Abstract
Aims
Heart failure patients with reduced and preserved left ventricular (LV) ejection fraction (EF) show reduced exercise capacity. We explored the relationship between exercise capacity and systolic and diastolic myocardial function in heart failure patients.
Methods and results
Exercise capacity, by peak oxygen uptake (VO2), was assessed in 100 patients (56 ± 12 years, NYHA functional class: 2.5 ± 0.9, EF: 42 ± 19%). LV systolic function, as EF and global longitudinal strain (GLS), and right ventricular function were assessed by echocardiography. Left atrial volume index and the ratio of peak early diastolic filling velocity (E) to early diastolic mitral annular velocity (e′) were measures of diastolic function. Thirty-seven patients had heart failure with preserved EF (HFpEF), defined as EF ≥50% and echocardiographic diastolic dysfunction. LV GLS and peak pulmonary arterial systolic pressure were independently correlated to peak VO2 in the total study population and in HFpEF separately. LV GLS was superior to EF in identifying patients with impaired peak VO2 <20 mL/kg/min as shown by receiver operating characteristic analyses [areas under curves 0.93 (0.89–0.98) vs. 0.85 (0.77–0.93), P < 0.05]. In patients with HFpEF, GLS was reduced below normal (−17.5 ± 3.2%) and correlated to E/e′ (R = 0.45, P = 0.005) and left atrial volume index (R = 0.48, P = 0.003), while EF did not.
Conclusion
GLS correlated independently to peak VO2 in patients with reduced and preserved EF and was superior in identifying patients with reduced exercise capacity. In HFpEF, systolic function by GLS was impaired. There was a significant relationship between diastolic function and GLS, confirming a coupling between diastolic and longitudinal systolic function in HFpEF.
Keywords: speckle tracking echocardiography, diastolic function, heart failure, myocardial mechanics, exercise testing
Introduction
At least half of patients with heart failure have reduced left ventricular (LV) ejection fraction (EF). The remaining are defined as having heart failure with preserved EF (HFpEF)1 with as poor prognosis as those with reduced EF.2 Cardiopulmonary exercise testing (CPX) with peak oxygen uptake (VO2) is a strong predictor of mortality, in the healthy population and in heart failure patients including HFpEF.3–7 Despite vast and consistent data on CPX's prognostic value, it remains underutilized.7 Previous studies have failed to find a relationship between LV systolic function by EF and exercise capacity8–10 while diastolic function11–13 and right ventricular (RV) function14–16 have been shown to correlate well. LV systolic function is an established prognostic marker, and it is unclear why CPX apparently is not correlated to EF. Echocardiographic strain is an accurate method for quantifying systolic function,17 is shown superior to EF in detecting early reduction in myocardial systolic function,18 and has prognostic impact.19,20 We explored the relationship between exercise capacity and myocardial mechanics in heart failure patients with preserved and reduced EF. We hypothesized that exercise capacity is more closely related to LV strain than to EF. In HFpEF, diastolic dysfunction is considered to be the underlying abnormality1 and has been shown to predict mortality.21 We hypothesized that patients with HFpEF also show mild systolic dysfunction detectable by echocardiographic strain.
Methods
Patient characteristics and clinical data
This single-centre, cross-sectional study included clinically stable patients referred to the Department of Cardiology, Oslo University Hospital, Norway for symptoms and signs of heart failure. Exclusion criteria were chronic lung disease, severe valvular disease, or anaemia.1
All patients were clinically examined and assessed by New York Heart Association (NYHA) functional classification. Blood samples were collected for analysis of N-terminal pro-B-type natriuretic peptide (NT-proBNP) (n = 81). All patients gave written informed consent. The study complied with the Declaration of Helsinki and was approved by the Regional Committees for Medical Research Ethics.
Cardiopulmonary exercise testing
All patients performed exercise testing with ventilatory expired gas analysis. Maximal, graded, exercise testing was performed on an upright electrical braked bicycle ergometer (Jäger ER900; Viasys Healthcare GmbH, Hochberg, Germany) starting at 20 W, with the pedal rate kept at 60 rotations/min and stepwise increments of 10–20 W/min. The test ended by patients' symptoms of exhaustion, if obtaining maximal physical level or by signs of severe pathology. VO2, CO2 production, and ventilation were measured on a breath-to-breath basis and calculated by the software MVmax 229 (Viasys Healthcare GmbH). Gas exchange values were obtained throughout the baseline rest, exercise, and early recovery period and were averaged from 20 s intervals. Peak VO2 and respiratory exchange ratio (RER) were measured in the last 20 s at maximal exercise. Patients with peak RER ≤ 1.1 were not included in the study. Heart rate and 12-lead electrocardiogram were continuously recorded, and cuff blood pressure was measured every minute. We defined peak VO2 as the highest VO2 obtained during an adequately performed test.3 At peak exercise, we recorded Watts, metabolic equivalents, heart rate, and blood pressure.
Echocardiography
Echocardiography was performed the same day as CPX using Vivid 7 and Vivid E9 system (GE Healthcare, Horten, Norway) and analysed offline (EchoPAC®). We calculated LV diameters by M-mode and LV EF by Simpson's biplane method. RV function was assessed by tricuspid annular plane systolic excursion (TAPSE), tricuspid lateral annular systolic velocity (S′) by pulsed tissue Doppler, and fractional area change (FAC).22 Peak pulmonary arterial systolic pressure (PASP) was estimated as the sum of peak RV-right atrial gradient from the tricuspid valve regurgitant jet and right atrial pressure on the basis of size and collapse of inferior vena cava.22
LV and RV longitudinal strains were analysed by speckle tracking echocardiography, with frame rate ≥50 s−1. From apical four-chamber, two-chamber, and long-axis view, peak longitudinal strains from each of the 16 LV segments, either negative or positive, were averaged to LV global longitudinal strain (GLS).18,19 The RV strain was averaged from the three RV free wall segments from apical four-chamber view.23
Diastolic function parameters were peak early diastolic filling (E) and late diastolic filling (A) velocities, E/A ratio, E deceleration time, early diastolic septal mitral annular velocity (e′) (averaged from three cardiac cycles), and E/e′ as an index of LV filling pressure.24 Left atrial area and volume index were calculated from apical four-and two-chamber views, using area–length formula. E/A ratio, E deceleration time, e′, E/e′, and left atrial volume index were classified as normal or abnormal adjusted for age.24 Diastolic dysfunction was fulfilled if at least two of these indices were abnormal.1,24
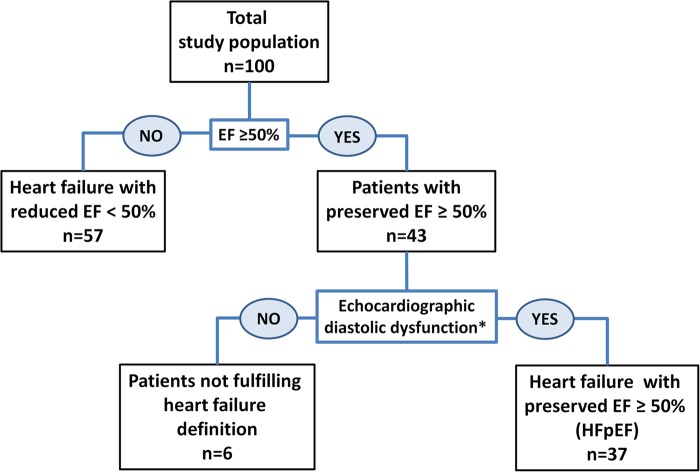
Patients with heart failure symptoms, EF ≥50%, and fulfilling echocardiographic criteria for diastolic dysfunction were defined as having HFpEF (Figure 1).
Figure 1.
The total study population (top) and the separated HFpEF group (lower right) were studied. Six patients with preserved EF (lower left) did not fulfil echocardiographic diastolic dysfunction and were not included for analysis in the HFpEF group (the asterick refers to McMurray et al.1 and Nagueh et al.24).
Two independent investigators (N.E.H. and K.H.H.) analysed echocardiographic recordings blinded to clinical data.
Statistical analysis
Continuous data were presented as mean ± SD. Comparisons of means were analysed by Student's t-test (SPSS 18, SPSS Inc., Chicago, IL, USA). Bivariate correlations were assessed by Pearson coefficient for normally distributed continuous parameters.
The multivariate linear regression for assessing independent correlations to peak VO2 was performed by including clinically relevant, significant variables from the univariate model. Strong collinearity (R ≥ 0.50 or R ≤ −0.50) was found between parameters of LV function (GLS and EF) and RV function (FAC and RV strain) and LV function and NYHA classification in the total study population. In the HFpEF population, there were strong collinearites between GLS and NT-proBNP and GLS and TAPSE. These parameters were therefore not included in multivariate analyses together.
Receiver operating characteristic (ROC) curves were created and areas under curves were calculated for the ability of GLS, EF, RV strain, and E/e′ to identify patients with peak VO2 <20 mL/kg/min. The ROC curve value closest to the upper left corner was defined to provide optimal sensitivity and specificity for each parameter. Two-sided P-values <0.05 were considered significant for all analyses.
Results
Clinical characteristics
We included 100 patients (Table 1). Forty-seven patients had coronary artery disease, 34 dilated cardiomyopathy, and 3 had hypertrophic cardiomyopathy. The remaining 16 patients had heart failure symptoms and signs secondary to hypertension, diabetes, or other co-morbidities. Thirty-seven patients fulfilled the HFpEF definition.
Table 1.
Patient characteristics
| Total study population (n = 100) | Patients with HFpEF (n = 37) | |
|---|---|---|
| Age (years) | 56 ± 12 | 58 ± 11 |
| Gender (male/female) (n) | 73/27 | 25/12 |
| HR (bpm) | 74 ± 15 | 77 ± 16 |
| Systolic BP (mmHg) | 116 ± 26 | 135 ± 21 |
| Diastolic BP (mmHg) | 71 ± 14 | 79 ± 11 |
| BMI (kg/m2) | 26 ± 4 | 26 ± 4 |
| NYHA class | 2.5 ± 0.9 | 2.0 ± 0.5 |
| Diabetes (n) | 13 (13%) | 5 (14%) |
| Hypertension (n) | 25 (25%) | 15 (41%) |
| NT-proBNP (pmol/L) (n = 81) (n = 24) | 464 ± 612 | 115 ± 164 |
| Aetiology of heart failure | ||
| Ischaemic heart disease (n) | 47 (47%) | 22 (60%) |
| Dilated CM (n) | 34 (34%) | 0 |
| Hypertrophic CM (n) | 3 (3%) | 2 (5%) |
| CM from hypertension, diabetes, and other (n) | 16 (16%) | 13 (35%) |
| CPX parameters | ||
| Peak exercise (Watt) | 102 ± 62 | 136 ± 59 |
| Peak exercise (METs) | 4.4 ± 1.8 | 5.7 ± 2.0 |
| HR at peak exercise (bpm) | 125 ± 25 | 133 ± 20 |
| Systolic BP at peak exercise (mmHg) | 154 ± 49 | 187 ± 34 |
| Diastolic BP at peak exercise (mmHg) | 84 ± 30 | 92 ± 21 |
| Peak VO2 (mL/kg/min) | 15.4 ± 6.3 | 20.1 ± 6.9 |
| VE/VCO2 (n = 76) | 30.7 ± 7.8 | 26.1 ± 4.0 |
| Echocardiographic parameters | ||
| LV ESV index (mL/m2) | 49 ± 44 | 14 ± 5 |
| LV EDD index (mm/m2) | 32 ± 7 | 26 ± 2 |
| LV EF (%) | 42 ± 19 | 62 ± 7 |
| LV GLS (%) | −11.9 ± 6.6 | −17.5 ± 3.2 |
| TAPSE (cm) | 1.6 ± 0.9 | 1.8 ± 0.6 |
| RV S′ (cm/s) | 8.6 ± 3.3 | 9.4 ± 3.1 |
| Peak PASP (mmHg) | 38 ± 13 | 31 ± 10 |
| RV FAC (%) | 37 ± 14 | 48 ± 11 |
| RV strain (%) | −14.9 ± 6.5 | −22.8 ± 5.7 |
| E (cm/s) | 78 ± 28 | 72 ± 23 |
| E deceleration time (ms) | 206 ± 76 | 245 ± 75 |
| E/A ratio | 1.97 ± 1.30 | 1.53 ± 1.08 |
| e′ (cm/s) (n = 92) | 5.8 ± 2.5 | 7.1 ± 2.0 |
| E/e′ (n = 92) | 17 ± 12 | 11 ± 5 |
| Left atrial area (cm2) | 28.1 ± 9.0 | 24.4 ± 7.4 |
| Left atrial volume index (mL/m2) | 56 ± 26 | 45 ± 22 |
A, late diastolic filling velocity; BMI, body mass index; BP, arterial blood pressure; bpm, beats per minute; CM, cardiomyopathy; CPX, cardiopulmonary exercise testing; E, peak early diastolic filling velocity; e′, early diastolic mitral annular velocity(septal); EDD, end diastolic diameter; EF, ejection fraction; ESV, end systolic volume; FAC, fractional area change; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LV, left ventricular; METs, metabolic equivalents; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PASP, pulmonary arterial systolic pressure; RV, right ventricular; S′, tricuspid lateral annular systolic velocity; TAPSE, tricuspid annular plane systolic excursion; VE/VCO2, minute ventilation/carbon dioxide production; VO2, oxygen uptake.
Cardiopulmonary exercise testing
Mean peak VO2 was 15.4 ± 6.3 mL/kg/min in the total population and 20.1 ± 6.9 mL/kg/min in HFpEF patients (Table 1).
Echocardiographic findings
Forty-three patients had preserved EF >50% (Table 1). Diastolic dysfunction was diagnosed in 37 of these, while 6 patients with preserved EF did not fulfil echocardiographic diastolic dysfunction and were not included in the HFpEF group (Figure 1). In the total study population, GLS was clearly reduced (−11.9 ± 6.6%). In HFpEF, GLS was subnormal (−17.5 ± 3.2%) indicating impaired systolic LV function.25–27 Measures of RV function were normal or slightly below normal reference values22 (Table 1). As expected, LV filling pressures as indexed by E/e′ were elevated in both the total study population and HFpEF patients. Mean frame rate was 58 ± 19 s−1. Intra- and inter-observer, intra-class correlation coefficients for strain analyses were 0.94 and 0.90, respectively.
Myocardial function and exercise capacity
Total study population
Parameters of myocardial systolic and diastolic function correlated to peak VO2 (Table 2 and Figure 2, upper panel). GLS and peak PASP were independently correlated to exercise capacity by multivariate analysis (Table 2). In separate multivariate analysis including RV function, RV strain showed an independent correlation to exercise capacity (Supplementary data online, Table S1).
Table 2.
Univariate (R) and multivariate analyses (β) of correlations between exercise capacity by peak VO2 and clinical and echocardiographic parameters in the total study population (left) and the HFpEF patients (right)
| Relation to peak VO2 | Total study population (n = 100) |
Patients with HFpEF (n = 37) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate correlation |
Multivariate regression |
Univariate correlation |
Multivariate regression |
|||||
| R | P-value | β (95% CI) | P-value | R | P-value | β (95% CI) | P-value | |
| Age, per 5 years | 0.13 | 0.20 | −0.37 (−0.85 to 0.12) | 0.13 | −0.01 | 0.99 | −1.07 (−2.07 to −0.07) | 0.04 |
| NYHA class | −0.77 | <0.001 | −0.65 | <0.001 | ||||
| NT-proBNP, per 10 pmol/L | −0.35 | 0.002 | −0.02 (−0.04 to 0.01) | 0.25 | −0.47 | 0.02 | ||
| LV EDD index | −0.43 | <0.001 | 0.08 (−0.16 to 0.33) | 0.50 | 0.12 | 0.48 | ||
| LV EF | 0.62 | <0.001 | −0.01 (−0.13 to 0.11) | 0.87 | 0.08 | 0.62 | ||
| LV GLS | −0.63 | <0.001 | −0.42 (−0.79 to −0.04) | 0.03 | −0.50 | 0.002 | −0.80 (−1.49 to −0.11) | 0.02 |
| TAPSE | 0.27 | 0.008 | 0.39 (−1.18 to 1.96) | 0.62 | 0.49 | 0.003 | ||
| RV S′ | 0.33 | 0.001 | 0.02 (−0.34 to 0.38) | 0.92 | 0.35 | 0.04 | 0.54 (−0.10 to 1.19) | 0.09 |
| Peak PASP | −0.55 | <0.001 | −0.15 (−0.28 to −0.02) | 0.02 | −0.49 | 0.002 | −0.35 (−0.68 to −0.02) | 0.04 |
| RV FAC | 0.54 | <0.001 | 0.35 | 0.03 | 0.08 (−0.11 to 0.28) | 0.38 | ||
| RV strain | −0.50 | <0.001 | −0.34 | 0.05 | ||||
| E | −0.28 | 0.04 | −0.39 | 0.02 | ||||
| E deceleration time | 0.48 | <0.001 | 0.34 | 0.04 | ||||
| E/A | −0.43 | <0.001 | −0.26 | 0.13 | ||||
| e′ | 0.46 | <0.001 | 0.20 | 0.24 | ||||
| E/e′ | −0.41 | <0.001 | 0.06 (−0.05 to 0.18) | 0.28 | −0.43 | 0.008 | 0.12 (−0.44 to 0.69) | 0.66 |
| Left atrial area | −0.40 | <0.001 | −0.28 | 0.09 | ||||
| Left atrial volume index | −0.41 | <0.001 | −0.03 (−0.08 to 0.02) | 0.16 | −0.28 | 0.09 | ||
A, late diastolic filling velocity; β, beta regression coefficient; CI, confidence interval; E, peak early diastolic filling velocity; e′, early diastolic mitral annular velocity(septal); EDD, end diastolic diameter; EF, ejection fraction; FAC, fractional area change; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; LV, left ventricular; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PASP, pulmonary arterial systolic pressure; R, Pearson coefficient; RV, right ventricular; S′, tricuspid lateral annular systolic velocity; TAPSE, tricuspid annular plane systolic excursion; VO2, oxygen uptake.
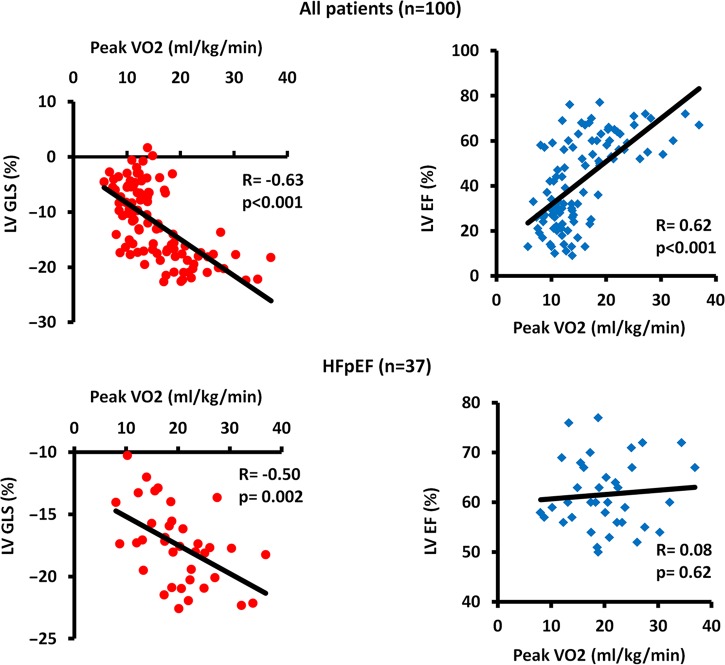
Figure 2.
Relationship between exercise capacity and LV systolic function. Upper panels show correlations between peak VO2 and LV GLS (left) and LV EF (right) in the total study population. Lower panels show that in patients with HFpEF peak VO2 correlates to LV GLS (left) but not to LV EF (right).
ROC analyses showed that GLS had significantly better ability to detect reduced peak VO2 of <20 mL/kg/min compared with EF, RV strain, and E/e′ (Figure 3). A GLS value of −17.3% had excellent sensitivity of 0.89 (95% CI 0.79–0.95) and specificity of 0.91 (95% CI 0.71–0.99) to identify patients with a peak VO2 of <20 mL/kg/min.
Figure 3.
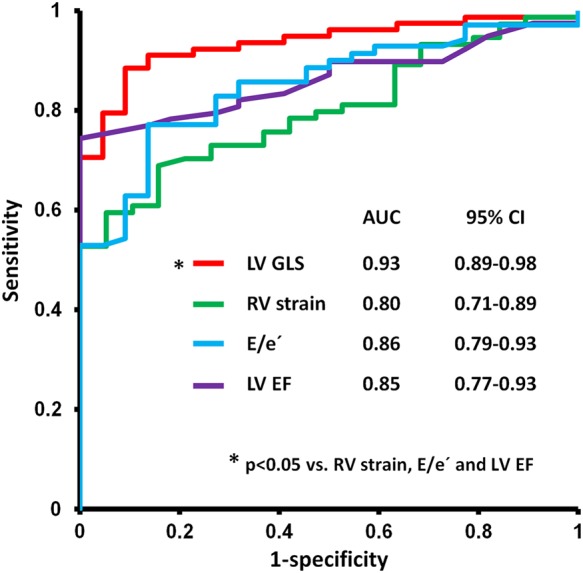
Receiver operating characteristic curve analyses showing that GLS is superior to other myocardial function parameters to identify patients with a peak VO2 of <20 mL/kg/min (n = 100). AUC, area under curve; CI, confidence interval; E, peak early diastolic filling velocity; e′, early diastolic mitral annular velocity (septal).
E/e′ and left atrial volume index correlated to GLS (R = 0.63, P < 0.001 and R = 0.44, P < 0.001) and to EF (R = −0.56, P < 0.001 and R = −0.46, P < 0.001).
In patients with reduced EF <50%, peak VO2 did neither correlate to EF nor GLS nor RV function.
Patients with HFpEF
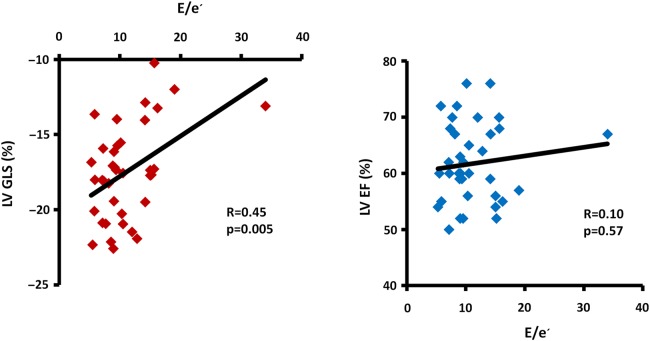
GLS and E/e′ correlated to peak VO2, while EF did not (Table 2 and Figure 2, lower panel). Furthermore, GLS was an independent predictor of peak VO2 (Table 2). GLS correlated moderately to E/e′ (R = 0.45, P = 0.005) (Figure 4) and to left atrial volume index (R = 0.48, P = 0.003). In contrast, EF correlated neither to E/e′ (R = 0.10, P = 0.57) (Figure 4) nor to left atrial volume index (R = −0.04, P = 0.80). This may indicate a relation between diastolic function and systolic longitudinal LV function detectable by strain only.
Figure 4.
Relationship between LV systolic function and diastolic function by E/e′ in patients with HFpEF (n = 37). LV GLS correlates to diastolic function (left) while ejection fraction (EF) does not (right). If excluding the patient with E/e′ 34 appearing as an outlier in the figure, the results remain unchanged; GLS correlates to E/e′ (R = 0.42, P = 0.01) while EF does not (R = −0.03, P = 0.88). E, peak early diastolic filling velocity; e′, early diastolic mitral annular velocity (septal).
N-terminal pro-B-type natriuretic peptide
NT-proBNP was increased above normal values (Table 1) and correlated to peak VO2 (Table 2). In the total population, NT-proBNP had a moderate correlation to both GLS and EF (R = 0.36, P = 0.001 and R = −0.40, P < 0.001). Interestingly in HFpEF patients, NT-proBNP correlated strongly to GLS (R = 0.60, P = 0.002) but not to EF (R = 0.03, P = 0.88).
Discussion
Our study provide novel data showing that LV longitudinal function by GLS was independently correlated to exercise capacity. GLS was better than LV EF to identify patients with impaired exercise capacity. Considering the strong relationship between exercise capacity and cardiac prognosis, these results are in line with recent reports showing the strong prognostic power of GLS.19,20 Myocardial strain may have the potential to identify reduced exercise capacity and poor prognosis at an early disease stage when traditional parameters fail. In HFpEF patients, GLS demonstrated reduced systolic function, emphasizing that the term ‘heart failure with preserved EF’ should not be equalled to heart failure with preserved systolic function. Furthermore, GLS related to diastolic function, indicating a coupling of systolic and diastolic function in HFpEF.
Myocardial function and exercise capacity
Previous studies have failed to find a relationship between peak VO2 and systolic LV function by EF,8–10 and rather found a correlation to RV function.14–16 In line with previous observations, we found strong correlations between exercise capacity and both RV function and diastolic function (Table 2 and Supplementary data online, Table S1). Our novel finding was that LV function by strain also related strongly to exercise capacity. LV GLS was independently correlated to exercise capacity both in the total population and in HFpEF patients (Table 2).
Current recommendations for CPX in heart failure define a peak VO2 of ≥20 mL/kg/min as normal based on the Weber classification.7,28 GLS was the best parameter to identify patients with a peak VO2 of <20 mL/kg/min. Therefore, GLS might be useful to discriminate patients with normal from those with moderately and severely reduced exercise capacity. (Figure 3)
In patients with HFpEF, GLS was correlated to exercise capacity and to NT-proBNP, both of which have prognostic impact in HFpEF.3,29 A few studies have shown correlations between myocardial strain and exercise capacity in patients with heart failure with reduced EF < 40%.16,30 Our study is the first study showing a strong correlation between systolic function and exercise capacity in patients with HFpEF. No relationship was observed between EF and exercise capacity nor between EF and NT-proBNP in HFpEF patients. This confirms EF's low sensitivity and limited quantitative value in patients with preserved EF.31 Our findings suggest that LV longitudinal function by strain should be the preferred method to quantify LV systolic function in patients with HFpEF.
Systolic–diastolic coupling in HFpEF
Others have reported that diastolic function correlates better to exercise capacity than systolic function.11–13 In contrast to our study, these studies did not include patients with HFpEF. Systolic function by GLS was impaired in our HFpEF patients. Recent studies have indicated the presence of systolic dysfunction in HFpEF.32,33 Our results underscore these findings and confirm that preserved EF is not equal to normal systolic function. Our correlations between GLS and diastolic parameters indicate a close coupling between systolic and diastolic function in HFpEF and are in accordance with an experimental study demonstrating that the diastolic parameter e′ is dependent on and related to systolic function through restoring forces.34
Clinical applications
EF is a strong predictor of outcome in patients with clearly reduced myocardial systolic function. Through its relation to exercise capacity, we suggest that GLS may have a similar prognostic function in HFpEF patients. Current recommendations for CPX define the assessment of peak VO2 as a primary prognostic marker.7 However, despite the strong scientific evidence supporting CPX, CPX is underutilized in clinical practice due to costs and lack of equipment, time, and competence. Echocardiography is a routine examination in the evaluation of all patients with verified or suspected cardiac disease. In specific clinical settings, strain assessment could work as a first-line examination, avoiding unnecessary CPX in stable patients and repeated CPX in unstable patients.35 Strain echocardiography might be a useful tool in the diagnostic workup of HFpEF.
Limitations
Our study was a cross-sectional study, and we did not relate our findings to clinical outcome. Follow-up studies should be performed to directly compare echocardiographic measures to exercise capacity and outcome. Also, our HFpEF population was relatively small and our findings should be confirmed in larger populations.
The prognostic value of peak VO2 between 15 and 20 mL/kg/min is debated. Our proposal to use strain as a surrogate for peak VO2 to predict prognosis in early disease stages should therefore be confirmed in future studies.
The study population was heterogeneous in functional level, symptom score, and disease stage. Hence, the level of myocardial impairment assessed by echocardiography ranged from near normal till severely reduced.
In separate analyses of patients with reduced LV function (EF < 50%), EF, GLS, and RV function were not correlated to exercise capacity. We believe that the heterogeneous aetiologies and pathologies and the co-morbidities in patients with severe myocardial dysfunction interfere with the relations found at earlier stages of myocardial disease, as also shown by others.8–10
Due to strong correlations between LV and RV function parameters and the limited number of observations, we did not include these in multivariate regression analyses together. Therefore, we were not able to interpret whether LV function was correlated to exercise capacity independently of RV function.
We used the absolute peak VO2 values in our study as recommended to assess heart failure severity and prognosis.7 The percentage of predicted peak VO2 has been used previously, but it has not shown superiority.3,36
We correlated echocardiographic parameters at rest to exercise parameters. Previous studies have shown better correlation between exercise parameters and stress echocardiographic LV filling pressures.37 However, the fact that we demonstrated a relationship between exercise capacity and resting myocardial function may strengthen the clinical importance of our findings.
Lack of NT-proBNP analyses in some patients may have influenced our data.
Conclusions
Longitudinal LV function by GLS was independently correlated to peak VO2, and GLS was superior to identify patients with exercise capacity below normal values. Through its strong relation to the established prognostic marker exercise capacity, we propose that GLS may help with early identification of patients with poor prognosis.
In patients with HFpEF, GLS was impaired and was related to reduced diastolic function and reduced exercise capacity. These results emphasize that there is systolic dysfunction in patients with preserved EF and the presence of a tight coupling of systolic and diastolic function in HFpEF patients.
Supplementary data
Supplementary data are available at European Heart Journal – Cardiovascular Imaging online.
Funding
This work was supported by the Research Council of Norway funding the Center for Cardiological Innovation [203489 to N.E.H. and T.E.] and the South-Eastern Norway Regional Health Authority [2011094 to K.H.H.]. Funding to pay the Open Access publication charges for this article was provided by the Norwegian Research Council.
Acknowledgements
We appreciate the help from M.P. Ribe, study nurse, Oslo University Hospital.
Conflict of interest: None declared.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 2.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–7. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 3.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 5.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005;46:1883–90. doi: 10.1016/j.jacc.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 6.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 7.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012;33:2917–27. doi: 10.1093/eurheartj/ehs221. [DOI] [PubMed] [Google Scholar]
- 8.Dubach P, Myers J, Dziekan G, Goebbels U, Reinhart W, Muller P, et al. Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. J Am Coll Cardiol. 1997;29:1591–8. doi: 10.1016/s0735-1097(97)82540-5. [DOI] [PubMed] [Google Scholar]
- 9.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol. 1981;47:33–9. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 10.Smart N, Haluska B, Leano R, Case C, Mottram PM, Marwick TH. Determinants of functional capacity in patients with chronic heart failure: role of filling pressure and systolic and diastolic function. Am Heart J. 2005;149:152–8. doi: 10.1016/j.ahj.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Davies SW, Fussell AL, Jordan SL, Poole-Wilson PA, Lipkin DP. Abnormal diastolic filling patterns in chronic heart failure—relationship to exercise capacity. Eur Heart J. 1992;13:749–57. doi: 10.1093/oxfordjournals.eurheartj.a060251. [DOI] [PubMed] [Google Scholar]
- 12.Tabet JY, Logeart D, Geyer C, Guiti C, Ennezat PV, Dahan M, et al. Comparison of the prognostic value of left ventricular filling and peak oxygen uptake in patients with systolic heart failure. Eur Heart J. 2000;21:1864–71. doi: 10.1053/euhj.2000.2174. [DOI] [PubMed] [Google Scholar]
- 13.Terzi S, Dayi SU, Akbulut T, Sayar N, Bilsel T, Tangurek B, et al. Value of left atrial function in predicting exercise capacity in heart failure with moderate to severe left ventricular systolic dysfunction. Int Heart J. 2005;46:123–31. doi: 10.1536/ihj.46.123. [DOI] [PubMed] [Google Scholar]
- 14.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–53. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 15.Hummel YM, Bugatti S, Damman K, Willemsen S, Hartog JW, Metra M, et al. Functional and hemodynamic cardiac determinants of exercise capacity in patients with systolic heart failure. Am J Cardiol. 2012;110:1336–41. doi: 10.1016/j.amjcard.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Salerno G, D'Andrea A, Bossone E, Scarafile R, Riegler L, Di SG, et al. Association between right ventricular two-dimensional strain and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy. J Cardiovasc Med (Hagerstown) 2011;12:625–34. doi: 10.2459/JCM.0b013e328349a268. [DOI] [PubMed] [Google Scholar]
- 17.Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106:50–6. doi: 10.1161/01.cir.0000019907.77526.75. [DOI] [PubMed] [Google Scholar]
- 18.Gjesdal O, Helle-Valle T, Hopp E, Lunde K, Vartdal T, Aakhus S, et al. Noninvasive separation of large, medium, and small myocardial infarcts in survivors of reperfused ST-elevation myocardial infarction: a comprehensive tissue Doppler and speckle-tracking echocardiography study. Circ Cardiovasc Imaging. 2008;1:189–96. doi: 10.1161/CIRCIMAGING.108.784900. , 2. [DOI] [PubMed] [Google Scholar]
- 19.Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A, et al. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr. 2012;25:667–73. doi: 10.1016/j.echo.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Sarvari SI, Haugaa KH, Anfinsen OG, Leren TP, Smiseth OA, Kongsgaard E, et al. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J. 2011;32:1089–96. doi: 10.1093/eurheartj/ehr069. [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Gjesdal O, Hopp E, Vartdal T, Lunde K, Helle-Valle T, Aakhus S, et al. Global longitudinal strain measured by two-dimensional speckle tracking echocardiography is closely related to myocardial infarct size in chronic ischaemic heart disease. Clin Sci (Lond) 2007;113:287–96. doi: 10.1042/CS20070066. [DOI] [PubMed] [Google Scholar]
- 26.Lancellotti P, Cosyns B, Zacharakis D, Attena E, Van CG, Gach O, et al. Importance of left ventricular longitudinal function and functional reserve in patients with degenerative mitral regurgitation: assessment by two-dimensional speckle tracking. J Am Soc Echocardiogr. 2008;21:1331–6. doi: 10.1016/j.echo.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009;2:382–90. doi: 10.1161/CIRCIMAGING.108.811620. [DOI] [PubMed] [Google Scholar]
- 28.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213–23. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 29.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Donal E, Coquerel N, Bodi S, Kervio G, Schnell F, Daubert JC, et al. Importance of ventricular longitudinal function in chronic heart failure. Eur J Echocardiogr. 2011;12:619–27. doi: 10.1093/ejechocard/jer089. [DOI] [PubMed] [Google Scholar]
- 31.Palmieri V, Palmieri EA, Arezzi E, Innelli P, Sabatella M, Ferrara LA, et al. Peak exercise oxygen uptake and left ventricular systolic and diastolic function and arterial mechanics in healthy young men. Eur J Appl Physiol. 2004;91:664–8. doi: 10.1007/s00421-003-0999-8. [DOI] [PubMed] [Google Scholar]
- 32.Morris DA, Boldt LH, Eichstadt H, Ozcelik C, Haverkamp W. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail. 2012;5:610–20. doi: 10.1161/CIRCHEARTFAILURE.112.966564. [DOI] [PubMed] [Google Scholar]
- 33.Yip GW, Zhang Q, Xie JM, Liang YJ, Liu YM, Yan B, et al. Resting global and regional left ventricular contractility in patients with heart failure and normal ejection fraction: insights from speckle-tracking echocardiography. Heart. 2011;97:287–94. doi: 10.1136/hrt.2010.205815. [DOI] [PubMed] [Google Scholar]
- 34.Opdahl A, Remme EW, Helle-Valle T, Lyseggen E, Vartdal T, Pettersen E, et al. Determinants of left ventricular early-diastolic lengthening velocity: independent contributions from left ventricular relaxation, restoring forces, and lengthening load. Circulation. 2009;119:2578–86. doi: 10.1161/CIRCULATIONAHA.108.791681. [DOI] [PubMed] [Google Scholar]
- 35.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aaronson KD, Mancini DM. Is percentage of predicted maximal exercise oxygen consumption a better predictor of survival than peak exercise oxygen consumption for patients with severe heart failure? J Heart Lung Transplant. 1995;14:981–9. [PubMed] [Google Scholar]
- 37.Podolec P, Rubis P, Tomkiewicz-Pajak L, Kopec G, Tracz W. Usefulness of the evaluation of left ventricular diastolic function changes during stress echocardiography in predicting exercise capacity in patients with ischemic heart failure. J Am Soc Echocardiogr. 2008;21:834–40. doi: 10.1016/j.echo.2007.12.008. [DOI] [PubMed] [Google Scholar]