Abstract
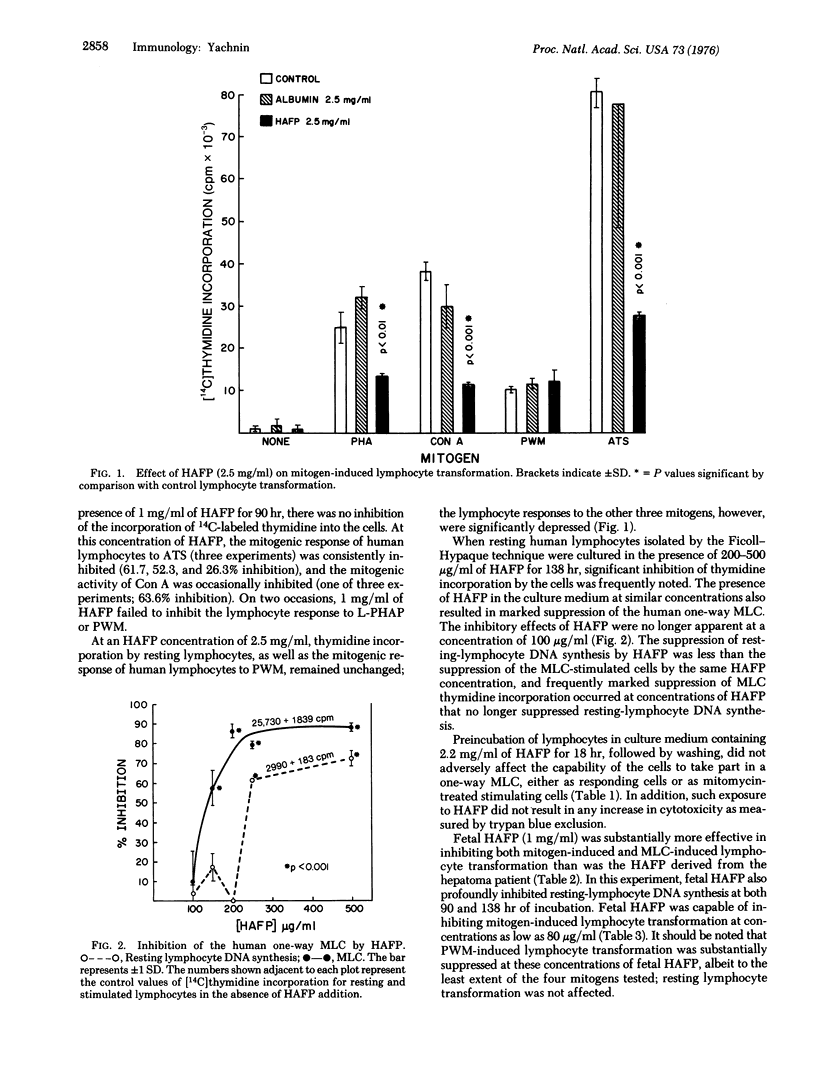
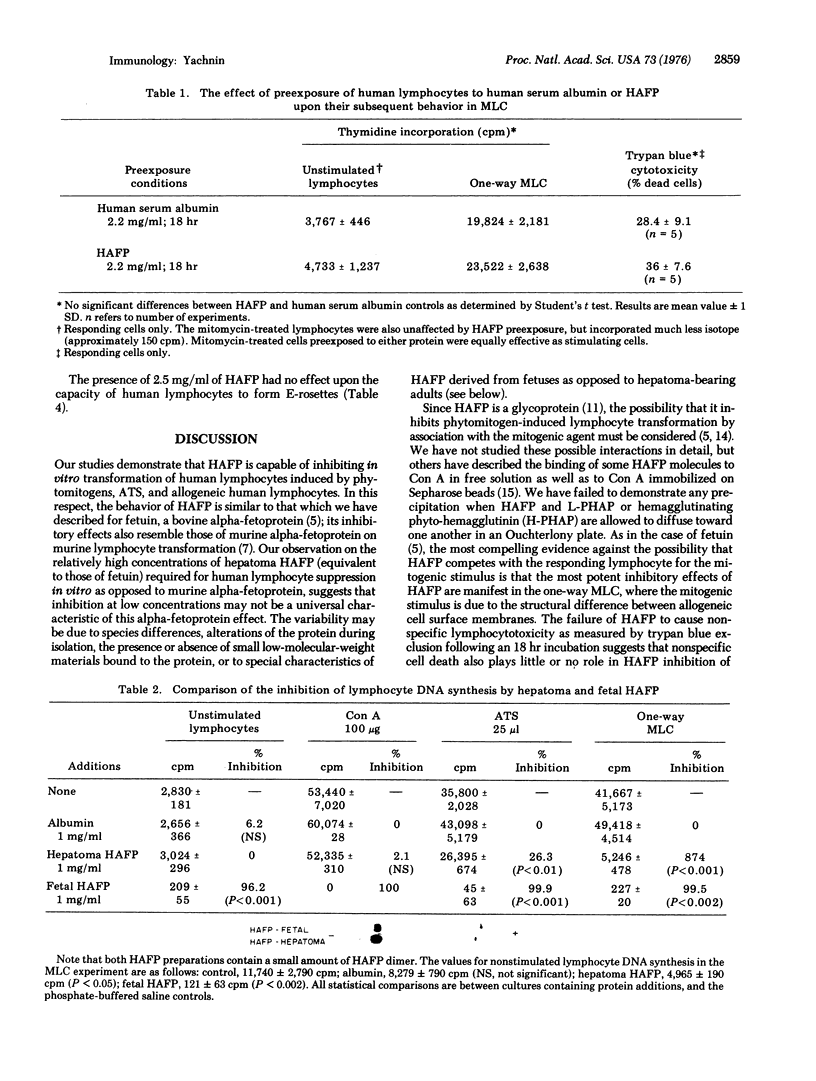
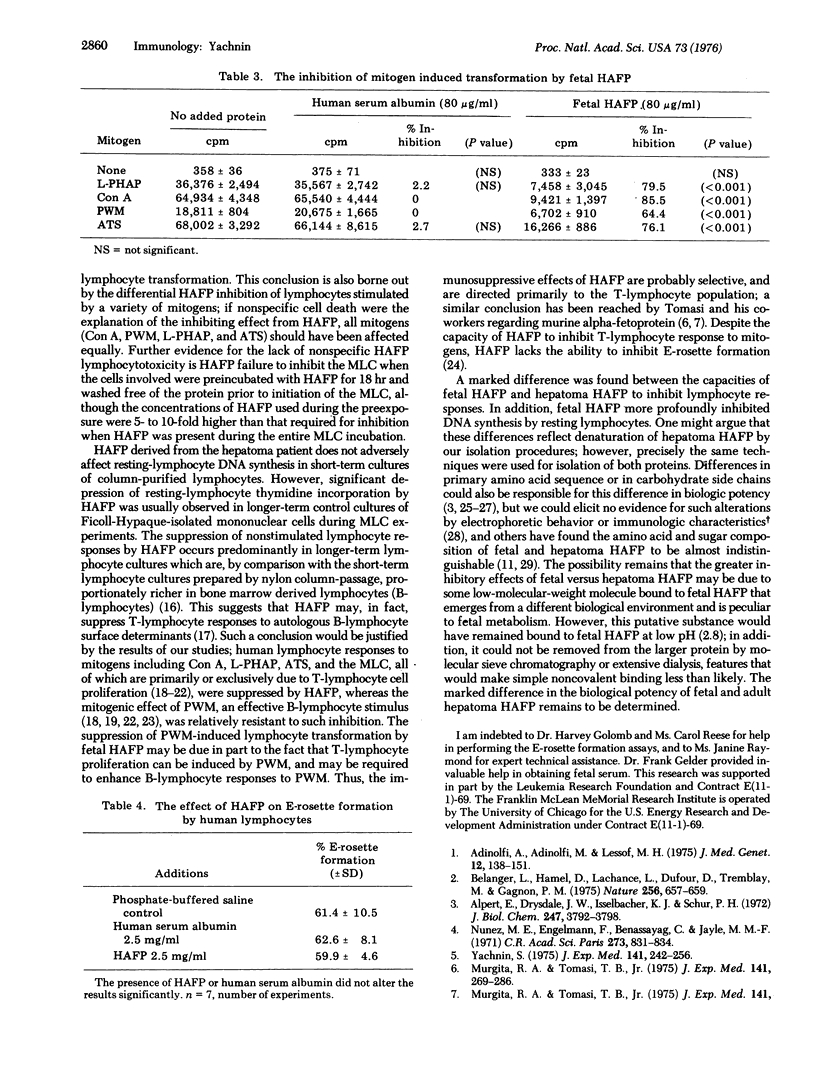
We have studied the effects of human alpha-fetoprotein (HAFP), isolated from the serum and ascitic fluid of a hepatoma-bearing patient, on the in vitro transformation of human peripheral blood lymphocytes by a variety of mitogenic stimuli. At a concentration of 2.5 mg/ml, HAFP inhibited the lymphocyte response to phytohemagglutinin, concanavalin A, and rabbit anti-human thymocyte serum, but failed to inhibit the response to pokeweed mitogen. HAFP was able to inhibit the one-way mixed lymphocyte culture at concentrations of 250-500 mug/ml, but failed to inhibit at 100 mug/ml. Exposure of lymphocytes to 2.2 mg/ml of HAFP for 18 hr did not result in significant lymphocytotoxicity, and such cells washed free of HAFP were fully capable of participating in the mixed lymphocyte culture. HAFP did not inhibit lymphocyte E-rosette formation. Fetal HAFP was more effective in inhibiting human lymphocyte responses than hepatoma HAFP. These experiments support the suggestion that HAFP plays an important immunoregulatory role during fetal development, possibly through the suppression of thymus-derived lymphocyte responses to antigenic stimuli; they also suggest that there are important differences in the biological properties of hepatoma and fetal HAFP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi A., Adinolfi M., Cohen S. Isolation and characterization of human foetal alpha-globulin (alpha 1F) from foetal and hepatoma sera. Biochim Biophys Acta. 1971 Nov 19;251(2):197–207. doi: 10.1016/0005-2795(71)90103-6. [DOI] [PubMed] [Google Scholar]
- Adinolfi A., Adinolfi M., Lessof Alpha-feto-protein during development and in disease. J Med Genet. 1975 Jun;12(2):138–151. doi: 10.1136/jmg.12.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen L. W., Svenson R. H., Yachnin S. Purification of mitogenic proteins derived from Phaseolus vulgaris: isolation of potent and weak phytohemagglutinins possessing mitogenic activity. Proc Natl Acad Sci U S A. 1969 Jun;63(2):334–341. doi: 10.1073/pnas.63.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert E., Drysdale J. W., Isselbacher K. J., Schur P. H. Human -fetoprotein. Isolation, characterization, and demonstration of microheterogeneity. J Biol Chem. 1972 Jun 25;247(12):3792–3798. [PubMed] [Google Scholar]
- Belanger L., Hamel D., Lachance L., Dufour D., Tremblay M., Gagnon P. M. Hormonal regulation of alpha1 foetoprotein. Nature. 1975 Aug 21;256(5519):657–659. doi: 10.1038/256657a0. [DOI] [PubMed] [Google Scholar]
- Braylan R., Variakojis D., Yachnin S. The Sézary syndrome lymphoid cell: abnormal surface properties and mitogen responsiveness. Br J Haematol. 1975 Dec;31(4):553–564. doi: 10.1111/j.1365-2141.1975.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Response of human thymus-derived (T) and non-thymus-derived (B) lymphocytes to mitogenic stimulation in vitro. Eur J Immunol. 1974 Mar;4(3):193–199. doi: 10.1002/eji.1830040308. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Greaves M., Janossy G., Doenhoff M. Selective triggering of human T and B lymphocytes in vitro by polyclonal mitogens. J Exp Med. 1974 Jul 1;140(1):1–18. doi: 10.1084/jem.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Siegal F. P. Letter: In vitro inhibition of E rosettes by human amniotic fluid. N Engl J Med. 1975 Aug 7;293(6):302–303. doi: 10.1056/NEJM197508072930614. [DOI] [PubMed] [Google Scholar]
- Gustine D. L., Zimmerman E. F. Developmental changes in microheterogeneity of foetal plasma glycoproteins of mice. Biochem J. 1973 Mar;132(3):541–551. doi: 10.1042/bj1320541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häyry P., Andersson L. C., Nordling S., Virolainen M. Allograft response in vitro. Transplant Rev. 1972;12:91–140. doi: 10.1111/j.1600-065x.1972.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgita R. A., Tomasi T. B., Jr Suppression of the immune response by alpha-fetoprotein on the primary and secondary antibody response. J Exp Med. 1975 Feb 1;141(2):269–286. doi: 10.1084/jem.141.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E., Engelmann F., Benassayag C., Jayle M. F. Identification et purification préliminaire de la faeto-protéine liant les aestrogènes dans le sérum de rats nouveau-nés. C R Acad Sci Hebd Seances Acad Sci D. 1971 Aug 30;273(9):831–834. [PubMed] [Google Scholar]
- Opelz G., Kiuchi M., Takasugi M., Terasaki P. I. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975 Nov 1;142(5):1327–1333. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Janossy G., Greaves M. F. Selective stimulation of IgM synthesis in mouse B lymphocytes by pokeweed mitogen. Nat New Biol. 1972 Jan 5;235(53):21–23. doi: 10.1038/newbio235021a0. [DOI] [PubMed] [Google Scholar]
- Polet H., Spieker-Polet H. Serum albumin is essential for in vitro growth of activated human lymphocytes. J Exp Med. 1975 Oct 1;142(4):949–959. doi: 10.1084/jem.142.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M., Pihko H., Vuopio P. Studies of carcino-fetal proteins. II. Biochemical comparison of -fetoprotein from human fetuses and patients with hepatocellular cancer. Int J Cancer. 1971 Sep 15;8(2):283–288. doi: 10.1002/ijc.2910080213. [DOI] [PubMed] [Google Scholar]
- Yachnin S., Allen L. W., Baron J. M., Svenson R. H. The potentiation of phytohemagglutinin-induced lymphocyte transformation by cell-cell interaction; a matrix hypothesis. Cell Immunol. 1972 Apr;3(4):569–589. doi: 10.1016/0008-8749(72)90120-7. [DOI] [PubMed] [Google Scholar]
- Yachnin S. Fetuin, an inhibitor of lymphocyte transformation. The interaction of fetuin with phytomitogens and a possible role for fetuin in fetal development. J Exp Med. 1975 Jan 1;141(1):242–256. doi: 10.1084/jem.141.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnin S., Svenson R. H. The immunological and physicochemical properties of mitogenic proteins derived from Phaseolus vulgaris. Immunology. 1972 May;22(5):871–883. [PMC free article] [PubMed] [Google Scholar]
- Yachnin S. The potentiation and inhibition by autologous red cells and platelets of human lymphocyte transformation induced by pokeweed mitogen concanavalin A, mercuric chloride, antigen, and mixed leucocyte culture. Clin Exp Immunol. 1972 May;11(1):109–124. [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E. F., Madappally M. M. Sialyltransferase: regulation of alpha-foetoprotein microheterogeneity during development. Biochem J. 1973 Jul;134(3):807–810. doi: 10.1042/bj1340807. [DOI] [PMC free article] [PubMed] [Google Scholar]