Abstract
A previous study demonstrated that the amount of Candida spp. in saliva is higher in children with sickle-cell disease. The results from a recent study demonstrate its participation in the etiology of dental caries.
Objective
This study assessed caries-associated virulence (production of acid, extracellular polysaccharides, proteins and metabolic activity) of biofilms from Candida albicans isolated from saliva of patients with sickle-cell anemia in comparison to isolates obtained from matched healthy children.
Material and Methods
The isolates were previously obtained from 25 children (4-6 years) and their matched controls (healthy children). One isolate of C. albicans per children was used, totaling 25 isolates per group. The C. albicans biofilms were grown for five days and analyzed regarding the production of lactic acid, extracellular polysaccharides, proteins and metabolic activity. The production of lactic acid was determined by the enzymatic method. The concentration of extracellular polysaccharides was determined by the phenol-sulphuric acid method, and the concentration of the protein was analyzed using the QuantiPro BCA kit. The XTT reduction was used to verify the metabolic activity. The data were analyzed with GraphPad Prism at 5%.
Results
The Mean±standard deviation for acid production, extracellular polysaccharides, proteins and metabolic activity of isolates from sickle-cell group was, respectively: 7.1±5.0 mmol/L; 15.6±2.5 μg glucose/mg biofilm; 7,503±3,097 μg/mL; A490 3.5±0.7. For isolates from control group the values obtained were: 3.5±3.3 mmol/L; 12.8±3.4 μg glucose/mg biofilm; 4,995±682 μg/mL; A490 3.4±0.5. The C. albicans isolates from patients with sickle-cell anemia produced a significantly greater quantity of acids (p=0.025), polysaccharides (p=0.025) and proteins (p=0.047) compared with the isolates from control group. However, there was no difference in metabolic activity (XTT) between groups (p=0.750).
Conclusion
The C. albicans biofilms from patients with sickle-cell anemia presented a greater caries-associated virulence than isolates from healthy children.
Keywords: Sickle cell anemia, Biofilms, Candida spp, Dental caries
INTRODUCTION
The oral microbiota plays an important role in the development of dental caries. The microorganisms that have been associated with this disease include Streptococcus mutans, Lactobacilli and Candida albicans, among others 1 , 15 .
C. albicans is a highly acid-tolerant yeast. It is able to secrete organic acids and reduce the salivary pH to a value lower than that produced by Streptococcus mutans 15 , 17 . The results from a recent study demonstrate its participation in dental caries etiology 3 , 9 , 29 .
Sickle-cell anemia is the most common hereditary disease in Brazil. Due to the high susceptibility to acquire infections and high mortality and morbidity associated with septicemia or secondary meningitis, children with sickle-cell anemia are submitted to prophylactic antibiotic therapy with penicillin 11 , 12 . Buccal manifestations of sickle-cell disease include orofacial pain, midfacial overgrowth, malocclusions, enamel hypomineralization and dental caries 14 .
Matos, et al. 20 (2014) demonstrated that even though salivary levels of Streptococcus mutans and Lactobacilli of patients with sickle-cell anemia were similar to healthy children, the quantity of yeasts in saliva was significantly higher in children with sickle-cell anemia compared with the control group. In addition to the quantitative aspect, it is important to assess the qualitative aspects, since there are no data in the scientific literature on this aspect. Therefore, the aim of this study was to assess caries-associated virulence (production of acid, extracellular polysaccharides, proteins and metabolic activity) of biofilms from Candida albicans isolated from saliva of patients with sickle-cell anemia in comparison with isolates obtained from matched healthy children.
MATERIAL AND METHODS
The study was conducted on clinical isolates previously obtained by Matos, et al. 20 (2014). The collection of the samples by this author was authorized by the Institutional Review Board from Univ. Estadual Paulista – UNESP, São José dos Campos (#082/2007), and followed the ethical principles and guidelines that regulate studies on human beings, according to the Declaration of Helsinki. Parents gave written informed consent prior to the stimulated saliva collection. The isolates were obtained from 25 children (4-6 years) and their matched controls (healthy children). One isolate of C. albicans per children was used, totaling 25 isolates per group.
The selected patients were treated in the ambulatory of the Discipline of Hematology, Department of Medicine of the Federal University of São Paulo – UNIFESP-EPM, aged 4 to 6 years, male and female, diagnosed with sickle-cell anemia (SS) by clinical and laboratory examinations. The children were under antibiotic therapy with penicillin for at least six months prior to the saliva collection. For the control group, healthy children matched by gender, age and oral status (DMFT) with the sickle-cell anemia group were selected. The Healthy children of the control group did not have any systemic diseases and did not take antibiotics or antifungal drugs in the last 60 days prior to the saliva collection. The mean±sd DMFT for sickle-cell patients was 1.44±1.96 and for control group children was 2.36±3.09. For DMFT, the mean±sd was 2.00±1.85 and 1.32±1.52 for sickle-cell and control children, respectively 20 .
The study excluded uncontrolled diabetic individuals, patients wearing orthodontic appliances or pacifiers, using antidepressants or presenting lesions that suggested the presence of oral candidiasis.
Fungal strains and growth conditions
The isolates were identified using the API 20C AUX kit (Biomérieux, Marcy l’Etoile, Rhône, France) and maintained in Sabouraud dextrose agar at 4°C.
For the experiments, the microorganisms were plated in brain heart infusion (BHI) agar (Himedia, Mumbai, Maharashtra, India) and cultured for 24 h. A suspension was prepared in 0.9% saline solution (NaCl; Vetec, Duque de Caxias, RJ, Brazil), according to the McFarland 0.5 scale (approximately 5x10 6 colony forming units (CFU)/ml).
The next step was to inoculate 100 mL of the suspensions in 10 mL of BHI broth (Himedia, Mumbai, Maharashtra, India) supplemented with PIPES buffer (1,4-piperazinediethanesulfonic acid; Sigma Aldrich, Saint Louis, Missouri, USA) and 0.2% glucose (Vetec, Duque de Caxias, RJ, Brazil). For the analysis of the production of acids, extracellular polysaccharides and proteins, biofilms were grown for five days in the bottom of 24-wells plates (TPP, Trasadingen, Schaffhausen, Switzerland) containing 3 mL of the inoculum. For the metabolic activity test, biofilms were grown in the bottom of 96-wells plates (TPP, Trasadingen, Schaffhausen, Switzerland) containing 200 µL of the inoculum. The culture medium was renewed once a day by carefully removing the old culture medium and adding a fresh one. All incubations were performed at 37°C in aerobiosis, and all experiments were performed in duplicate.
Determination of lactic acid production
Among the final products of oral microorganisms, lactic acid was analyzed because it is the strongest acid metabolite, presenting a pKa (acid dissociation constant) of 3.810. In the fifth day of the biofilm growth process, the medium was renewed and the plates were incubated for further 3.5 h. Lactic acid analysis was carried out in the culture media (BHI broth).
The amount of lactic acid was determined by the enzymatic method 13 . In 96-wells plates, 85 mL of 1 mol/L glycine (Sigma Aldrich, Saint Louis, Missouri, USA), 85 mL of 0.8 mol/L hydrazine sulfate (Sigma Aldrich, Saint Louis, Missouri, USA), 20 mL of 26 mmol/L b-nicotinamide adenine dinucleotide (Sigma Aldrich, Saint Louis, Missouri, USA) and 10 mL of BHI broth or L-lactic acid standards (0.2 to 3.0 mmol/L; Sigma Aldrich, Saint Louis, Missouri, USA) were added. The absorbance was read at 340 nm (A1). Then, 10 mL of 1.0 g/L L-lactic dehydrogenase were added. After 1 h at room temperature, a new reading was performed at 340 nm (A2). The quantity of lactic acid (DL) was obtained by the equation DL=A2-A1. The DL values were converted to mmol/L of lactic acid, according to the readings of L-lactic acid standards.
Determination of extracellular polysaccharides production
A model modified from Koo, et al. 18 (2003) was used to analyze extracellular polysaccharides production. Biofilms were resuspended in 2 mL of 0.9% NaCl by vigorous pipetting. The content of the wells was transferred to pre-weighed tubes and centrifuged for 10 min at 8,000×g.
The precipitate was vacuum-dried with diphosphorus pentoxide (Sigma Aldrich, Saint Louis, Missouri, USA) 24 . After 12 h at room temperature, the dry weight of the biofilms was obtained. Then,1 mol/L of NaOH (Vetec, Duque de Caxias, RJ, Brazil) was added (10 μL/g of dry weight). The samples were homogenized for 1 min, agitated for 3 h at room temperature and centrifuged for 1 min at 11,000×g 23. A solution of 75% cooled ethanol (Vetec, Duque de Caxias, RJ, Brazil) was added to the supernatant in a volume equivalent to three times the volume of supernatant. After precipitation at -4°C for 12 h, the suspension was centrifuged and the precipitate was resuspended in 1.0 mol/L NaOH2.
The concentration of alkali-soluble polysaccharides was determined by the phenol-sulfuric acid method 8 . In the next step, 10 μL of the samples were pipetted in test tubes in duplicate. Following, 0.5 mL of 5% phenol (Vetec, Duque de Caxias, RJ, Brazil) and 2.5 mL of concentrated sulfuric acid (Vetec, Duque de Caxias, RJ, Brazil) were added. The absorbance (λ=490 nm) was assessed after 20 min using a spectrophotometer (Micronal, São Paulo, SP, Brazil). Glucose was used as standard. The values were converted to μg of glucose/dry weight of biofilm.
Determination of extracellular matrix proteins production
To analyze the protein content in the biofilm matrix, the biofilms were resuspended in ultrapure water, sonicated (30 s/30 W) and vortexed for 2 min. The suspension was centrifuged (2,599×g; 10 min; 4°C) and the supernatant was filtered in a 0.2 μm nitrocellulose membrane (TPP, Trasadingen, Schaffhausen, Switzerland) 28 . Quantification of proteins was performed using BCA kit (Bicinchoninic Acid, Sigma Aldrich, Saint Louis, Missouri, USA). The plates were incubated for 1 h at 60°C. The absorbance was analyzed (λ=570 nm) and the protein concentration (μg/mL) was calculated.
Evaluation of metabolic activity of biofilms
The metabolic activity of biofilms was assessed by XTT (2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide; Sigma Aldrich, Saint Louis, Missouri, USA) assay 15 using a XTT solution (1 mg/mL in PBS, sterilized by filtration) and menadione (0.4 mmol/L in acetone; Sigma Aldrich, Saint Louis, Missouri, USA). The biofilms were rinsed and 200 µL of XTT-menadione solution (5:1) were added to each well. The plate was incubated for 3 h at 37°C and the absorbance values were read at 490 nm.
Statistical analysis
The data were analyzed using the software GraphPad Prism version 3.02 (GraphPad Software Inc., San Diego, CA, USA). To use the most adequate test, a normality test of the sample distribution was performed, analyzing the adherence to the normal curve. Since the data presented normal and homogeneous distribution, two-tailed unpaired t test was applied, at a significance level of 5%.
RESULTS
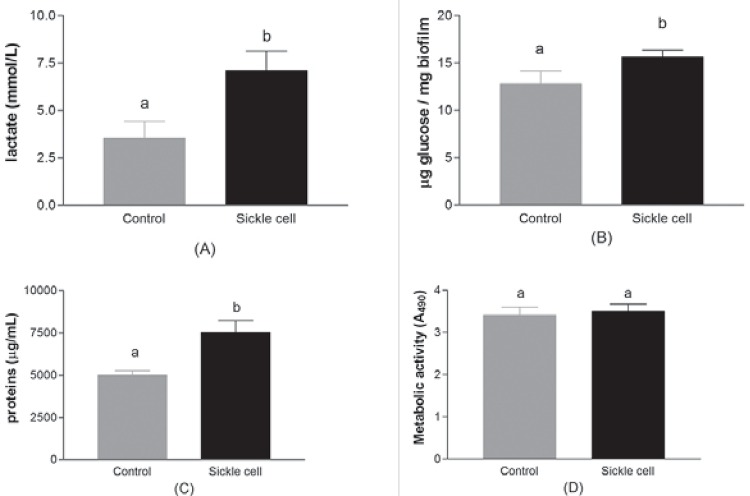
Figure 1 presents the quantification of acid production, extracellular alkali-soluble polysaccharides, extracellular proteins and metabolic activity in biofilms formed by C. albicans clinical isolates. The mean±sd for acid production, extracellular alkali-soluble polysaccharides, extracellular proteins and metabolic activity of isolates from sickle-cell anemia group were, respectively: 7.1±5.0 mmol/L; 15.6±2.5 μg glucose/mg biofilm; 7503±3097 μg/mL; A490 3.5±0.7. For isolates from the healthy control group the values obtained were: 3.5±3.3 mmol/L; 12.8±3.4 μg glucose/mg biofilm; 4995±682 μg/mL; A490 3.4±0.5.
Figure 1. Cariogenicity of C. albicans biofilms isolated from children with sickle-cell disease and their matched healthy controls (n=25 isolates per group). A) Concentration of lactic acid (mmol/L-1); B) Concentration of alkali-soluble polysaccharides (μg glucose/mg dry biofilm); C) Concentration of proteins (µg/mL); D) Metabolic activity (XTT). Bars represent the standard deviation. Different letters indicate statistically significant differences (unpaired t test, p<0.05).
The data demonstrated that C. albicans isolates from patients with sickle-cell anemia are able to produce a significantly greater quantity of acids (p=0.025), extracellular polysaccharides (p=0.025) and extracellular proteins (p=0.047) compared with the isolates from healthy control group. However, there was no difference in metabolic activity (XTT) between the studied groups (p=0.750).
DISCUSSION
Despite the increased risks of patients with sickle-cell anemia to acquire infections, few studies about the oral microbiota of pediatric patients with sickle-cell anemia submitted to prolonged antibiotic therapy are available. Matos, et al. 20 (2014) demonstrated an increase of yeasts colonization in pediatric patients with sickle-cell anemia and a reduction in the buffer capacity of saliva from these children compared to the control group (healthy patients). In a recent study, Charone, et al. 3 (2012) demonstrated that C. albicans isolated from HIV+ patients are able to demineralize enamel in vitro. However, the cariogenicity of these isolates were not compared to the cariogenicity of isolates obtained from healthy patients.
C. albicans phenotypical evaluations were done in the present study because it was the only specie found in higher concentrations in the saliva of sickle-cell disease patients when compared to healthy volunteers 20 . This acidogenic, aciduric and heterofermentative yeast is frequently isolated from the oral cavity. It is also able to form biofilms and to adapt to several habitats 21 . The greater cariogenic features of clinical isolates obtained from patients with sickle-cell anemia may be partly due to the prolonged antibiotic therapy — which might select more virulent species — and to the innate low immunity of sickle-cell disease patients.
The production of lactic acid is one of the most important phenotypical feature that should be analyzed when studying the cariogenicity of strains. In the present study, although the pH of the spent media was not analyzed, isolates from sickle-cell disease patients produced two times more lactic acid than isolates from the healthy control group. C. albicans is known to substantially reduce environmental pH by the production of acids and to survive at low pH levels 17 , 27 . The pH drop promoted by acid production is related to the appearance of carious lesions. Nikawa, et al. 22 (2003) demonstrated that the capacity of C. albicans to dissolve hydroxyapatite is nearly 20 times greater than S. mutans. Recently, Szabó, et al. 29 (2014) demonstrated that Candida spp. are able to dissolve dentine and release calcium to the medium at different rates. These results show that Candida spp. are actively involved in demineralization of dental tissues. Moreover, as the rate of acid production is influenced by the buffer capacity of the saliva, the results obtained in the present study, associated with the low buffer capacity observed by Matos, et al. 20 (2014), may produce significant effects in children with sickle-cell disease. As Matos, et al. 20 (2014) matched healthy patients in DMFT, it is not possible to analyze the impact of these differences in caries level. A future study may be conducted analyze this finding in more detail.
The extracellular matrix is the most critical component of biofilms, because it is responsible for maintaining the cells adhered 4 . The biofilm matrix of C. albicans is mainly composed by β 1,3-glucan that builds up its structure, provides antifungal resistance and also provides sites for GtfB activity in dual species biofilms with S. mutans 9 . Therefore, the greater quantity of polysaccharides observed in C. albicans biofilms from sickle-cell anemia patients characterizes the formation of a denser and thicker biofilm 5 , 6 , which would enhance the adherence of microorganisms and would have a greater resistance to antimicrobial drugs penetration 3 , 7 , 9 , 29 . However, the impact of the higher amount of extracellular polysaccharides on biofilm thickness and on antimicrobial resistance should be assessed further. Recently, Falsetta, et al. 9 (2014) showed that matrix extracellular polysaccharides also mediate biofilm formation from different species. Particularly, the coexistence of S. mutans and C. albicans induces the expression of important virulence factors from both species.
The XTT assay has been routinely used for the quantification of Candida spp. biofilms 25 , 26 . One of the limitations of this technique is that it only analyzes the metabolic activity of the cells present in the upper layers of mature biofilms, which are more active due to the presence of more nutrients 30 . This may be one of the reasons that contributed to the lack of statistically significant differences in the metabolic activity from different groups, together with the limited comparison of different strains 19 .
Several studies have highlighted the presence of Candida spp. in carious lesions. Recent studies are demonstrating that this yeast plays an important role in the etiology of caries 3 , 16 , 29 . The present results show that C. albicans is a potentially cariogenic microorganism due to the high concentrations of acid, extracellular polysaccharides and proteins produced in biofilms. These data highlight the need to further investigate the influence of Candida spp. on caries etiology, especially in the presence of systemic diseases, as in the case of sickle-cell anemia.
The capacity to produce acids and extracellular polysaccharides are important characteristics for recognized cariogenic microorganisms, as S. mutans and Lactobacillus spp. This is the first study that assessed phenotypic characteristics of oral isolates from patients with sickle-cell anemia. The present results are helpful to elucidate the impact of selection promoted by the prolonged use of penicillin on caries-associated virulence of microorganisms. Due to the high morbidity and economic difficulties caused by this disease, community programs of early and multidisciplinary diagnosis are required, and the active participation of the dentist is important to implement and encourage preventive measures.
CONCLUSION
Biofilms of C. albicans clinical oral isolates obtained from children with sickle-cell anemia presented a greater production of lactic acid, extracellular polysaccharides and proteins.
ACKNOWLEDGMENTS
This study was supported by the São Paulo Research Foundation (FAPESP), grant #2009/14097-0. Translation was supported by FUNDUNESP (grant # 557-01-DCP - FUNDUNESP). The authors thank Dr. Maria Stella Figueiredo and Dr. Josefina Aparecida Pellegrini Braga for allowing the samples collection at the Paulista School of Medicine of the Federal University de São Paulo.
REFERENCES
- 1.Carvalho FG, Silva DS, Hebling J, Spolidorio LC, Spolidorio DM. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol. 2006;51:1024–1028. doi: 10.1016/j.archoralbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Ccahuana-Vásquez RA, Tabchoury CP, Tenuta LM, Del Bel Cury AA, Vale GC, Cury JA. Effect of frequency of sucrose exposure on dental biofilm composition and enamel demineralization in the presence of fluoride. Caries Res. 2007;41:9–15. doi: 10.1159/000096100. [DOI] [PubMed] [Google Scholar]
- 3.Charone S, Portela MB, Chagas MS, Araújo Soares RM, Araújo Castro GF. Biofilm of Candida albicans from oral cavity of an HIV-infected child: challenge on enamel microhardness. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:500–504. doi: 10.1016/j.oooo.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Cury JA, Rebelo MAB, Del Bel Cury AA, Derbyshire MT, Tabchoury CP. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000;34:491–497. doi: 10.1159/000016629. [DOI] [PubMed] [Google Scholar]
- 5.Dashper SG, Reynolds EC. Effects of organic acid anions on growth, glycolysis and intracellular pH of oral streptococci. J Dent Res. 2000;79:90–96. doi: 10.1177/00220345000790011601. [DOI] [PubMed] [Google Scholar]
- 6.Dibdin GH, Shellis RP. Physical and biochemical studies of Streptococcus mutans sediments suggest new factors linking the cariogenicity of plaque with its extracellular polysaccharide content. J Dent Res. 1988;67:890–895. doi: 10.1177/00220345880670060101. [DOI] [PubMed] [Google Scholar]
- 7.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois M, Grilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 9.Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald RJ, Adams BO, Sandham HJ, Abhyankar S. Cariogenicity of a lactate dehydrogenase-deficient mutant of Streptococcus mutans serotype c in gnotobiotic rats. Infect Immun. 1989;57:823–826. doi: 10.1128/iai.57.3.823-826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaston MH, Verter J. Sickle cell anemia trial. Stat Med. 1990;9:45–51. doi: 10.1002/sim.4780090111. [DOI] [PubMed] [Google Scholar]
- 12.Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher J, Presbury G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 13.Gutmann I, Wahlefeld W. L-(+)-Lactate determination with lactate dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Verlag Chemie-Academic Press; 1974. pp. 1464–1468. [Google Scholar]
- 14.Javed F, Correa FO, Nooh N, Almas K, Romanos GE, Al-Hezaimi K. Orofacial manifestations in patients with sickle cell disease. Am J Med Sci. 2013;345:234–237. doi: 10.1097/MAJ.0b013e318265b146. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Samaranayake LP, Samaranayake Y, Yip HK. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49:789–798. doi: 10.1016/j.archoralbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Klinke T, Guggenheim B, Klimm W, Thurnheer T. Dental caries in rats associated with Candida albicans. Caries Res. 2011;45:100–106. doi: 10.1159/000324809. [DOI] [PubMed] [Google Scholar]
- 17.Klinke T, Kneist S, Soet JJ, Kuhlisch E, Mauersberger S, Forster A, et al. Acid production by oral strains of Candida albicans and lactobacilli. Caries Res. 2009;43:83–91. doi: 10.1159/000204911. [DOI] [PubMed] [Google Scholar]
- 18.Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn DM, Balkis M, Chandra J, Mukherjee PK, Ghannoum MA. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin Microbiol. 2003;41:506–508. doi: 10.1128/JCM.41.1.506-508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matos BM, Ribeiro ZE, Balducci I, Figueiredo MS, Back-Brito GN, Mota AJ, et al. Oral microbial colonization in children with sickle cell anaemia under long-term prophylaxis with penicillin. Arch Oral Biol. 2014;59:1042–1047. doi: 10.1016/j.archoralbio.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Moreira D, Spolidório DM, Rodrigues JA, Boriollo MF, Pereira CV, Rosa EA, et al. Candida spp. biotypes in the oral cavity of school children from different socioeconomic categories in Piracicaba-SP, Brazil. Braz Oral Res. 2001;15:187–195. doi: 10.1590/s1517-74912001000300003. [DOI] [PubMed] [Google Scholar]
- 22.Nikawa H, Yamashiro H, Makihira S, Nishimura M, Egusa H, Furukawa M, et al. In vitro cariogenic potential of Candida albicans. Mycoses. 2003;46:471–478. doi: 10.1046/j.0933-7407.2003.00888.x. [DOI] [PubMed] [Google Scholar]
- 23.Nobre dos Santos M, Melo dos Santos L, Francisco SB, Cury JA. Relationship among dental plaque composition, daily sugar exposure and caries in the primary dentition. Caries Res. 2002;36:347–352. doi: 10.1159/000065959. [DOI] [PubMed] [Google Scholar]
- 24.Pearce EI, Hancock EM, Gallagher IH. The effect of fluorhydroxyapatite in experimental human dental plaque on its pH, acid production and soluble calcium, phosphate and fluoride levels following glucose challenge. Arch Oral Biol. 1984;29:521–527. doi: 10.1016/0003-9969(84)90073-6. [DOI] [PubMed] [Google Scholar]
- 25.Ramage G, Vandewalle K, Wickes BL, López-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001;18:163–170. [PubMed] [Google Scholar]
- 26.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 27.Samaranayake LP, Hughes A, Weetman DA, MacFarlane TW. Growth and acid production of Candida species in human saliva supplemented with glucose. J Oral Pathol. 1986;15:251–254. doi: 10.1111/j.1600-0714.1986.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 28.Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Medical Mycology. 2009;47:681–689. doi: 10.3109/13693780802549594. [DOI] [PubMed] [Google Scholar]
- 29.Szabó B, Majoros L, Papp-Falusi E, Szabó Z, Szabó J, Márton I, et al. Studies on the possible aetiological role of different Candida species in pathogenesis of dentine caries by monitoring the calcium release from tooth particles. Acta Microbiol Immunol Hung. 2014;61:11–17. doi: 10.1556/AMicr.61.2014.1.2. [DOI] [PubMed] [Google Scholar]
- 30.Xie Z, Thompson A, Kashleva H, Dongani-Bagtzoglou A. A quantitative real-time RT-PCR assay for mature C. albicans biofilms. 9310.1186/1471-2180-11-93.BMC Microbiol. 2011;11 doi: 10.1186/1471-2180-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]