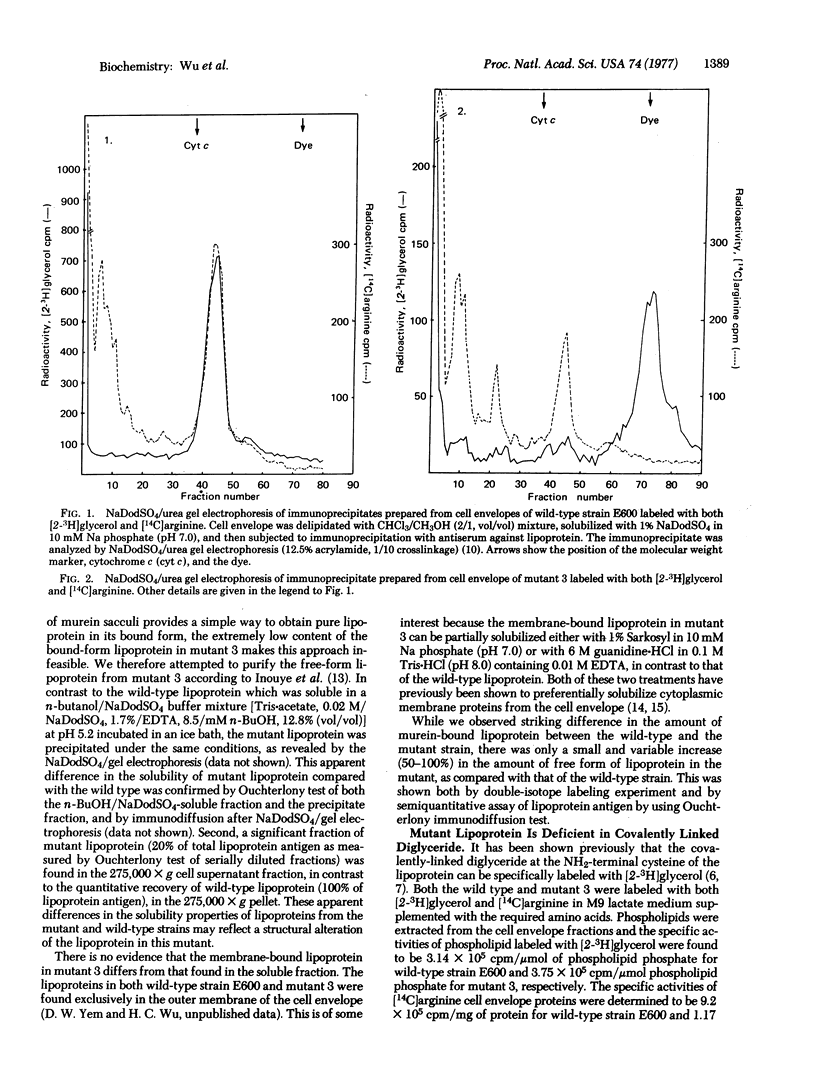
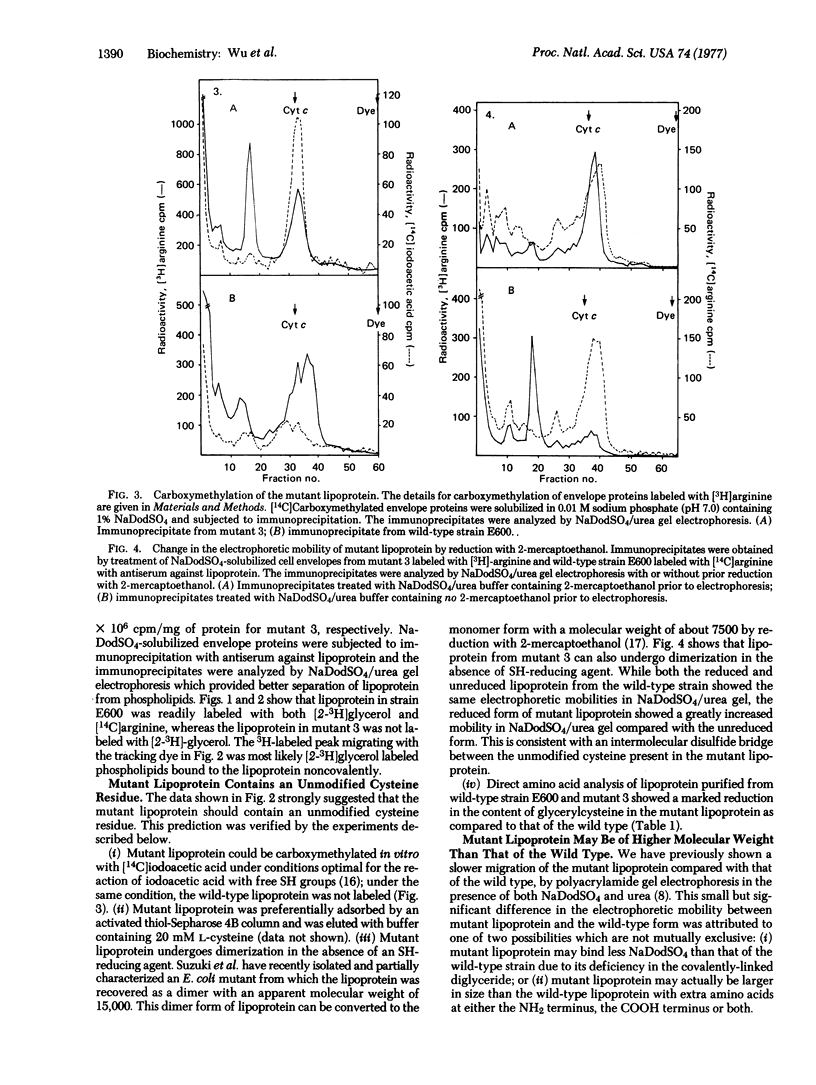
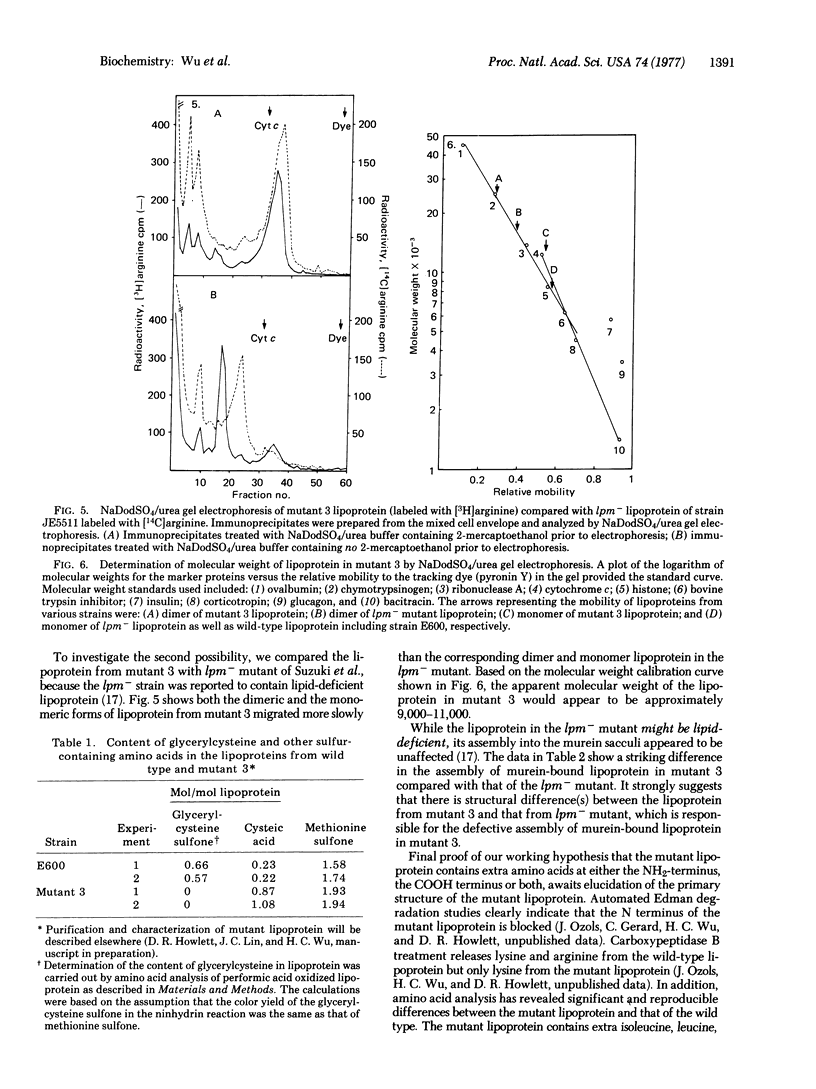
Abstract
A lipoprotein mutant of E. coli K-12 has been characterized. The mutant lipoprotein was found to differ from the wild-type lipoprotein in the following respects: (i) it is present in an appreciable amount in the soluble fraction (275,000 X g supernatant); (ii) it lacks the covalently-linked diglyceride; (iii) it contains an unmodified cysteine which can be carboxymethylated in vitro; (iv) it undergoes dimerization and the dimer can be converted into monomeric form by reduction with 2-mercaptoethanol; (v) both the monomeric form and especially the dimeric form of the mutant lipoprotein migrate more slowly than the corresponding forms of wild-type lipoprotein in sodium dodecyl sulfate/urea polyacrylamide gel electrophoresis; and (vi) the mutant lipoprotein is not assembled into the murein sacculi, and this results in a greatly reduced amount of bound-form lipoprotein in the mutant. These data strongly suggest that the mutation has affected the primary structure of lipoprotein, in such a way that it is not modified normally, leading to the production of a structurally-altered lipoprotein deficient in covalently-linked lipid as well as a defective assembly of the altered lipoprotein into the rigid layer of the cell envelope.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Bosch V. Sequence of the murein-lipoprotein and the attachment site of the lipid. Eur J Biochem. 1972 Jun 23;28(1):51–69. doi: 10.1111/j.1432-1033.1972.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Hirashima A., Inouye M. Puromycin-resistant biosynthesis of a specific outer-membrane lipoprotein of Escherichia coli. J Bacteriol. 1976 Apr;126(1):183–191. doi: 10.1128/jb.126.1.183-191.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Wu H. C., Venkateswaran P. S., Inouye M. Two forms of a structural lipoprotein in the envelope of Escherichia coli. Further characterization of the free form. J Biol Chem. 1973 Aug 25;248(16):5654–5659. [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Inoyye S., Takeishi K., Lee N., DeMartini M., Hirashima A., Inouye M. Lipoprotein from the outer membrane of Escherichia coli: purification, paracrystallization, and some properties of its free form. J Bacteriol. 1976 Jul;127(1):555–563. doi: 10.1128/jb.127.1.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Wu H. C. Biosynthesis and assembly of envelope lipoprotein in a glycerol-requiring mutant of Salmonella typhimurium. J Bacteriol. 1976 Mar;125(3):892–904. doi: 10.1128/jb.125.3.892-904.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldow C., Robertson J., Rothfield L. Purification of bacterial membrane proteins. The use of guanidinium thiocyanate and urea. J Membr Biol. 1972;10(2):137–152. doi: 10.1007/BF01867850. [DOI] [PubMed] [Google Scholar]
- Ozols J. Amino acid sequence of rabbit liver microsomal cytochrome b5. J Biol Chem. 1970 Oct 10;245(19):4863–4874. [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Iketani H., Campisi J., Hirashima A. Novel mutation that causes a structural change in a lipoprotein in the outer membrane of Escherichia coli. J Bacteriol. 1976 Sep;127(3):1494–1501. doi: 10.1128/jb.127.3.1494-1501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Lin J. J. Escherichia coli mutants altered in murein lipoprotein. J Bacteriol. 1976 Apr;126(1):147–156. doi: 10.1128/jb.126.1.147-156.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



