Abstract
Purpose:
To compare different preparation methods for a suitable amniotic membrane (AM) extract containing a given amount of growth factors.
Methods:
In this interventional case series, we dissected the AM from eight placentas within 24 hours after delivery, under clean conditions. After washing and mixing, AM extracts (AMEs) were prepared using pulverization and homogenization methods, and different processing and storing conditions. Main outcome measures were the amount of added protease inhibitor (PI), the relative centrifugal force (g), in-process temperature, repeated extraction times, drying percentage, repeated pulverization times, and the effect of filtering with 0.2 μm filters. Extract samples were preserved at different temperature and time parameters, and analyzed for hepatic growth factor (HGF) and total protein using ELISA and calorimetric methods, respectively.
Results:
The extracted HGF was 20% higher with pulverization as compared to homogenization, and increased by increasing the PI to 5.0 μl/g of dried AM. Repeating centrifugation up to 3 times almost doubled the extracted HGF and protein. Storing the AME at −170° for 6 months caused a 50% drop in the level of HGF and protein. Other studied parameters showed no significant effect on the extracted amount of HGF or total protein.
Conclusion:
Appropriate extraction methods with an adequate amount of PI increases the level of extractable components from harvested AMs. To achieve the maximal therapeutic effects of AMEs, it is necessary to consider the half-life of its bioactive components.
Keywords: Amniotic Membrane, Case Series, Growth Factor, Extraction Methods
INTRODUCTION
Today, use of the amniotic membrane (AM) is one of the most common strategies in the treatment of ocular surface diseases. The AM is the innermost layer of the placenta, and includes a single layer of epithelial cells on the surface, a thick basal membrane, and an avascular stroma. One of the unique properties of AM is that it allows easy proliferation of epithelial cells, and the healing process becomes completed with minimum inflammation, angiogenesis, and scarring. In addition to mechanical protection, the AM has antiangiogenic effects which has been documented in several animal and cell studies.[1] The AM also contains protease inhibitors (PIs), which promote healing of corneal alkali burns by inhibiting the inflammatory reaction to proteolytic injury.[2] The main source for antiinflammatory and antiangiogenic factors of the AM seems to be the epithelial cells.[3] A surgically grafted AM is commonly used in the treatment of ocular surface burns and many other destructive diseases, such as acute toxic epidermal necrolysis/Stevens-Johnson syndrome.[4] Furthermore recently, some authors have used fibrin glues for AM transplantation, which is a sutureless technique and appears easier with possibly lower morbidity.[5,6] Topical use of AM extract (AME) is a much more simpler approach and would obviate the need for surgical intervention in many ocular conditions. Many researchers suggest AME can provide results similar to that of AM grafting.[7] In corneal alkali injury, the AME appears to have a greater healing effect than autologous serum.[8] Today, AME is available as packaged lyophilized powder by a limited number of producers, which is being investigated in few studies. Exclusive studies comparing different AME preparation methods are scarce as well. Such information is needed for more extensive studies, especially in countries where packaged lyophilized powder is not available.
In the present investigation, we compared different methods of preparing extracts from the AM, and their effects on the total amount of protein and hepatic growth factor (HGF) in the extract. HGF is an acidic protein growth factor extractable from the AM with potent mitogenic effects.[9] We also tested the stability of the HGF under different periods and temperatures.
METHODS
The Institutional Review Board of Noor Ophthalmology Research Center approved the study.
Amniotic Membrane Preparation
The AMs were harvested from the placenta of healthy women delivered by elective cesarean section at term who did not have a high risk pregnancy. The mothers were screened for hepatitis B and C, HIV and syphilis, and their consent for the use of their placenta was obtained before delivery.
A total of eight placentas, delivered <24 hours before, were selected, and the AMs were harvested under clean conditions. First, the AMs were rinsed with water and cleared of blood and gelatinous matters, and the excess water was squeezed off. Then, the AMs were weighed.
Drying
Amniotic membrane drying was done at two stages, and AM samples were used to compare the effect of drying on the amount of extractable HGF. In the first stage, each AM was partially dried (PD) with gauze. Then, 10% of each AM was separated as partially dried AM (PDAM) and mixed together. The mixture weighed 19.2 g. No further drying was done on the PDAM sample. The next stage of drying was done with the remaining AMs as follows: epithelium free AMs were laid on drying paper, and after water absorption, they were transferred to the next paper until maximum water absorption was achieved. These AMs were used as completely dried AM (CDAM) in the rest of the study. The amount of drying for PDAM was 40.4% and for CDAM was 61%.
Sample Preparation for the Homogenization Process
About 10% of the each CDAM was separated and mixed together (wt = 11.5 g). This sample was used later as CDAM for homogenization (CDAM-Hom).
Preparing the Pulverized Sample
The remaining AMs were pulverized using the following method, and used later as pulverized CDAM (CDAM-Pul).
For the purpose of pulverization, AMs were placed in 10 × 5 cm plastic bags, 0.2 cm thick, and each pack was immersed in liquid nitrogen for 20-30 min. Later, they were crushed in a mortar prechilled to −85°C, and grinded immediately for 2-3 min using a pulverizer (Johnson, CG, 1010ssj/2786, Hong Kong), and turned into a soft powder.
The PDAM underwent the same process as well (PDAM-Pul).
Assessment of the Effect of the AMEs Preparation Process on the Amount of the Extracted Total Protein and HGF
The level of HGF in different samples was determined using ELISA (Awareness ELISA reader 2100) method with the R and D immunoassay kit. The total protein was measured using the pyrogallol red colorimetric method (Roche/Hitachi 902 auto-analyser, Japan). Each sample was tested twice and the mean values were considered.
Assessment of the Effect of Different PI Concentrations on the Amount of the Total Protein and HGF Extractable from AM
Five lidded test tubes were labeled as PI 0, PI 2.5, PI 5, PI 10, and PI 20, and added 0, 2.5, 5, 10, and 20 μL of PI (SIGMA/P8340) to these tubes, respectively, followed by 2 g of CDAM-Pul and 2 mL of balanced salt solution (BSS). These tubes were placed horizontally on the rotator, set at 200 revolutions per minute (rpm) for 20 min at 4°C followed by centrifuging at 5,000 rpm (g = 3,500) for 20 min at 4°C. The supernatants labeled as SP0, SP2.5, SP5, SP10, and SP20, were stored at −170°C for 1 day before analyses for HGF and total protein levels.
Assessment of the Effect of Different Centrifuge Speeds and Temperatures on the Amount of the Total Protein and HGF Extractable from AM
Four lidded test tubes were labeled as g1, g2, T1, and T2, and added 2 g of CDAM-Pul and 2 mL BSS to each.
To examine the role of centrifuge speed, the g1 and g2 tubes were horizontally spun on the rotator for 20 min at 200 rpm at 4°C. Then, g1 was centrifuged for 20 min at 14,000 rpm (g = 25,000) at 4°C, and g2 was centrifuged for 20 min at 5,000 rpm (g = 3,500) at 4°C. The supernatants labeled as S-g1 and S-g2 were stored at −170°C for 1 day before analyses for HGF and total protein levels
To examine the role of in-process temperature, T1 and T2 tubes were spun at 200 rpm and centrifuged at 5,000 rpm (g = 3,500) for 20 min at 4°C and 20°C, respectively. The supernatants labeled as ST1 and ST2 were stored at −170°C for 1 day before analyses for HGF and protein levels.
Assessment of the Effect of Repeated Extraction Times on the Amount of the Total Protein and HGF Extractable from AM
Three lidded test tubes were labeled as E1, E2, E3, and added 5 g of CDAM-Pul in each. To the E1 tube, we added 5 mL BSS and 25 μL PI, then the tube was spun at 200 rpm and centrifuged at 5,000 rpm (g = 3,500) for 20 min at 20°C. The collected supernatant was labeled SE1.
In E2, 3 mL BSS added and 15 μL of PI. Spinning and centrifuging was done as described for tube E1, and the supernatant was labeled S1E2. Then 2 mL BSS and 10 μL PI was added to the sediment, mixed, and the tube was spun at 200 rpm and centrifuged similarly. The collected supernatant (S2E2) was mixed with the previous one (S1E2), and collectively labeled SE2. The above process was repeated 3 times with test tube E3. First 2 mL BSS and 10 μL PI was added. In the second stage, again 2 mL BSS and 10 μL PI, and in the 3rd stage, 1 mL BSS and 5 μL PI was added. The three supernatants (S1E3, S2E3, and S3E3) were mixed and labeled SE3. SE1, SE2, and SE3 were stored at −170°C for 1 day before analyses for HGF and protein levels.
Assessment of the Effect of the Amount of Drying on the Amount of the Total Protein and HGF Extractable from AM
Two lidded test tubes were labeled as completely dried (CD) and partially dried (PD). In the CD tube, we placed 3 g CDAM-Pul, 3 mL BSS, and 15 μL PI. In the PD tube, considering one less stage of drying, we placed 4.6 g PDAM-Pul, 1.4 mL BSS, and 15 μL PI. Both tubes were spun for 20 min at 200 rpm and then centrifuged for 20 min at 5000 rpm (g = 3500) at 4°C. The supernatants were labeled stearoyl-CoA desaturase (SCD) and standard purified diet (SPD), and stored at −170°C for 1 day before analyses for HGF and protein levels.
Assessment of the Effect of Repeated Pulverization Times on the Amount of the Total Protein and HGF Extractable from AM
Four lidded test tubes were labeled as P1, P2, P3, and P4. We weighed 20 g of CDAM-Pul and placed 2 g in P1. The rest was placed in a 5 × 10 cm plastic bag, immersed in liquid nitrogen, and pulverized as before. A total of 2 g of the generated powder was placed in P2, and the remaining powder was pulverized once more to get 2 g of pulverized powder for P3 and a 4th time for P4. To each of these four tubes, 2 mL BSS and 10 μL PI was added, and they were spun for 20 min at 200 rpm and centrifuged for 20 min at 5,000 rpm (g = 3,500) at 4°C. The supernatants were labeled SP1, SP2, SP3, and SP4, and stored at −170°C for 1 day before analyses for HGF and protein levels.
Assessment of the Effect of Pulverization and Homogenization Methods; the Use of 0.2μ Cellulose Acetate Filter; Freeze-Thaw; and the AMEs Storage Time and Temperature on the Amount of the Total Protein and HGF Extractable from AM
A total of 10 g of the AM previously considered for (CDAM-Hom) was cut into small pieces with scissors, added 10 mL BSS and 50 μL PI, homogenized 3 times with ice jacket (Heidolph homogenizer, DIAX 900, Sigma-Aldrich, USA), each time for 90 s, and then spun at 200 rpm for 20 min and centrifuged at 5,000 rpm (g = 3,500) for 20 min. The supernatant was labeled S-Hem and stored at −170°C for 1 day before analyses for HGF and protein levels
A total of 10 g of CDAM-Pul was mixed with 10 mL BSS and 50 μL PI, spun at 200 rpm for 20 min, and then centrifuged at 5,000 rpm (g = 3,500) for 20 min. The supernatant was labeled S-Pul and stored at −170°C for 1 day before analyses for HGF and protein levels
-
To assess the effect of 0.2 μ cellulose acetate filter: 1 ml S-Pul was filtered through a 0.2 μm cellulose acetate filter (FP30/0.2 CA-S, Whatman, England), labeled as S-Pul-F, stored at −170°C for 1 day before analyses for HGF and protein levels
- To assess the effect of repeated freeze-thaw (FT): Three microtubes were filled with 0.5 mL S-Pul each. The first tube underwent FT with liquid nitrogen twice (S-Pul-FT2), the second tube 5 times (S-Pul-FT5), and the third tube 10 times (S-Pul-FT10). These three samples were stored at −170°C for 6 days before analyses for HGF and protein levels
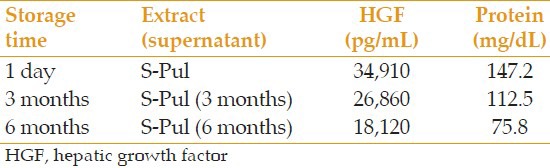
- To assess the effect of storage time on the amount of HGF and total protein: 1 mL S-Pul was poured into 2 microtubes with lids; labeled as S-Pul (3 month) and S-Pul (6 month) and stored at −170°C for 3 and 6 months, respectively, before analyses for HGF and protein levels
- To assess the effect of the AME storage temperature: Five lidded microtubes were filled with 2.5 mL S-Pul and labeled S-Pul (−170), S-Pul (−20), S-Pul (4), S-Pul (20), and S-Pul (37) to be stored for 6 days at − 170, −20, 4, 20, and 37°C, respectively, before analyses for HGF and protein levels.
RESULTS
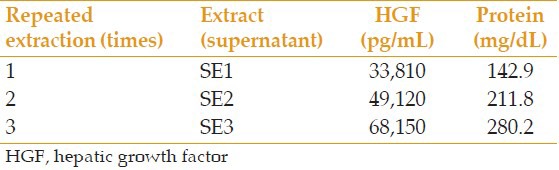
All prepared extracts were analyzed for the level of HGF and total protein. In samples S-g1 and S-g2, which were centrifuged at 14,000 rpm (g = 25,000) and 5,000 rpm (g = 3,500) respectively, the level of HGF was 32,830 and 34,100 pg/mL, respectively, and the amount of total protein was 142.8 and 146.9 mg/dL, respectively. In samples ST1 and ST2, which were processed at 4°C and 20°C, respectively, the level of HGF was 34,240 and 35,110 pg/mL, respectively, and the amount of total protein was 148.3 and 150.0 mg/dL, respectively. Results of analyses of extracts prepared with different concentrations of PI are summarized in Table 1. Results with repeated pulverization times and extraction times are presented in Tables 2 and 3, respectively.
Table 1.
Comparison of the effect of different PI concentrations on the amount of extractable HGF and protein
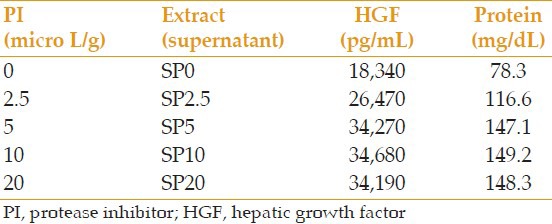
Table 2.
Comparison of the repeated times of pulverization on the amount of extractable HGF and protein
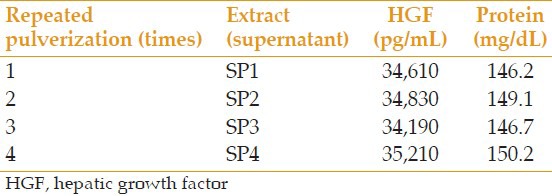
Table 3.
Comparison of the repeated extraction times on the amount of extractable HGF and protein
In S-Pul and S-Hem samples prepared for the comparison of pulverization and homogenization, HGF level was 34,310 and 28,280 pg/mL, respectively, and the total protein was 144.7 and 118.9 mg/dL, respectively. The differences in the HGF and total protein content of S-Pul and S-Pul-F samples which were prepared to assess the effect of filtration were negligible with HGF levels of 33,960 and 32,890 pg/mL and total protein of 143.9 and 140.3 mg/dL, respectively. Table 4 demonstrates the effect of repeated FT times on the level of HGF and total protein. The effects of storage temperature and storage time are summarized in Tables 5 and 6, respectively.
Table 4.
Comparison of the effect of repeated FT times on the amount of extractable HGF and protein
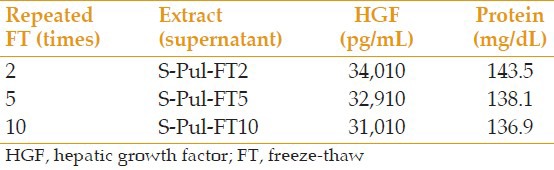
Table 5.
Comparison of the effect of storage temperature (6 days) on the amount of extractable HGF and protein
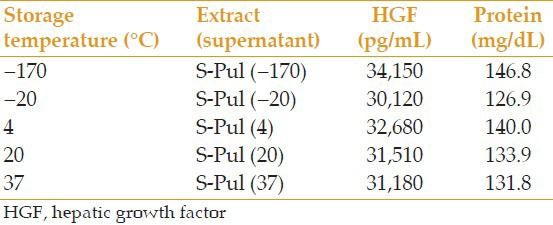
Table 6.
Comparison of the effect of storage time on the amount of extractable HGF and protein
In terms of the effect of the level of drying, SPD and SCD had HGF levels 34,580 pg/mL and 36,120 pg/mL, and their total protein content was 145.0 and 153.9 mg/dL respectively.
DISCUSSION
In this study, we compared different AME preparation methods and factors affecting HGF and total protein levels under different time and temperature conditions.
According to Table 1, 5 μl PI per each gram of dried AM seems to provide the necessary and sufficient amount of HGF and total protein. With amounts <5 μl/g, the level of HGF and protein decreased proportionate to the amount of PI, and the level of HGF almost halved when PI was not used. Increasing PI amounts over 5 μl/g of dried AM had no effect on the HGF and total protein level. If 5 μl PI is used per gram of dried AM in the extraction process, there would be no need to perform the processes at low temperature. The extracted HGF from the process done at 20°C was even slightly more than that of 4°C, the difference was not significant, though. Also, the extracted HGF with 5,000 rpm (g = 3,500) and 14,000 rpm (g = 25,000) processes showed no significant difference. In other studies, AME is prepared at different spinning speeds, and their similar results further support this observation.[10,11]
We compared homogenization and pulverization preparation methods, and the extractable factors achieved with pulverization were 20% more than that with homogenization. This is probably because the use of liquid nitrogen causes greater damage to the AM cell wall, and thus more protein and HGF emerge. Meanwhile, conducting the process with the pulverization method is easier and less time consuming.
It is also possible to extract more factors without any extra cost by adding BSS to PI and pulverized AM in several stages, and mixing the supernatants after repeated centrifuging. By increasing the number of times this process is done from once to 3 times, the extractable HGF almost doubled. Increasing the repeated pulverization times had no significant effect on the level of HGF. Furthermore, the AM drying phase, which is very time consuming, seemed to have no significant effect on the level of extractable HGF, and we can extract the same amount of HGF with partial drying.
We compared different storage conditions of AME. Although the stability of different growth factors vary, our findings indicated that HGF, which is an essential mitogen in AM, is relatively resistant to repeated FT. In terms of storage temperature conditions, if other measures such as adding preservatives are taken to prevent the growth of microbes in different temperatures, storing the extract at 20°C is not different from the 2°C to 8°C in the fridge, or even the −20°C in the freezer. However, keeping the extract at −170°C causes the least drop in HGF after 6 days.
The drop in HGF over time, while stored at −170°C, was 26% and 49% after 3 and 6 months, respectively. This is indicative for the instability of the factors over time even when stored at −170°C. Therefore, when the extract is not lyophilized, especially in research projects, this drop must be calculated according to the storage time, and taken into account when diluted samples are prepared.
Tseng has conducted valuable research on AME preparation[12] in which it is suggested to use 5 μl PI per gram of pulverized AM presenting agreement with our findings. He also assumed it is possible to use different centrifuge speeds and in-process temperatures, and according to our observations, the amount of HGF and total protein did not change by varying these conditions.
In the study by Tseng, both homogenization and pulverization preparation methods are suggested without comparing the extractable amount of factors. Our study shows that pulverization leads to 20% more extracted HGF and total protein.
Hu et al recently published an article which showed the positive effects of AME on treatment of rabbit corneas with herpes simplex keratitis.[13] Although, AME is still not approved for human use, it is under investigation and its therapeutic effect for ocular surface disease has been confirmed in different studies.[14] The details of its preparation techniques, however, have not been published in full.
In the study by Liang et al on 14 eyes, AME significantly increased the healing rate of acute chemical wounds. In their study, AME did not lead to complete healing of chronic wounds, however significantly decreased the diameter of the epithelial defect.[11] A similar study was conducted by Sheha et al on six human eyes with acute chemical burns; in addition to increasing the healing rate and preventing cicatericial complications, AME also significantly reduced inflammation, and the patient's pain and discomfort.[15] In this study, AME was prepared under sterile conditions. According to our results, the use of the 0.2 μm filter for sterilizing the extract had no considerable effect on the level of HGF and protein. This means that by using a 0.2 μm cellulose acetate filter, there is probably no need to conduct all preparation stages under sterile conditions of a laminar hood.
In the study by Koizumi et al, the amount of HGF per gram AM was 44,700 ± 33,700 pg; this translates to a range between 11,000 and 78,400 pg/g AM.[16] In our study, the amount of extracted HGF under different conditions ranged between 18,340 (without using PI) and 68,150 (with the three stage extraction), and the amounts of total protein with these two conditions were 78.3 and 280.2 mg/dL, respectively, which are comparable to the total protein extracted by Tseng (137.0–146.7 mg/dL).[12]
It must be noted that our basis of comparison of different AME preparation methods was only the amount of extractable HGF and total protein. Obviously, there are other growth factors in addition to HGF, and nonmitogen molecules including PI enzymes, as well as several unknown factors which may contribute to the healing effect of AM, and these may be affected in the different processing stages of AM. Further studies with these different extracts can determine which method is more appropriate and cost-effective for the preparation of AME in appropriate concentration.
For testing the efficacy of the extract solution which we obtained from AMs, it was added to cell culture media and its effect on cell growth was analyzed, which will be published, later.
In conclusion, appropriate extraction methods with an adequate amount of PI increases the level of extractable components from harvested amniotic membranes. To achieve the maximal therapeutic effects of AM extracts, it is necessary to consider the half life of its bioactive components.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jiang A, Li C, Gao Y, Zhang M, Hu J, Kuang W, et al. In vivo and in vitro inhibitory effect of amniotic extraction on neovascularization. Cornea. 2006;25:S36–S40. doi: 10.1097/01.ico.0000247211.78391.af. [DOI] [PubMed] [Google Scholar]
- 2.Ha SW, Kim JS, Cheong TB, Kim JC. Therapeutic effect of amniotic membrane extract on keratitis following corneal alkali burn. J Korean Ophthalmol Soc. 2001;42:1555–1561. [Google Scholar]
- 3.Kamiya K, Wang M, Uchida S, Amano S, Oshika T, Sakuragawa N, et al. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res. 2005;80:671–679. doi: 10.1016/j.exer.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Warkad VU, Sahu SK, Das S. Amniotic membrane grafting in the management of acute toxic epidermal necrolysis/Stevens Johnson syndrome. Am J Ophthalmol. 2011;151:381–382. doi: 10.1016/j.ajo.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Kheirkhah A, Casas V, Raju VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008;145:787–794. doi: 10.1016/j.ajo.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheirkhah A, Casas V, Blanco G, Li W, Hayashida Y, Chen YT, et al. Amniotic membrane transplantation with fibrin glue for conjunctivochalasis. Am J Ophthalmol. 2007;144:311–313. doi: 10.1016/j.ajo.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 7.Guo Q, Hao J, Yang Q, Guan L, Ouyang S, Wang J. A comparison of the effectiveness between amniotic membrane homogenate and transplanted amniotic membrane in healing corneal damage in a rabbit model. Acta Ophthalmol. 2011;89:e315–e319. doi: 10.1111/j.1755-3768.2010.02097.x. [DOI] [PubMed] [Google Scholar]
- 8.Shahriari HA, Tokhmehchi F, Reza M, Hashemi NF. Comparison of the effect of amniotic membrane suspension and autologous serum on alkaline corneal epithelial wound healing in the rabbit model. Cornea. 2008;27:1148–1150. doi: 10.1097/ICO.0b013e318173138a. [DOI] [PubMed] [Google Scholar]
- 9.Wolf HK, Zarnegar R, Oliver L, Michalopoulos GK. Hepatocyte growth factor in human placenta and trophoblastic disease. Am J Pathol. 1991;138:1035–1043. [PMC free article] [PubMed] [Google Scholar]
- 10.Choi JA, Choi JS, Joo CK. Effects of amniotic membrane suspension in the rat alkali burn model. Mol Vis. 2011;17:404–412. [PMC free article] [PubMed] [Google Scholar]
- 11.Liang L, Li W, Ling S, Sheha H, Qiu W, Li C, et al. Amniotic membrane extract for acute ocular chemical burns. Clin Experiment Ophthalmol. 2009;37:855–863. doi: 10.1111/j.1442-9071.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- 12.Tseng S. Purified amniotic membrane compositions and methods of use. United State Patent Application. 20070071740. [Google Scholar]
- 13.Hu N, Yang L, Guan H. Effects of amniotic membrane extraction on rabbit corneas with herpes simplex keratitis. Adv Intell Soft Comput. 2012;134:307–313. [Google Scholar]
- 14.Kenyon KR. Amniotic membrane: Mother's own remedy for ocular surface disease. Cornea. 2005;24:639–642. doi: 10.1097/01.ico.0000178220.12977.7e. [DOI] [PubMed] [Google Scholar]
- 15.Sheha H, Hashemi H, Liang L, Ramzy M, ZaKi A. Amniotic Membrane Extract for Acute Ocular Chemical Burns. Journal of American Science. 2010;6:427–433. [Google Scholar]
- 16.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]


