and has reported reliability of .98 for Full Scale IQ, with high test–retest reliability, and correlates .92 with the Wechsler Adult Intelligence Scale-III (WAIS; Pearson Assessment, Inc., San Antonio, TX). The measure yields scores for Full Scale IQ, Verbal IQ, and Performance IQ. The WASI was individually administered by a trained research technician under the supervision of a licensed doctoral level neuropsychologist.
2.3. Procedure
Given the relatively large number of performance-based tests and self-report measures administered, testing occurred over two consecutive days in order to reduce participant fatigue. During the first testing session, participants completed demographic forms, consent forms, the MSCEIT, EQ-i, and SREIS. On the second day of testing, participants completed the WASI and IGT. All testing took place at McLean Hospital in a private testing room. All assessments were administered according to published instructions and procedures. Complete data from ten participants were not available for either the WASI or the IGT due to computer malfunctions or participant discontinuation, so those data were excluded from the analyses reported below.
3. Results
3.1. IGT performance
Performance on the IGT was scored by dividing the task into 5 blocks of twenty trials (e.g., block 1 = cards 1–20, block 2 = cards 21–30, etc.). A net score was calculated for each block by subtracting the number of cards chosen from the high-risk decks (A′ and B′) from the number of cards chosen from the low-risk decks (C′ and D′). Positive net scores reflected overall advantageous decision-making, while a negative value reflected overall disadvantageous decision-making for each block.
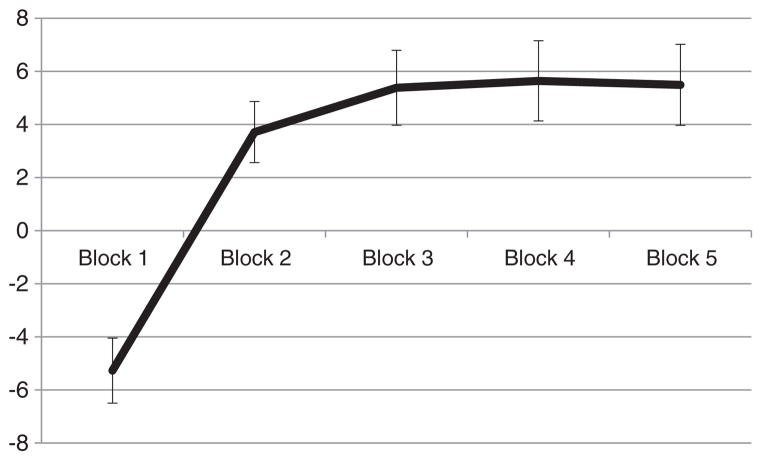
Consistent with prior reports in healthy samples, participants showed the typical learning curve indicative of increased advantageous decision-making across the 5 blocks of the IGT (see Bechara, 2004; Bechara et al., 2000), with scores sharply improving over the first 2 blocks and plateauing during blocks 3–5 (see Fig. 1). To statistically test for this learning curve, a repeated-measures analysis of variance (ANOVA) was conducted, with an independent variable of block and a dependent variable of the net scores. There was a main effect of block, F(3,166) = 17.67, p < .001 (Greenhouse–Geisser corrected). Post-hoc Least Significant Difference (LSD) tests revealed that block 1 performance (M = −5.27, SD = 9.08) was significantly worse than performance on blocks 2 through 5 (block 2: M = 3.71, SD = 8.53; block 3: M = 5.38, SD =10.46; block 4: M = 5.64, SD = 11.20; block 5: M = 5.49, SD = 11.30; p < .001 for each comparison). No other significant differences emerged.
Fig. 1.
IGT performance (+/−1 SEM) as a function of block. The y-axis denotes the number of cards chosen from the “good” decks minus cards chosen from the “bad” decks.
3.2. Zero-order correlations
Bivariate Pearson correlations were run to test for linear associations between performance on the IGT and the IQ and EI measures. Means (M), standard deviations (SD) and intercor-relations are listed in Table 1. IGT performance (IGT Total) was significantly correlated with Full Scale IQ (r = .47, p < .001), including both Verbal IQ (r = .40, p = .002) and Performance IQ (r = .45, p = .001). IGT performance was also significantly correlated with the MSCEIT (r = .36, p = .007). When examining the constituent branches of the MSCEIT, IGT Total score was significantly correlated with Facilitating (r = .28, p = .038) and Understanding (r = .43, p = .001), but not Managing (r = .11, p = .415); a nonsignificant trend emerged for the Perceiving branch (r = .24, p = .082). However, there were no significant correlations between IGT performance and the two self-report measures of emotional intelligence (EQ-i: r = .01, p = .937; SREIS: r = .23, p = .099; see Table 1 for results by IGT block).
Table 1.
Means, standard deviations, and correlations for investigated variables.
| M | SD | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. MSCEIT | 103.06 | 12.09 | .11 | .32* | .52** | .52** | .43** | .36** | .06 | .25 | .23 | .30* | .35** |
| 2. EQ-i | 101.42 | 13.63 | – | .50** | .22 | .17 | .24 | .01 | .00 | −.02 | .03 | .00 | .02 |
| 3. SREIS | 73.06 | 8.04 | – | .30* | .37** | .17 | .23 | .02 | .18 | .14 | .21 | .20 | |
| 4. IQ — Full | 111.10 | 16.08 | – | .92** | .91** | .47** | −.08 | .31* | .39** | .45** | .46** | ||
| 5. IQ — Verbal | 110.40 | 15.76 | – | .69** | .40** | −.09 | .26 | .33* | .39** | .43** | |||
| 6. IQ — Perf. | 108.85 | 15.43 | – | .45** | −.04 | .31* | .39** | .43** | .41** | ||||
| 7. IGT Total | 14.95 | 34.96 | – | .34** | .59** | .72** | .87** | .85** | |||||
| 8. Block 1 | −5.27 | 9.08 | – | .10 | −.10 | .10 | .18 | ||||||
| 9. Block 2 | 3.71 | 8.53 | – | .34* | .33* | .36** | |||||||
| 10. Block 3 | 5.38 | 10.46 | – | .63** | .49** | ||||||||
| 11. Block 4 | 5.64 | 11.20 | – | .78** | |||||||||
| 12. Block 5 | 5.49 | 11.30 | – |
Note: MSCEIT = Mayer–Salovey–Caruso Emotional Intelligence Test; EQ-i = Bar-On Emotional Quotient Inventory; SREIS = Self-Rated Emotional Intelligence Scale; IQ-Full/Verbal/Performance = Wechsler Abbreviated Scale of Intelligence — Full-Scale/Verbal IQ/Performance IQ; IGT = Iowa Gambling Task (blocks 1–5 and Total score).
p < .05.
p < .01.
3.3. Partial correlations
Next, given the significant bivariate correlations between EI and IQ scores (see Table 1), a series of partial correlations was conducted to test the association between IGT performance (net score on blocks 1–5) and scores on each EI measure (MSCEIT, EQ-i and SREIS total scores) after controlling for Full Scale IQ. As illustrated in Fig. 2, after controlling for Full Scale IQ, there were no significant associations between any of the three EI measures and IGT performance across any blocks (for MSCEIT, all r < .16 & p > .27; for EQ-i, all r < .02 & p > .45; for SREIS, all r < .10 & p > .46).
Fig. 2.
The partial correlation of IGT performance (blocks 1–5) with our measures of IQ and EI. Each bar represents a partial r: i.e., the correlation between EI scores and IGT performance, after controlling for Full Scale IQ; and the correlation between Full Scale IQ and IGT performance, controlling for scores on the MSCEIT, SREIS, or EQ-i. **p < .01; *p < .05.
In contrast, when controlling for the different measures of EI, Full Scale IQ remained significantly associated with several IGT performance variables. Specifically, when controlling for MSCEIT scores, Full Scale IQ was significantly associated with IGT performance in blocks 3–5 (block 3, r = .33; p = .015; block 4, r = .36; p = .008; block 5, r = .34; p = .011). When controlling for EQ-i scores, Full Scale IQ was significantly associated with IGT performance in blocks 2–5 (block 2, r = .33; p = .016; block 3, r = .40; p = .003; block 4, r = .46; p < .001; block 5, r = .46; p < .001). Similarly, when controlling for SREIS scores, Full Scale IQ was significantly associated with IGT performance in blocks 2–5 (block 2, r = .28; p = .042; block 3, r = .37; p = .006; block 4, r = .42; p = .002; block 5, r = .43; p = .001).
3.4. Hierarchical multiple regressions
Separate hierarchical multiple regression analyses were performed to examine the incremental contribution of the combined EI measures over IQ in predicting IGT performance. Similarly, we tested whether IQ remained significantly associated with IGT performance after controlling for all three EI measures simultaneously (and providing accompanying R2 change values). Given that several of our predictor variables were significantly correlated (see Table 1), Tolerance values were examined to test for issues of multicollinearity in our models. It has been suggested that Tolerance values of 0.2 or below are worthy of concern (Field, 2009; Menard, 1995). In the below models, Tolerance values ranged from .68 to .95, thus indicating no significant multicollinearity. All predictor variables were forced into entry in our multiple regression models.
The first set of hierarchical multiple regressions predicted IGT performance (i.e., net score on IGT blocks 1–5 and IGT Total score) from Full Scale IQ (Step 1) and our three EI measures (Step 2). Six separate multiple regressions were conducted, predicting IGT performance in blocks 1–5 and Total IGT score separately. As presented in Table 2, in none of the multiple regressions did the combined EI measures (Step 2) predict a significant amount of variance above and beyond Full Scale IQ scores (Step 1).
Table 2.
Hierarchical multiple regression analyses testing incremental contribution of combined EI measures over IQ in predicting IGT performance.
| Criterion (IGT block) | Regression step | Predictor | ΔR2 | ΔF | Sig F. change (p values) |
|---|---|---|---|---|---|
| Block 1 | 1 | IQ | .006 | .30 | ns |
| 2 | EI | .015 | .26 | ns | |
| Block 2 | 1 | IQ | .099 | 5.81 | .019 |
| 2 | EI | .037 | .72 | ns | |
| Block 3 | 1 | IQ | .155 | 9.70 | .003 |
| 2 | EI | .008 | .17 | ns | |
| Block 4 | 1 | IQ | .202 | 13.41 | .001 |
| 2 | EI | .033 | .72 | ns | |
| Block 5 | 1 | IQ | .209 | 14.01 | <.001 |
| 2 | EI | .035 | .76 | ns | |
| IGT Total | 1 | IQ | .218 | 14.73 | <.001 |
| 2 | EI | .044 | .99 | ns |
Note: IQ = Wechsler Abbreviated Scale of Intelligence — Full Scale IQ; IGT = Iowa Gambling Task. EI = All three EI measures entered in Step/block 2 (Mayer–Salovey–Caruso Emotional Intelligence Test; Bar-On Emotional Quotient Inventory; Self-Rated Emotional Intelligence Scale).
ns = nonsignificant.
Finally, as presented in Table 3, we conducted a similar set of six hierarchical multiple regressions with Steps 1 and 2 reversed. Specifically, we predicted IGT performance (i.e., net score on IGT blocks 1–5 and IGT Total score) from our three EI measures (Step 1) followed by Full Scale IQ (Step 2). Even after controlling for all three EI measures, Full Scale IQ remained a significant predictor of IGT Total score and performance in blocks 3–5 (it should be noted that, when IQ was not statistically controlled, the combined EI measures did significantly predict IGT performance on block 5 and for IGT Total score).
Table 3.
Hierarchical multiple regression analyses testing incremental contribution of IQ over combined EI measures in predicting IGT performance.
| Criterion (IGT block) | Regression step | Predictor | ΔR2 | ΔF | Sig F. change |
|---|---|---|---|---|---|
| Block 1 | 1 | EI | .004 | .07 | ns |
| 2 | IQ | .017 | .86 | ns | |
| Block 2 | 1 | EI | .089 | 1.67 | ns |
| 2 | IQ | .047 | 2.70 | ns | |
| Block 3 | 1 | EI | .061 | 1.10 | ns |
| 2 | IQ | .103 | 6.12 | .017 | |
| Block 4 | 1 | EI | .120 | 2.31 | .087 |
| 2 | IQ | .115 | 7.53 | .008 | |
| Block 5 | 1 | EI | .142 | 2.82 | .048 |
| 2 | IQ | .101 | 6.70 | .013 | |
| IGT Total | 1 | EI | .155 | 3.11 | .034 |
| 2 | IQ | .106 | 7.21 | .010 |
Note: IQ = Wechsler Abbreviated Scale of Intelligence — Full Scale IQ; IGT = Iowa Gambling Task. EI = All three EI measures entered in Step/block 1 (Mayer–Salovey–Caruso Emotional Intelligence Test; Bar-On Emotional Quotient Inventory; Self-Rated Emotional Intelligence Scale).
ns = nonsignificant.
4. Discussion
The present study sought to directly compare the relative influence of cognitive intelligence (IQ) versus emotional intelligence (EI) on IGT performance. Both IQ and the performance-based measure of EI (MSCEIT) were significantly correlated with IGT performance, whereas neither of the self-reported measures (EQ-i and SREIS) was associated with the IGT. It is interesting to note that the MSCEIT evidenced relatively small correlations with the two EI measures that were not associated with IGT performance (MSCEIT-EQ-i r = .11; MSCEIT-SREIS r = .32), whereas the EQ-i and SREIS exhibited a significant and relatively strong association with one another (r = .50).
A series of hierarchical regression analyses revealed that most of the variance in IGT performance was accounted for by IQ rather than by EI measures. Specifically, the MSCEIT failed to contribute significantly above and beyond IQ in the prediction of IGT performance. On the other hand, as hypothesized, IQ contributed uniquely to the prediction of IGT even after controlling for scores on EI measures. Our results build upon and further advance those of Demaree et al. (2010) who reported that IQ, but not EI, was associated with IGT performance. However, the current investigation augments the previous findings by also addressing several limitations of the prior work. In particular, whereas the study by Demaree and colleagues relied on a single, self-report measure of EI, the current study used several EI measures, including popular self-report measures (EQ-i & SREIS), as well as the most commonly used performance-based measure (MSCEIT). In addition, to assess cognitive ability, the Demaree et al. study relied on the Mill Hill Vocabulary Scale rather than on one of the widely used “gold standard” measures of IQ (i.e., Wechsler or Stanford–Binet intelligence scales). Accordingly, to address this limitation, in the current study we employed an abbreviated version of the Wechsler scales. Third, Demaree et al. used a convenience sample of undergraduate students, while our sample consisted of a relatively diverse group of participants with a broader age and education range.
Our work is also consistent with other recent studies that have found associations between better IGT performance and greater executive function (e.g., Brand, Recknor, Grabenhorst, & Bechara, 2007) and possessing explicit knowledge of the advantageous strategy (e.g., Maia & McClelland, 2004; also see Roca et al., 2010 for finding linking IGT performance to “g”). Taken together, these results suggest that IGT performance may recruit more deliberate, cognitive processes than implicit, emotional ones. As noted above, the observation that VMPFC lesion patients perform poorly on the IGT – and do not evidence anticipatory SCRs (Bechara et al., 1996) –was interpreted as evidence that such individuals lacked integrity of the neural circuitry needed to generate somatic marker biasing-signals that could guide selection of cards from the “good” decks. However, emerging evidence suggests that poor performance on the IGT may be alternatively explained by cognitive, rather than by emotion-based, deficits. As noted by Demaree et al. (2010), the standard IGT requires reversal learning – a cognitive skill – to be successful on the task. Specifically, the “bad” decks in fact provide larger rewards and no losses early in the IGT, and losses only accrue after several trials, thus requiring an eventual shift to the “good” decks (which provide relatively small wins, but even smaller losses). Fellows and Farah (2005) tested VMPFC lesion patients in a variant of the IGT and found that the poor performance of these patients seemed to be attributable to a specific deficit in reversal learning.
Contrary to the present findings, it should be noted that there are prior studies supporting the claim that emotion-based processes drive successful IGT performance. For example, other studies have failed to find significant associations between IGT performance and either executive functions or intelligence (see Toplak et al., 2010 for a review). A key strength of the current study, however, was the fact that competing measures of EI and IQ were directly compared in the same investigation. Undoubtedly, more research is warranted to fully disentangle the relative role of, and interaction between, cognitive and emotional processes in driving IGT performance.
Of course, it is important to note that current EI measures assess a variety of emotion-related constructs (Webb et al., 2013), and it may be that they do not adequately tap the implicit, emotion-based processes proposed by the SMH that may be relevant to decision-making. It should be highlighted that the MSCEIT was significantly correlated with performance on the IGT, indicating that those with higher emotional intelligence as measured by the MSCEIT did perform better on the task (although, as noted above, this relationship was no longer significant after controlling for IQ). Interestingly, the two MSCEIT branches that correlated most strongly with success on the IGT were those designed to assess the ability to use emotional information to facilitate thought (Facilitating) and the ability to understand emotions and their development in oneself and others (Understanding). In line with the SMH, one could argue that those who are especially adept at noticing emotional fluctuations in themselves (including “emotional hunches” regarding advantageous response options) and using that information to facilitate decision-making would do particularly well on the IGT. In addition, although the emotional intelligence field is relatively young, a growing body of research has begun to investigate the underlying neural substrates of EI. Interestingly, the regions identified seem to overlap with critical regions of the SMC, in particular the VMPFC (Killgore & Yurgelun-Todd, 2007; Killgore et al., 2013; Krueger et al., 2009). Thus, these findings provide at least some evidence that EI and SMC may have partially overlapping neural substrates.
The fact that the MSCEIT was significantly associated with IGT performance, but that this association was no longer significant after controlling for IQ is intriguing. The MSCEIT has been shown to correlate with measures of cognitive intelligence, including IQ (e.g., Mayer, Roberts, & Barsade, 2008; Webb et al., 2013). Thus, one interpretation of our finding is that the aspects of the MSCEIT that predict IGT performance are the more “cognitive” features of the measure.
Ultimately, more research is also needed to establish the ecological validity of the IGT task. Namely, to what extent does IGT performance in fact mirror “real-world” decision-making, as its developers intended. To the extent that the IGT does bear relevance to decision-making in real life, our findings, along with those of others (e.g., Demaree et al., 2010), may highlight the contribution of cognitive capacities over emotional intelligence in contributing to at least some domains of “real-world” decision-making.
4.1. Limitations
Several limitations of the current study should be noted. First, our sample size was relatively small, limiting statistical power. Nevertheless, we found a number of significant and intriguing relationships in line with our hypotheses. Second, we used the WASI rather than the full-length WAIS. Although the WASI has been to shown to correlate very highly with the WAIS, it still would have been ideal to use the full-length measure. Finally, although we used several of the most commonly used measures of EI, the field is relatively new and debate persists regarding the validity of existing measures in adequately capturing EI (Webb et al., 2013). Additional research is clearly needed to further operationalize EI and to refine existing measures, or develop new measures, to more adequately assess this complex construct and investigate its role in relevant domains of decision-making.
References
- Bar-On R. Bar-On emotional quotient inventory: User’s manual. Toronto: Multi-Health Systems; 2002. [Google Scholar]
- Bar-On R. Bar-On Emotional Quotient Inventory: A Measure of Emotional Intelligence - Technical Manual. North Tonawanda, NY: Multi-Health Systems; 2004. [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behaviour. 2005;52(2):336–372. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1294. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Brackett MA, Rivers SE, Shiffman S, Lerner N, Salovey P. Relating emotional abilities to social functioning: A comparison of self-report and performance measures of emotional intelligence. Journal of Personality and Social Psychology. 2006;91(4):780–795. doi: 10.1037/0022-3514.91.4.780. [DOI] [PubMed] [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: Correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. Journal of Clinical and Experimental Neuropsychology. 2007;29(1):86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes error: Emotion, reason and the human brain. New York: Avon; 1994. pp. 350–412. [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society, B: Biological Sciences. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR. William James and the modern neurobiology of emotion. In: Evans D, Cruse P, editors. Emotion, evolution and rationality. Oxford: Oxford University Press; 2004. pp. 3–14. [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Somatic markers and the guidance of behaviour: Theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. pp. 217–229. [Google Scholar]
- Demaree HA, Burns KJ, DeDonno MA. Intelligence, but not emotional intelligence, predicts Iowa Gambling Task performance. Intelligence. 2010;38:249–254. [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: A critical evaluation. Neuroscience and Biobehavioral Reviews. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. 3. Thousand Oaks, CA: Sage Publications, Inc; 2009. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Biometrics research. New York State Psychiatric Institute; 2001. Structured Clinical Interview for DSM-IV-TR-Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) [Google Scholar]
- Guillaume S, Jollant F, Jaussent I, Lawrence N, Malafosse A, Courtet P. Somatic markers and explicit knowledge are both involved in decision-making. Neuropsychologia. 2009;47(10):2120–2124. doi: 10.1016/j.neuropsychologia.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Thinking, fast and slow. Macmillan; 2011. [Google Scholar]
- Killgore WD, Schwab ZJ, Tkachenko O, Webb CA, DelDonno SR, Kipman M, et al. Emotional intelligence correlates with functional responses to dynamic changes in facial trustworthiness. Social Neuroscience. 2013;8(4):334–346. doi: 10.1080/17470919.2013.807300. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Neural correlates of emotional intelligence in adolescent children. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(2):140–151. doi: 10.3758/cabn.7.2.140. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, McCabe K, Strenziok M, Zamboni G, Solomon J, et al. The neural bases of key competencies of emotional intelligence. Proceedings of the National Academy of Sciences. 2009;106(52):22486–22491. doi: 10.1073/pnas.0912568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, McClelland JL. A reexamination of the evidence for the somatic marker hypothesis: What participants really know in the Iowa Gambling Task. PNAS. 2004;101(45):16075–16080. doi: 10.1073/pnas.0406666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JD, Roberts RD, Barsade SG. Human abilities: Emotional intelligence. Annual Review of Psychology. 2008;59(1):507–536. doi: 10.1146/annurev.psych.59.103006.093646. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso D. MSCEIT technical manual. Toronto, Ontario, Canada: Multi-Health Systems; 2002. [Google Scholar]
- Mayer JD, Salovey P, Caruso D, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3:97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- Menard S. Sage University paper series on quantitative applications in the social sciences. Thousand Oaks, CA: Sage; 1995. Applied logistic regression analysis; pp. 07–106. [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133(1):234–247. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, Hall LE, Haggerty DJ, Cooper JT, Golden CJ, et al. Development and validation of a measure of emotional intelligence. Personality and Individual Differences. 1998;25(2):167–177. [Google Scholar]
- Toplak ME, Sorge GB, Benoit A, West RF, Stanovich KE. Decision-making and cognitive abilities: A review of associations between Iowa Gambling Task performance, executive functions, and intelligence. Clinical Psychology Review. 2010;30(5):562–581. doi: 10.1016/j.cpr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Vastfjall D, Slovic P. Cognition and emotion in judgment and decision making. Handbook of Cognition and Emotion. 2013:252. [Google Scholar]
- Webb CA, Schwab ZJ, Weber M, DelDonno S, Kipman M, Weiner MR, et al. Convergent and divergent validity of integrative versus mixed model measures of emotional intelligence. Intelligence. 2013;41:149–156. [Google Scholar]