Abstract
Background
A growing number of studies on the associations between nicotinamide adenine dinucleotide phosphate (NADPH) oxidase p22phox C242T polymorphism and risk of ischemic cerebrovascular disease have recently been published, but the results remain inconsistent.
Material/Methods
We performed an updated meta-analysis to evaluate this association. Eight case-control studies were included, involving 2045 cases and 2102 controls. Heterogeneity was assessed by the Q test and the I2 statistic. Begg and Egger’s tests were conducted to evaluate publication bias. Odds ratio (OR) was tested to identify the associations.
Results
Significant associations between p22phox gene C242T polymorphism and ischemic cerebrovascular disease (ICVD) risk were observed in the allelic genetic model (OR=1.33, 95% confidence interval [CI] 1.00–1.77, p=0.048). No statistical significant association was found in the dominant model (OR=0.74, 95% CI 0.54–1.02, p=0.064) and recessive model (OR=1.40, 95% CI 0.89–2.19, p=0.146). Subgroup analysis showed an association in European populations for recessive model (OR=2.13, 95% CI 1.06–4.26, p=0.034) and no significant evidence of association in Asian populations was found (dominant model: OR=0.64, 95% CI 0.41–1.00, p=0.05; recessive model: OR=0.98, 95% CI 0.53–1.81, p=0.948; allelic model: OR=1.51, 95% CI 0.98–2.32, p=0.061).
Conclusions
p22phox gene C242T polymorphism was associated with ICVD risk in the allelic genetic model, as well as in European populations for recessive model. No evidence showed association between p22phox gene C242T polymorphism and ICVD risk in the dominant model and recessive model. Furthermore, no association existed in Asian populations for any of the 3 genetic models and European populations in the dominant model and allelic model.
MeSH Keywords: Cerebrovascular Disorders; NADPH Oxidase; Polymorphism, Genetic
Background
The etiology of ischemic cerebrovascular disease (ICVD) is multifactorial. Many risk factors (e.g., hypertension, diabetes, hyperlipemia, smoking, and genetic factors [1]) have been shown to contribute to ICVD. Reactive oxygen species (ROS) are considered to play an important role in vascular disease [2–8] by influencing the process of atherosclerosis of cerebral arteries [9–11]. The cytosolic components of p47phox, p67phox, p40phox, Rac-1 [12,13], Nox2, and p22phox (2 membrane-bound subunits) are involved in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, an important source of superoxide. The gene encodes p22phox and is located on chromosome 16q24, containing some genetic variants [14–16]. Recently, an increasing number of studies have investigated the association of NADPH oxidase C242T polymorphism and ICVD. Inoue et al. [17] reported that T allele in the p22phox gene presented association with a low risk for coronary artery disease. In contrast, some studies suggested that the T allele in the p22phox gene is a risk factor of coronary artery disease [18,19]. Published meta-analyses have evaluated the association of p22phox gene polymorphism and ICVD and various studies have focused on this topic, but the results were conflicting. Thus, we carried out this meta-analysis to reassess the possible association between p22phox gene C242T polymorphism and ICVD.
Material and Methods
Database search
Systematic searches of the electronic databases Embase, PubMed, Chinese Biological Medical Literature database (CBM), and Chinese National Knowledge Infrastructure (CNKI) from January 2000 to December 2013 were performed. The search strategy was as follows: “NADPH oxidase”; “p22phox gene”; “ “C242T polymorphisms”; “ischemic cerebrovascular disease”. The search results were limited to humans. Potential related studies were screened by manual searching of the references articles.
Selection criteria
Study inclusion criteria were: (1) studies on the association between NADPH oxidase C242T polymorphism and ICVD; (2) study type: case-control or cohort; (3) healthy people as the control group; (4) raw data was available. Studies were excluded if they were: (1) duplicate publications. Studies that deviated from Hardy-Weinberg equilibrium (HWE) were not eliminated.
Data extraction
The following information was extracted from each included study by 2 authors (P. Li, C. Qin): country, first author, year, journal, number of cases and controls, allele frequencies, genotype frequencies, ethnicity, experimental design, diagnoses of ICVD. Disagreement was resolved by consensus.
Statistical analysis
The association between p22phox gene C242T polymorphism and ICVD risk was measured by odds ratio (OR) and 95% confidence intervals (CIs). Heterogeneity was evaluated by the chi-squared test and the inconsistency index (I2). It indicated evidence of heterogeneity between studies when P value was<0.10. Quantification of the heterogeneity was calculated by the I2 metric, which is independent of the number of studies in the meta-analysis [20]. A random effects model was used if significant heterogeneity existed; otherwise, fixed effects model was used. Subgroup analyses were conducted by ICVD subtypes and ethnicity. Sensitivity analysis was carried out by removing each single article if necessary. The publication bias between studies was assessed by the funnel plot and Egger’s test. All the statistical analyses were performed using Review Manager 5.2 and Stata 12.0.
Results
Study characteristics
Of all the publications, a total of 10 articles were eligible. One study [21] was a review article and was excluded. Another article [22] without original data was also excluded. We tried to contact the author for the raw data but no response came back, which limited our quantitative synthesis. Hence, a total of 8 studies [19,23–29] involving 2045 cases and 2102 controls were eligible according to our selection criteria. Three articles were written in Chinese [27–29] and the rest were in English. Of all these articles, six [19,23,24,27–29] were in Asian populations and two [25,26] were in European populations. All the included studies were conducted from 2000 to 2013. Etiology subtype analyses were conducted in 4 studies [19,23,24,26]. The baseline characteristics of the participants are shown in Table 1.
Table 1.
Baseline characteristics of included studies.
| First Author | Year | Country | Number | Age (y) | ||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||
| Ito | 2000 | Japan | 226 | 301 | 58±8 | 59±4 |
| Shimo | 2004 | Japan | 120 | 177 | 61.2±11.4 | 58.9±9.25 |
| Han | 2004 | China | 112 | 105 | 64.92±11.1 | 63.91±11.9 |
| Kuroda | 2007 | Japan | 1055 | 1055 | 70±10 | 70±10 |
| Genius | 2008 | Germany | 161 | 136 | 40.4±7.6 | 34.3±9.5 |
| Niemiec | 2010 | Poland | 70 | 50 | 8.48 ±5.44 | 9.0±6.1 |
| Chen | 2011 | China | 176 | 131 | 65±9.4 | 62±9.1 |
| Li | 2013 | China | 125 | 147 | 65.42±11.65 | 65.22 ±9.69 |
Meta-analysis and subgroup analysis
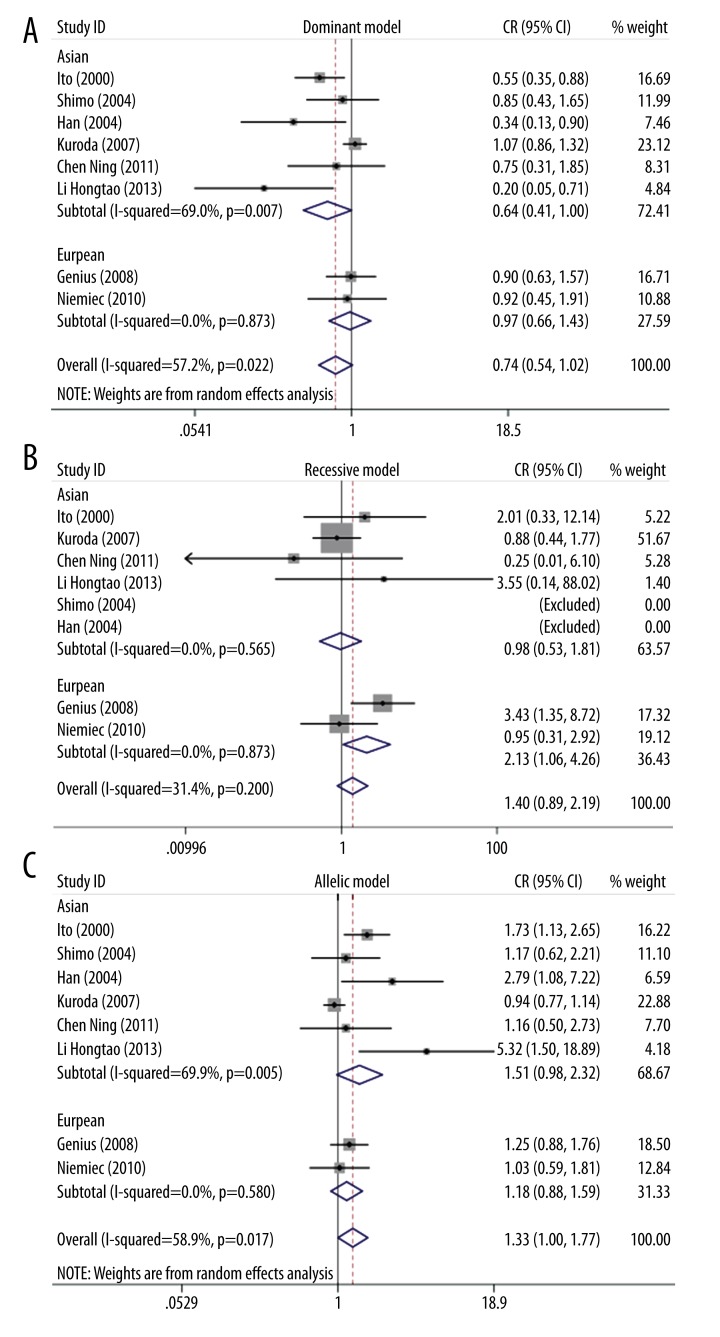
A total of 2045 cases and 2102 controls in the included studies were analyzed in our meta-analysis. Pooling the data of the 8 included studies, the overall results showed that p22phox gene C242T had no association with ICVD risk in the following genetic models: dominant model (OR=0.74, 95% CI 0.54–1.02, p=0.064) and recessive model (OR=1.40, 95% CI 0.89–2.19, p=0.146). The overall results of the allelic model (OR=1.33, 95% CI 1.00–1.77, p=0.048) was significantly associated with ICVD risk (Figure 1). The overall results are summarized in Table 2. A fixed effects model was used because no heterogeneity existed in the recessive model (I2=31.4%, p=0.2). If significant inter-study heterogeneity existed in the dominant model (I2=57.2%, p=0.022) and allelic model (I2=58.9%, p=0.017), the random effects model was used to calculate the pooled OR.
Figure 1.
Overall and subgroup forest plot of genetic models (A: dominant model; B: recessive model; C: allelic model).
Table 2.
Pooled analysis of genetic models.
| Genetic model | Cases (n) | Controls (n) | Fixed/Random effects OR (95% CI) | Heterogeneity test p value | I2 | P-value of test for overall effect |
|---|---|---|---|---|---|---|
| Dominant | 2045 | 2102 | 0.74 [0.54,1.02] | 0.022 | 57.2% | 0.064 |
| Recessive | 2045 | 2102 | 1.40 [0.89,2.19] | 0.2 | 31.4% | 0.146 |
| Allelic | 2045 | 2102 | 1.33 [1.00,1.77] | 0.017 | 58.9% | 0.048 |
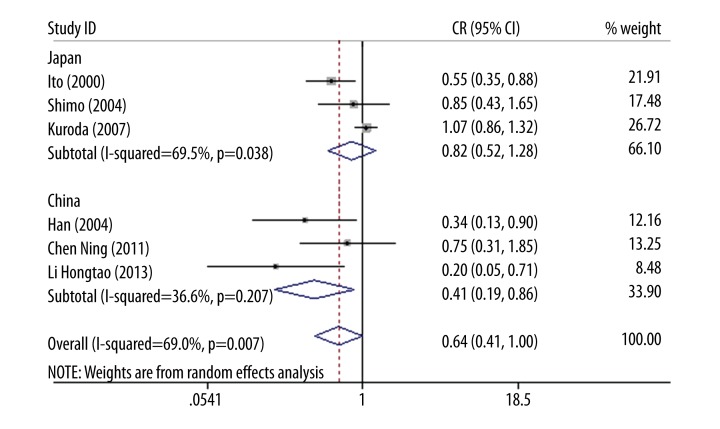
To explore the source of inter-study heterogeneity, subgroup analyses were conducted by ethnicity. A total of 6 studies [19,23,24,27–29] in Asian populations and 2 studies [25,26] in European populations were identified. No relevant association was found in p22phox gene C242T polymorphism and ICVD in both Asian populations (OR=0.64, 95% CI 0.41–1.00, p=0.05) and European populations (OR=0.97, 95% CI 0.66–1.43, p=0.88) for dominant model (Figure 1A). There was no heterogeneity in European populations (I2=0%, p=0.873), but significant heterogeneity was discovered in Asian populations (I2=69%, p=0.007). In the 6 Asian population studies, 3 studies [27–29] investigated people of Chinese descent and the other three [19,23,24] investigated people of Japanese descent. Thus, the 6 Asian population studies were divided into 2 subgroups for further analysis. The results indicated no heterogeneity in Chinese populations (I2=36.6%, p=0.207) but heterogeneity existed in Japanese populations (I2=69.5%, p=0.038) (Figure 2).
Figure 2.
Forest plot of Asian populations for dominant model.
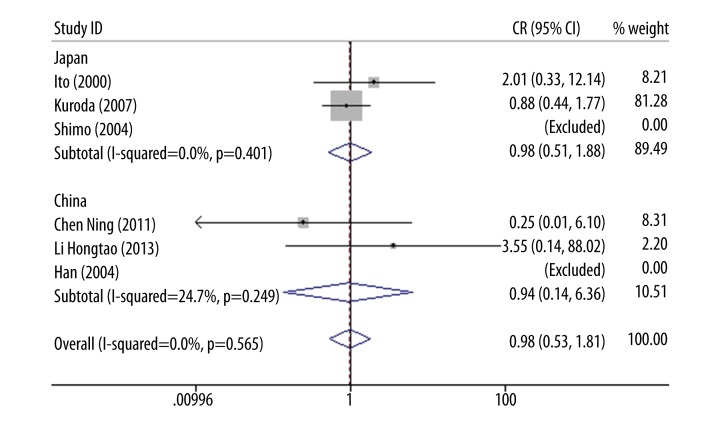
Results of subgroup analysis for recessive model indicated a pertinent association in p22phox gene C242T polymorphism and ICVD in European people (OR=2.13, 95% CI 1.06–4.26, p=0.034) but no relevant association in Asian populations (OR=0.98, 95% CI 0.53–1.81, p=0.948) was found (Figure 1B). A further analysis of Asian decent was conducted. However, no significant association was found between p22phox gene C242T polymorphism and ICVD in Chinese (OR=0.94, 95%CI: 0.14–6.36, p=0.949) or Japanese populations (OR=0.98, 95% CI 0.51–1.88, p=0.962) (Figure 3). No inter-study heterogeneity existed in either Chinese (I2=24.7%, p=0.249) or Japanese populations (I2=0%, p=0.401).
Figure 3.
Forest plot of Asian populations for recessive model (Japan: OR=0.98, 95%CI: 0.51–1.88, I2=0%; China: OR=0.94, 95%CI: 0.53–1.81, I2=24.7%).
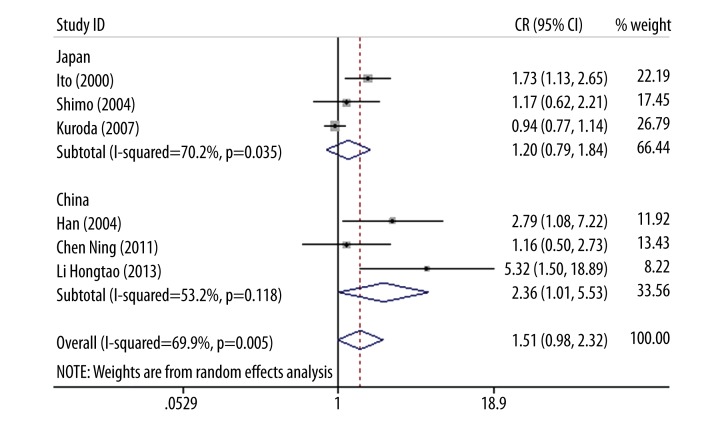
p22phox gene C242T polymorphism had no association with ICVD in Asian (OR=1.51, 95% CI 0.98–2.32, p=0.061) and European descent (OR=1.18, 95% CI: 0.88–1.59, p=0.266) (Figure 1C). No heterogeneity existed in European populations (I2=0%, p=0.58), but moderate heterogeneity was found in Asian populations (I2=69.9%, p=0.005). Further analysis of Asian groups for an allelic model showed significant association between p22phox gene C242T polymorphism and ICVD risk in Chinese populations (OR=2.36, 95% CI1.01–5.53, p=0.048), but no significant association existed in Japanese population (OR=1.20, 95% CI 079–1.84, p=0.386) (Figure 4).
Figure 4.
Forest plot of Asian populations for allelic model.
Sensitivity analysis
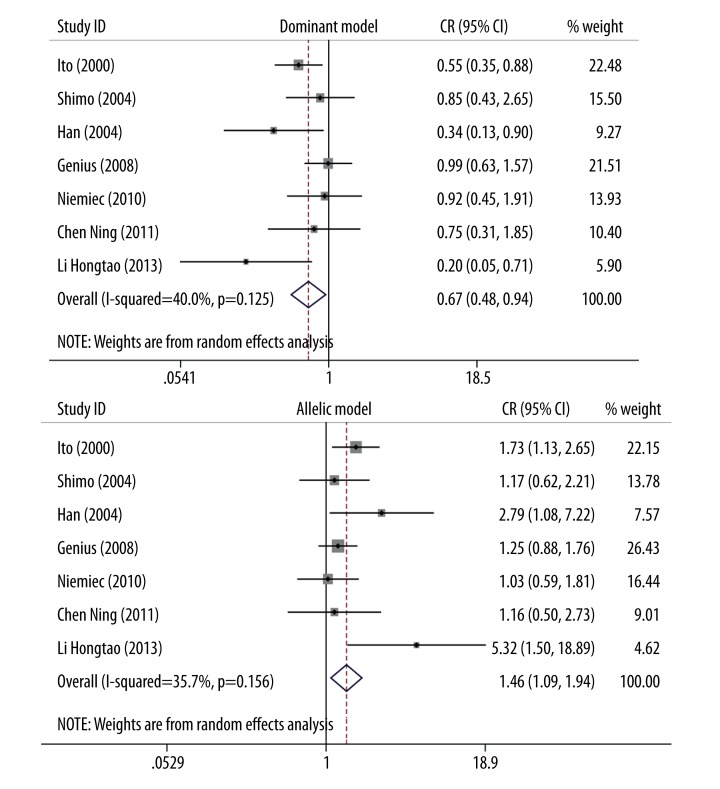
Sensitivity analysis was carried out by removing 1 individual study each time to evaluate the stability of the meta-analysis and explore the source of heterogeneity. Results indicated evidence of quite obvious impact on the overall pooled OR for the dominant model, recessive model, and allelic model, which demonstrated the meta-analysis results were stable. The I-square and p values of heterogeneity test of the results altered when 1 study was removed. No evidence of heterogeneity existed when this study, by Kuroda et al. [24] was removed (dominant model: I2=40%, p=0.125; allelic model: I2=35.7%, p=0.156), which indicated that this study might contribute to the inter-study heterogeneity (Figure 5). Results showed that the pooled ORs were stable when studies that deviated from HWE were excluded.
Figure 5.
Sensitivity analysis results (study by Kuroda et al. was removed; dominant model: I2=40%, p=0.125, OR=0.67, 95%CI 0.48–0.94; allelic model: I2=35.7%, p=0.156, OR=1.46, 95% CI 1.09–1.94).
Publication bias
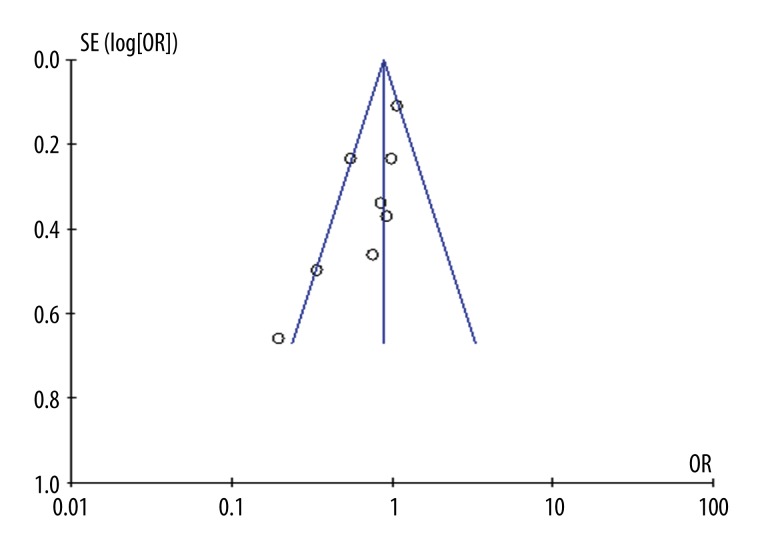
Funnel plot and Egger’s test were conducted to value the publication bias. The funnel plot revealed a potential publication bias (Figure 6).
Figure 6.
Funnel plot.
Discussion
The pathogenesis of ICVD is still unclear. The incidence of ICVD are known to be related to ethnic factors [1,30], which may reveal genetic or non-genetic differences. Recently, more and more studies have focused on the relationship between p22phox gene C242T polymorphism and ICVD risk. Published meta-analysis by Li et al. [31] indicated no evidence of association between p22phox gene C242T polymorphism and ICVD risk. The potential association between p22phox gene C242T polymorphism and ICVD has aroused great interest from the public. A variety of studies have been devoted to this topic, but the results are conflicting. Therefore, we conducted this meta-analysis to identify and reassess the association between p22phox gene C242T polymorphism and ICVD.
To our best knowledge, this is the most comprehensive meta-analysis thus far to assess the association between p22phox gene C242T polymorphism and ICVD risk with 8 studies included, involving 2045 ICVD cases and 2102 controls. Results obtained from the allelic model confirmed a positive association between p22phox gene C242T polymorphism and ICVD risk. The pooled results of the dominant model and recessive model indicated that p22phox gene C242T polymorphism was not associated with ICVD, which is consistent with the previously published meta-analyses.
To estimate the genetic effect on different population, subgroup analysis by ethnicity was conducted. According to the results, it seems to have a null hereditary effect in Asian populations for the dominant model, recessive model, and allelic model. In European populations, no significant association of genetic effect was found in the dominant model and allelic model. However, p22phox gene C242T polymorphism was associated with ICVD risk in the recessive model, which may indicate that European participants who inherited a recessive allele may have increased p22phox gene C242T polymorphism susceptibility. A further analysis in Asian groups was also conducted, revealing a null genetic effect in Chinese populations for the dominant and recessive model but a relevant association of ICVD in the allelic model. No significant association of p22phox gene C242T polymorphism and ICVD existed in Japanese populations for the following genetic model: dominant model, recessive model and allelic model.
Heterogeneity is a potential dilemma and is inevitable when combining the overall results of meta-analyses [32]. The I-square values fall within the range of 0–100%, with higher values demonstrating a greater degree of heterogeneity [33]. Moderate heterogeneity was observed in the dominant and allelic genetic models except for the recessive model when pooling the overall results of included studies. To explore the source of heterogeneity, subgroup analysis and sensitivity analysis were conducted.
The results of subgroup analysis by ethnicity showed that no heterogeneity existed in European populations for dominant and allelic genetic models. A moderate degree of inter-study heterogeneity existed in Asian populations for both dominant and allelic genetic models, which might contribute to the existence of heterogeneity. We then conducted a further subgroup analysis in Asian populations. Significant heterogeneity was observed in Japanese populations in both dominant and allelic genetic models, but no heterogeneity was found in Chinese populations in these 2 genetic models. Sensitivity analysis was conducted by pooling the overall data of included studies with 1 individual study eliminated each time. Heterogeneity existed when eliminating most of the included studies except for 1 study. When the study conducted by Kuroda et al. [24] was eliminated, the I-square and p-value for heterogeneity test clearly changed in both dominant and allelic genetic models (Figure 5) without statistical significance of heterogeneity. These findings indicated that inter-study heterogeneity might come from the study by Kuroda et al. In the 3 Japanese studies, 2 studies [19,23] selected healthy subjects who visited the hospital for regular check-ups as controls, while Kuroda et al. [24] chose control subjects from participants of an epidemiological study called the Hisayama study. This indicated that the source of the control group might cause heterogeneity. And inter-study heterogeneity might come from various selection criteria of cases and controls, different sample size, and variation in diagnostic method of subtypes. Many factors (even the environmental factors) could have led to the existence of heterogeneity.
Publication bias was measured. Potential publication bias existed, although we performed careful search strategies for published studies and data extraction. Relevant English and Chinese studies were included, but articles in other languages, which might have contributed to the results, were probably missed. The unpublished studies might also have resulted in publication bias.
Polymorphisms in p22phox gene have been reported to be associated with NADPH oxidase activity, which potentially contributes to the pathogenetic mechanisms of ICVD [30]. It has been reported that increased atherosclerosis had associations with increased ROS production and increased activity of NADPH oxidase subunits p22phox and nox2 [34]. Mutation of C→T may reduce susceptibility to cardiovascular diseases due to decreased oxidative stress in vasculature [35], and increased oxidative stress and NADH stimulated vascular superoxide production might possibly contribute to the development of atherosclerosis [36]. Specific risk factors, such as diabetes, may cause increased expression and activity of NADPH oxidase in vascular disease. Letonja et al. [30] explored aspects of NADPH oxidase C242T polymorphism in pathogenesis of carotid atherosclerosis in patients with type 2 diabetes, and found no significant association of C242T polymorphism in the NADPH gene with clinical parameters, including plaque score and intima-media thickness of the carotid artery, using the 75th percentile of intima-media thickness as a cut-off value. Factors like more frequent stable atherosclerotic plaques, lower CIMT, and lower risk for complications might cause such phenomena.
Several limitations of this meta-analysis must be considered. First of all, heterogeneity existed in the included studies. The presence of heterogeneity can result from various aspects such as sample population and selection criteria of cases and controls. Future studies with larger sample sizes are needed. Secondly, only published articles were analyzed; the unpublished significant findings or negative findings may also contribute to the overall results.
Conclusions
In conclusion, this meta-analysis indicated a significant association between p22phox gene C242T polymorphism and ICVD risk in the allelic genetic model. No statistically relevant association existed in the dominant model and recessive model. Furthermore, no relevant association was found in Asian populations for any of the 3 genetic models and European populations in the dominant model and allelic model. Results of subgroup analysis revealed that p22phox gene C242T polymorphism had a significant association with ICVD in European populations for the recessive model. Future studies were needed for further analysis of the association between p22phox gene C242T polymorphism and ICVD.
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
Source of support: Self financing
References
- 1.Starcevic JN, Petrovic D. Carotid intima media-thickness and genes involved in lipid metabolism in diabetic patients using statins – a pathway toward personalized medicine. Cardiovasc Hematol Agents Med Chem. 2013;11(1):3–8. doi: 10.2174/1871525711311010003. [DOI] [PubMed] [Google Scholar]
- 2.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27(6):1124–29. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 3.Custodis F, Baumhakel M, Schlimmer N, et al. Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117(18):2377–87. doi: 10.1161/CIRCULATIONAHA.107.746537. [DOI] [PubMed] [Google Scholar]
- 4.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11(4):791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He YY, Hsu CY, Ezrin AM, Miller MS. Polyethylene glycol-conjugated superoxide dismutase in focal cerebral ischemia-reperfusion. Am J Physiol. 1993;265(1 Pt 2):H252–56. doi: 10.1152/ajpheart.1993.265.1.H252. [DOI] [PubMed] [Google Scholar]
- 6.Heistad DD. Oxidative stress and vascular disease: 2005 Duff lecture. Arterioscler Thromb Vasc Biol. 2006;26(4):689–95. doi: 10.1161/01.ATV.0000203525.62147.28. [DOI] [PubMed] [Google Scholar]
- 7.Imaizumi S, Woolworth V, Fishman RA, Chan PH. Liposome-entrapped superoxide dismutase reduces cerebral infarction in cerebral ischemia in rats. Stroke. 1990;21(9):1312–17. doi: 10.1161/01.str.21.9.1312. [DOI] [PubMed] [Google Scholar]
- 8.Liu TH, Beckman JS, Freeman BA, et al. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol. 1989;256(2 Pt 2):H589–93. doi: 10.1152/ajpheart.1989.256.2.H589. [DOI] [PubMed] [Google Scholar]
- 9.Miller AA, Drummond GR, Sobey CG. Reactive oxygen species in the cerebral circulation: are they all bad? Antioxid Redox Signal. 2006;8(7–8):1113–20. doi: 10.1089/ars.2006.8.1113. [DOI] [PubMed] [Google Scholar]
- 10.Kim GH, Ryan JJ, Archer SL. The role of redox signaling in epigenetics and cardiovascular disease. Antioxid Redox Signal. 2013;18(15):1920–36. doi: 10.1089/ars.2012.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnholtz A, Nickenig G, Schulz E, et al. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99(15):2027–33. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 12.DeLeo FR, Renee J, McCormick S, et al. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest. 1998;101(2):455–63. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90(5):491–93. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soccio M, Toniato E, Evangelista V, et al. Oxidative stress and cardiovascular risk: the role of vascular NAD(P)H oxidase and its genetic variants. Eur J Clin Invest. 2005;35(5):305–14. doi: 10.1111/j.1365-2362.2005.01500.x. [DOI] [PubMed] [Google Scholar]
- 15.Ushio-Fukai M, Zafari AM, Fukui T, et al. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271(38):23317–21. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 16.Rae J, Noack D, Heyworth PG, et al. Molecular analysis of 9 new families with chronic granulomatous disease caused by mutations in CYBA, the gene encoding p22(phox) Blood. 2000;96(3):1106–12. [PubMed] [Google Scholar]
- 17.Inoue N, Kawashima S, Kanazawa K, et al. Polymorphism of the NADH/NADPH oxidase p22 phox gene in patients with coronary artery disease. Circulation. 1998;97(2):135–37. doi: 10.1161/01.cir.97.2.135. [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Duarte N, Wilcken DE, Wang XL. NADH/NADPH oxidase p22 phox C242T polymorphism and coronary artery disease in the Australian population. Eur J Clin Invest. 1999;29(9):744–48. doi: 10.1046/j.1365-2362.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 19.Ito D, Murata M, Watanabe K, et al. C242T polymorphism of NADPH oxidase p22 PHOX gene and ischemic cerebrovascular disease in the Japanese population. Stroke. 2000;31(4):936–39. doi: 10.1161/01.str.31.4.936. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7(1):51–61. doi: 10.1258/1355819021927674. [DOI] [PubMed] [Google Scholar]
- 21.San Jose G, Fortuno A, Beloqui O, et al. NADPH oxidase CYBA polymorphisms, oxidative stress and cardiovascular diseases. Clin Sci. 2008;114(3):173–82. doi: 10.1042/CS20070130. [DOI] [PubMed] [Google Scholar]
- 22.Khan U, Bevan S, Markus HS. NADPH oxidase polymorphisms in cerebral small vessel disease. Cerebrovasc Dis. 2007;24(1):135–38. doi: 10.1159/000103615. [DOI] [PubMed] [Google Scholar]
- 23.Shimo-Nakanishi Y, Hasebe T, Suzuki A, et al. Functional effects of NAD(P)H oxidase p22(phox) C242T mutation in human leukocytes and association with thrombotic cerebral infarction. Atherosclerosis. 2004;175(1):109–15. doi: 10.1016/j.atherosclerosis.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda J, Kitazono T, Ago T, et al. NAD(P)H oxidase p22(phox) C242T polymorphism and ischemic stroke in Japan: the Fukuoka Stroke Registry and the Hisayama study. Eur J Neurol. 2007;14(10):1091–97. doi: 10.1111/j.1468-1331.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- 25.Niemiec P, Zak I, Emich-Widera E, et al. The C242T polymorphism of the gene encoding cytochrome b-245 alpha is not associated with paediatric ischaemic stroke: family-based and case-control study. Neurologia I Neurochirurgia Polska. 2010;44(5):453–58. doi: 10.1016/s0028-3843(14)60135-3. [DOI] [PubMed] [Google Scholar]
- 26.Genius J, Grau AJ, Lichy C. The C242T polymorphism of the NAD(P)H oxidase p22(phox) subunit is associated with an enhanced risk for cerebrovascular disease at a young age. Cerebrovasc Dis. 2008;26(4):430–33. doi: 10.1159/000155639. [DOI] [PubMed] [Google Scholar]
- 27.Han HX LX, Zhang C. Correlation between NAD(P)H oxidase p22phox C242T polymorphism and ischemic cerebrovascular diseases. Cerebrovasc Dis Foreign Med Sci. 2004;(03):183–86. [Google Scholar]
- 28.Hongtao L, Fangping Y, Yingchun Z, et al. The relationship between NAD(P)H oxidase p22phox C242T polymorphism and stroke in the population of Han nationality of Shanghai city. Acta Universitatis Medicinalis Nanjing (Natural Science) 2013;(08):1081–86. [Google Scholar]
- 29.Chen Ning FY, Yayun Y, Peihua N. Correlation between NAD(P)H oxidase p22phox C242T polymorphism and cerebral infarction. Laboratory Medicine. 2011;(03):175–79. [Google Scholar]
- 30.Letonja MS, Nikolajevic-Starcevic J, Batista DC, et al. Association of the C242T polymorphism in the NADPH oxidase p22 phox gene with carotid atherosclerosis in Slovenian patients with type 2 diabetes. Mol Biol Rep. 2012;39(12):10121–30. doi: 10.1007/s11033-012-1886-3. [DOI] [PubMed] [Google Scholar]
- 31.Li BH, Zhang LL, Zhang BB, et al. Association between NADPH Oxidase p22(phox) C242T Polymorphism and Ischemic Cerebrovascular Disease: A Meta-Analysis. Plos One. 2013;8(2):e56478. doi: 10.1371/journal.pone.0056478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20(9):439–44. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Peddareddygari LR, Dutra AV, Levenstien MA, et al. An analysis of methylenetetrahydrofolate reductase and glutathione S-transferase omega-1 genes as modifiers of the cerebral response to ischemia. BMC Neurology. 2009;9:37. doi: 10.1186/1471-2377-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorescu D, Weiss D, Lassegue B, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105(12):1429–35. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 35.Guzik TJ, West NE, Black E, et al. Functional effect of the C242T polymorphism in the NAD(P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation. 2000;102(15):1744–47. doi: 10.1161/01.cir.102.15.1744. [DOI] [PubMed] [Google Scholar]
- 36.Hayaishi-Okano R, Yamasaki Y, Kajimoto Y, et al. Association of NAD(P)H oxidase p22 phox gene variation with advanced carotid atherosclerosis in Japanese type 2 diabetes. Diabetes Care. 2003;26(2):458–63. doi: 10.2337/diacare.26.2.458. [DOI] [PubMed] [Google Scholar]