Abstract
Many cationic lipids have been developed for lipid-based nanoparticles (LNPs) for delivery of siRNA and microRNA (miRNA). However, less attention has been paid to “helper lipids”. Here, we investigated several “helper lipids” and examined their effects on the physicochemical properties such as particle size and zeta potential, as well as cellular uptake and transfection efficiency. We found that inclusion of oleic acid (OA), an unsaturated fatty acid; into the LNP formulation significantly enhanced the delivery efficacy for siRNA and miRNA. For proof-of-concept, miR-122, a liver-specific microRNA associated with many liver diseases, was used as a model agent to demonstrate the hepatic delivery efficacy both in tumor cells and in animals. Compared to Lipofectamine 2000, a commercial transfection agent, OA containing LNPs delivered microRNA-122 in a more efficient manner with a 1.8-fold increase in mature miR-122 expression and a 20% decrease in Bcl-w, a target of microRNA-122. In comparison with Invivofectamine, a commercial transfection agent specifically designed for hepatic delivery, OA containing LNPs showed comparable liver accumulation and in vivo delivery efficiency. These findings demonstrated the importance of “helper lipid” components of the LNP formulation on the cellular uptake and transfection activity of siRNA and miRNA. OA containing LNPs are a promising nanocarrier system for the delivery of RNA-based therapeutics in liver diseases.
Keywords: Cationic lipid nanoparticles, Helper lipids, siRNA, microRNA, Hepatic delivery
1. Introduction
RNA interference (RNAi) can silence target gene expression and thereby block the production of disease-causing proteins [1–3]. Liver plays a pivotal role in the metabolism and its dysfunction can cause many diseases such as hepatitis [4], cirrhosis [5], alcohol damage [6] and primary liver cancer [7]. siRNAs and microRNAs (miRNAs) have been investigated as potential therapeutic agents for the treatment of various liver diseases, especially hepatocellular carcinoma (HCC) [8,9]. For example, microRNA-122 (miR-122) is the predominant liver miRNA, making up 70% of the total miRNA population [10,11]. It has been demonstrated that miR-122 plays a pivotal role in liver function and miR-122 loss in mice results in inflammation, fibrosis and cancer [10,11]. This indicates a potential therapeutic role for exogenous miR-122 delivery in the treatment of liver disease. Recently, we developed a cationic lipid nanoparticle (LNP) formulation for miR-122 delivery to restore deregulated gene expression in HCC cells.
Cationic lipid or lipid-like material-based nanoparticles are the most well studied nanocarriers for systemic delivery of siRNA and miRNA to the liver because of their relative safety and simplicity of production. Some have been utilized in clinical trials [12–16]. A typical cationic LNP formulation for in vivo delivery consists of cationic lipids, neutral lipids and PEG-lipids. LNPs are able to stabilize nucleic acids against enzymatic degradation, facilitate the cellular uptake and prolong the circulation half-life time of siRNA and miRNA [17]. Over the last decade, a variety of conditionally ionizable and permanently ionized cationic lipids have been extensively investigated for LNP mediated siRNA and miRNA delivery [18,19]. Neutral lipids such as cholesterol, dioleoylphosphatidyl ethanolamine (DOPE) and phosphatidylcholine (PC) are known as “helper lipids” ‘because of their ability to increase LNP stability [20] and decrease toxicity [21] from cationic lipids. Although considerable efforts have been made in developing novel cationic lipids, there are few reports on identifying more effective helper lipids for the delivery of siRNA or miRNA [18, 22–25].
In the present study, our goal is to develop LNP formulations for the enhanced in vivo delivery of siRNA/miRNA to hepatocytes. The cationic lipid, N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) was used to form a complex with siRNA/miRNA due to its high transfection activities in vitro and in vivo [26, 27]. We formulated a series of cationic LNPs differing in their helper lipids (cholesterol, PCs and unsaturated fatty acids) and studied their particle size, surface charge, cellular uptake and transfection activity in vitro, especially comparing oleic acid with phosphatidylcholine (PC). Finally, the optimal formulation was chosen to deliver miR-122 to HCC cells and mouse liver to study the transfection efficacy in vivo.
2. Materials and methods
2.1. Materials
N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), and hydrogenated L-α-phosphatidylcholine (HSPC) were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Other chemicals and reagents were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and were of analytical grade. Egg phosphatidylcholine (egg PC) was obtained from Lipoid (Newark, NJ, USA). All tissue culture media and supplies were obtained from Invitrogen (CA, USA). All other reagents were of analytical grade. Luciferase-targeted and negative control siRNA as well as FAM-siRNA scramble were obtained from Applied Biosystems (Austin, TX, USA). The Silencer® GL2+GL3 luciferase siRNA (siLuc, AM4629), control #1 siRNA (siNC, AM4611), FAM™-labeled negative control #1 siRNA (FAM-siRNA, AM4620), Cy3-labeled negative control #1 siRNA (Cy3-siRNA, AM4621), and miR-122 mimic (C-300591-05) were purchased from Dharmacon (Pittsburgh, PA). TaqMan MicroRNA reverse transcription kit was provided by Applied Biosystems (4366596).
2.2. Preparation of LNPs
The LNPs were first prepared by ethanol injection as described previously with minor modification [28]. Briefly, an ethanolic lipid solution composed of cationic lipid and various helper lipids (Tables 1 and 2) was injected into a stirring 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES) solution (20 mM HEPES, pH 7.4) at room temperature. Ethanol was removed by dialysis using a MWCO 10,000 Dalton Float-A-Lyzer (Spectrum Laboratories Inc., Ranco Dominguez, CA, USA) against HEPES buffer (20 mM HEPES, pH 7.4) for 2 h at room temperature. The resulting liposomes were then sterilized using a 0.22 μm filter (Fisher Scientific, Pittsburgh, PA, USA). Cationic liposomal nanoparticles containing siRNA were prepared by mixing cationic liposomes with an equal volume of siRNA in HEPES buffer (20 mM HEPES, pH 7.4) and incubated at room temperature for 15 min. The weight ratio of lipids to siRNA was 10/1.
Table 1.
Characterization of Cholesterol, PCs and unstaturated fatty acids containing particles
| Formulation | Components | Average particle size (nm) | Zeta potential (mV) (in water) | PDI | siRNA EE (%) |
|---|---|---|---|---|---|
| DOTMA-Chol (38%mol) | DOTMA/Chol/Chol-PEG=60/38/2 | 128.8 | 21.88 ± 1.61 | 0.14 | 89.7 |
| DOTMA-Chol (48%mol) | DOTMA/Chol/Chol-PEG=50/48/2 | 77.6 | 13.6 ± 1.27 | 0.18 | 93.5 |
| DOTMA-Chol (58%mol) | DOTMA/Chol/Chol-PEG=40/58/2 | 80.9 | 7.80 ± 0.93 | 0.16 | 92.3 |
| DOTMA-Chol (68%mol) | DOTMA/Chol/Chol-PEG=30/68/2 | 90.4 | 1.71 ± 0.47 | 0.21 | 90.8 |
| DOTMA-Chol (73%mol) | DOTMA/Chol/Chol-PEG=25/73/2 | 89.5 | 0.82 ± 0.162 | 0.20 | 88.4 |
| DOTMA-EggPC | DOTMA/EggPC/Chol/Chol-PEG=45/18/35/2 | 99.3 | 17.43 +/− 0.65 | 0.15 | 94.5 |
| DOTMA-DPPC | DOTMA/DPPC/Chol/Chol-PEG=45/18/35/2 | 40.5 | 15.90 ± 2.08 | 0.14 | 90.3 |
| DOTMA-HSPC | DOTMA/HSPC/Chol/Chol-PEG=45/18/35/2 | 222.8 | 15.30 ± 0.79 | 0.17 | 91.7 |
| DOTMA-POPC | DOTMA/POPC/Chol/Chol-PEG=45/18/35/2 | 128.4 | 8.33 ± 0.82 | 0.18 | 89.5 |
| DOTMA-DSPC | DOTMA/DSPC/Chol/Chol-PEG=45/18/35/2 | 73.1 | 22.57 ± 1.08 | 0.20 | 93.4 |
| DOTMA-OA | DOTMA/OA/Chol/Chol-PEG=45/18/35/2 | 37.5 | 2.01 ± 0.29 | 0.14 | 93.8 |
| DOTMA-LA | DOTMA/LA/Chol/Chol-PEG=45/18/35/2 | 68.2 | 4.12 ± 0.74 | 0.18 | 92.1 |
| DOTMA-LNA | DOTMA/LNA/Chol/Chol-PEG=45/18/35/2 | 108.3 | 3.20 ± 0.05 | 0.19 | 93.2 |
PDI: polydispersity index. Values are mean +/− SD. EE: encapsulation efficiency
2.3. Size, zeta potential and siRNA encapsulation efficiency
The particle size of siRNA-LNPs was determined by dynamic light scattering using a particle sizer BI-200SM (Brookhaven Instruments Corp., Holtsville, NY, USA) in an intensity-weighted mode. Following dilution in water, the zeta potentials (ζ) of siRNA-LNPs was measured on a ZetaPALS zeta potential analyzer (Brookhaven Instrument Corp., Holtsville, NY). The siRNA encapsulation efficiency of the formulations was measured using the Quant-iT™ RiboGreen® RNA Kit, according to the manufacturer’s instructions [29].
2.4. Cell culture
SK-Hep-1 cells with stable luciferase expression (SK-Hep-1 Luc) and HepG2 were grown in MEM culture medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin, at 37°C in a humidified atmosphere containing 5% CO2.
2.5. In vitro transfection and gene silencing studies
SK-Hep-1 cells, stably expressing the firefly luciferase gene, were plated at 2 × 104 cells per well in 48-well plates and grown to 60–70% confluent prior to transfection. Luciferase specific siRNA (Luci-siRNA) and negative control (NC siRNA) were formulated into LNPs. Cells were treated with various siRNA-LNPs at indicated concentrations and incubated for another 24 h at 37°C and 5% CO2. The cells were then washed with PBS and lysed. The luciferase activity for each well was determined using Luciferase Reagent (Promega) on a Berthold MicroLumatPlus LB96V plate luminometer. Luciferase down-regulation relative to a control was then determined for each condition. Lipofectamine 2000 (Invitrogen, CA, USA) was used as a positive control. Untreated cells were used as a negative control.
2.6. Cellular uptake study and endosomal release of siRNA by flow cytometry
Cy3 or FAM-siRNA was used to study cellular uptake of LNPs. A total of 6×104 cells were seeded in 24-well plates prior to treatment. The cells were rinsed three times with 500 μl phosphate buffered Saline (PBS) (pH=7.4) and fixed in 4% para-formaldehyde 24 h post transfection with free Cy3- or FAM-siRNA, or Cy3- or FAM-siRNA-LNP. The cell suspension was directly introduced into a Beckman Coulter EPICS XL (Beckman Coulter Inc., CA, USA) to determine the fluorescence intensity of Cy3 or FAM. For each cell sample, a minimum of 10,000 events were collected under the LIST mode.
2.7. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
Cryo-TEM imaging was performed at the Imaging Center of the Ohio State University. Briefly, samples were examined in a Philips CM120 microscope (Eindhoven, The Netherlands) at 120 kV, using an Oxford CT-3500 cooling holder and transfer station (Abingdon, England). Specimens were equilibrated in the microscope at below −178°C, then examined in the low-dose imaging mode to minimize electron beam radiation damage, and recorded at a nominal under-focus of 1–2 μm to enhance phase contrast. Images were recorded digitally by a Gatan 791 MultiScan CCD camera, and processed using the Digital Micrograph 3.1 software package.
2.8. Quantification of mature miR-122 and Bcl-w mRNA in HepG2 cells
The cells were washed with cold 1X PBS 48 h post transfection. Total RNA was extracted by Trizol according to manufacturer’s protocol (Invitrogen, 15596-018). To measure mature miR-122 expression, the total RNA was first reverse transcribed into cDNA using the TaqMan MicroRNA reverse transcription kit (Applied Biosystems). The qRT-PCR amplification of cDNA was then performed using TaqMan MicroRNA assay (Applied Biosystems). The miR-122 expression was determined by the ΔΔCT method and normalized to RNU6B (Applied Biosystems), which was the endogenous control in the corresponding samples, and relative to the untreated control cells. To measure the Bcl-w expression at the mRNA level, the total RNA was transcribed into cDNA using the first-strand cDNA synthesis kit (Invitrogen). The cDNA was then amplified by qRT-PCR (Applied Biosystems). Relative gene expression values were determined by the ΔΔCT method. Bcl-w expression was normalized to GAPDH (Applied Biosystems), which was the endogenous reference for the corresponding sample, and relative to the untreated control cells.
2.9. Experimental animals
The animal studies were carried out in accordance with the internal Institutional Animal Care and Use Committee guidelines at The Ohio State University. ICR mice of weight 18–20 g (age 4–6 weeks) were purchased from Harlan Laboratories, Inc. All experiments performed on animals were in accordance with and approved by the IACUC committee at The Ohio State University.
2.10. In vivo bio-distribution study of LNPs
ICR mice were given i.v. injections of Cy5-labeled oligodeoxynucleotide (Cy5-G3139) formulated in LNPs. After 4 h, mice were euthanized and tissues were collected and fixed in 4% paraformaldehyde for 6 h followed by 12 h incubation in a 30% sucrose/PBS solution at 4°C. Fixed tissue samples were then placed into block holders containing O.C.T. (ThermoFisher, Pittsburgh, PA) and snap-frozen on dry ice. The frozen blocks were sectioned using cryostat at 4 μm. Cellular outline and nuclei were stained with Alexa-488 phalloidin (Invitrogen, Carlsbad, CA) and DAPI (Vector, Burlingame, CA), respectively for 15 min at room temperature. Green fluorescence of actin filament, red fluorescence of Cy5-labeled G3139 and blue fluorescence of DAPI were detected by an Olympus FV1000 Filter Confocal Microscope (Olympus Optical Co., Tokyo, Japan).
2.11. Serological analyses of toxicity induced by DOTMA-OA LNPs containing miRNA
Serum was isolated from mice by cardiac puncture after CO2 asphyxiation and cervical dislocation following overnight fasting. Biochemical analyses of ALT, AST, BUN, and creatinine in serum were performed at The Ohio State University Comparative Pathology and Mouse Phenotyping Shared Resource using VetAce (Alfa Wassermann system).
2.12. In vivo delivery of miR-122 containing DOTMA-OA LNPs
miR-122 and miR-NC containing DOTMA-OA LNPs were administered to ICR mice by tail vein injection at 1.5 mg/kg. After 48 h, mice were euthanized and liver tissues were collected and quickly frozen in liquid nitrogen. The frozen liver tissues were ground to powders and total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). Quantification of miR-122 and Bcl-w mRNA expression were carried out using the same method as described above.
2.13. Statistical analysis
Data were analyzed by the analysis of variance (ANOVA). In vivo bio-distribution data was analyzed by a mixed effect model incorporating repeated measures for each subject [30]. Holm’s method was applied to adjust for multiplicity to control the overall family-wise error rate at α = 0.05 [31]. SAS software was used for data analysis (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Characterization of various helper lipids in DOTMA based cationic LNPs
Cholesterol and various PCs such as EggPC and DSPC are often chosen as helper lipids in the LNP formulations (Fig. 1). In this study, we selected the unsaturated fatty acids as the helper lipid components to investigate the effects of various helper lipids on the transfection activities (Fig. 1). In LNP based formulations, DOTMA and cholesterol-PEG (PEG-Chol) were used as the cationic lipid and PEG-lipid, respectively. We prepared 13 different LNP formulations termed as DOTMA-Chol (38%mol), DOTMA-Chol (48%mol), DOTMA-Chol (58%mol), DOTMA-Chol (68%mol), DOTMA-Chol (73%mol), DOTMA-EggPC, DOTMA-DPPC, DOTMA-HSPC, DOTMA-POPC, DOTMA-DSPC, DOTMA-OA, DOTMA-LA and DOTMA-LNA. Table 1 displays the particle size and surface charge of these LNP formulations. The measurements were obtained from dynamic light scattering and zeta potential analysis. All the DOTMA-Chol formulations with siRNA complexes resulted in ~100 nm particles in size. Increasing molar ratio of cholesterol from 38% to 73% contributed to a decrease of the zeta potential in a dose dependent manner. Particle size of LNP/siRNA complexes formed by DOTMA-PCs and siRNA was also around 100 nm. The zeta potential was in a range of 7.33~ 22.57mV. DOTMA-OA-siRNA, DOTMA-LA-siRNA and DOTMA-LNA-siRNA LNPs had particle sizes of 37.5, 68.2 and 108.3 nm, respectively and all the formulations showed a positive charge.
Fig. 1. Chemical structure of helper lipids.
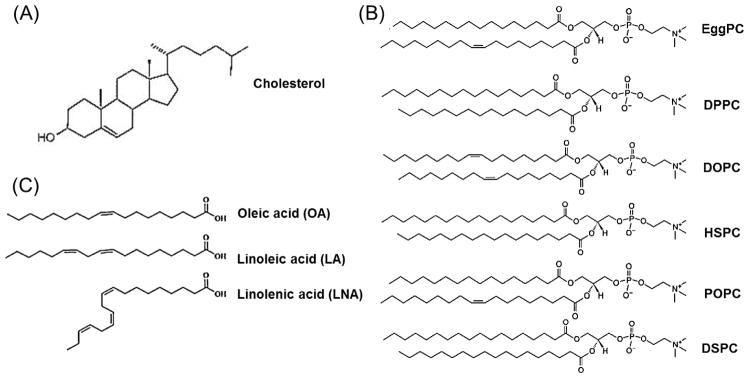
(A) Cholesterol, (B) various PCs (C) unsaturated fatty acids (OA, LA and LNA).
3.2. Effect of helper lipids on cellular uptake of siRNA containing cationic LNPs
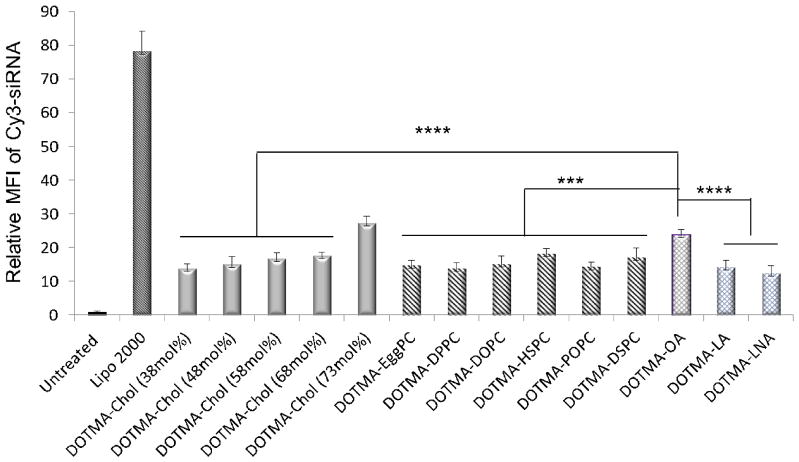
Cellular uptake is one of criteria to evaluate the transfection activities of siRNA mediated by LNPs. We first tested the effect of cholesterol content, selection of the type of PC or unsaturated fatty acid on cellular uptake of LNPs in vitro. The uptake of DOTMA-based LNPs containing various molar ratios of cholesterol, PCs and unsaturated fatty acids (Table 1) for siRNA delivery were evaluated in SK-Hep-1 cells. Nonspecific siRNA (siRNA-scramble) labeled by Cy3 loaded into DOTMA-based LNPs containing different helper lipids was applied to SK-Hep-1 cells. Flow cytometry was used to study the cellular uptake of Cy3-siRNA-LNPs at 4 h post-transfection. As shown in Fig. 2, The Mean fluorescence intensity (MFI) of Cy3-siRNA delivered by various DOTMA-based LNPs was significantly greater than the untreated control (p<0.0001) while lower than that mediated by Lipofectamine 2000 (Lipo 2000), a widely used commercial transfection agent for DNAs and siRNAs. The results showed no clear correlation between the transfection efficiency and the degree of saturation or acyl chain lengths of PCs. The fluorescence signals of cells transfected with Cy3-siRNA and OA containing LNPs were stronger than those transfected with Cy3-siRNA-PCs (p<0.001) or Cy3-siRNA-cholesterol (p<0.0001) LNPs except for the formulation with 73 mol% cholesterol. Cholesterol is known to influence bilayer fluidity, resulting in increased rigidity, enhanced bilayer stability against leakage [32] and improved vesicle resistance to aggregation [33]. However, a very high content of cholesterol may make the LNPs fragile and unstable [34]. In addition, OA containing DOTMA LNPs showed greater uptake than LA and LNA (p<0.0001). The OA is a potent helper lipid component and the introduction of OA in DOTMA based LNPs enhanced the intracellular uptake and transfection activity.
Fig. 2. Uptake of various helper lipids containing DOTMA LNPs in SK-Hep-1 cells.
Flow cytometry analysis of cellular uptake of Cy3-siRNA-NC delivered by various helper lipids containing DOTMA cationic LNPs 4 hrs. post-transfection at Cy3-siRNA-NC concentration of 100 nM. MFI of Cy3 averaged on 3 replicates (n=3; ***: P<0.001; ****: P<0.0001).
3.3. Examination of effect of the choice of helper lipids on siRNA transfection efficiency
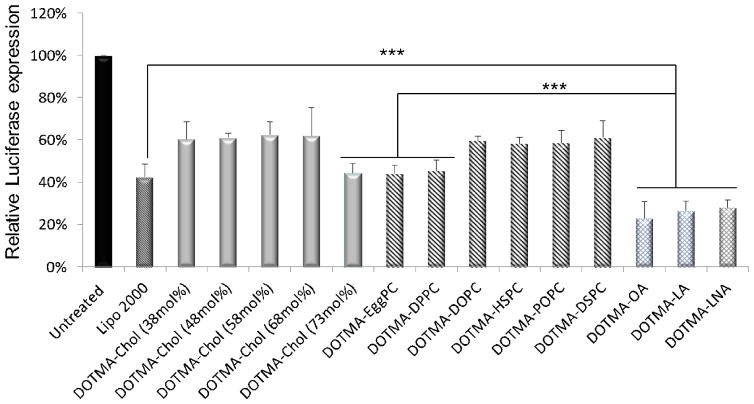
In general, high cellular uptake leads to high transfection efficiency. To further prove that a higher gene silencing efficiency could be achieved by the incorporation of OA, the transfection efficiency of DOTMA-based LNPs containing different helper lipids (Table 1) for siRNA delivery was evaluated in SK-Hep-1 cells stably expressing the luciferase gene. Luciferase specific and negative control siRNAs were encapsulated in DOTMA-based LNPs (Table 1) and delivered to cells. Luciferase expression was determined at 24 h after transfection. Consistent with the cellular uptake results presented above, luciferase expression of various DOTMA-based LNPs was significantly lower than untreated control (p<0.0001). As cholesterol content increased to 73 mol%, luciferase gene expression was significantly decreased resulting in efficiency similar to that of Lipo 2000. For PC containing LNPs, among all PC based LNPs, DOTMA-EggPC and DOTMA-DPPC LNPs showed higher gene silencing efficiency compared to the others (p<0.01). Fig. 3 also shows that the luciferase level, expressed relative to the level of untreated cells, significantly decreased when siRNAs were transfected with DOTMA-OA, LA and LNA LNPs. The efficiency was higher than that of the DOTMA-EggPC LNPs (p<0.001). The reduction of the luciferase level induced by the OA containing LNPs was approximately 15% better than Lipo 2000 (P<0.001). This may be due to the fact that negatively charged molecules such as oleic acid can dissociate nucleotide from cationic LNPs in the cytoplasm. These results confirmed our hypothesis of pH-dependent destabilization induced by OA in the DOTMA based LNPs [35]. There is no significant difference in the transfection efficiency among OA, LA and LNA formulations.
Fig. 3. Luciferase gene silencing mediated by various helper lipids containing DOTMA based LNPs in SK-Hep-1 cells.
Reduced luciferase expression in SK-Hep-1 cells transfected with siRNA formulated in various helper lipids containing DOTMA nanoparticles. Cells were transfected with 100 nM luciferase specific siRNA. The inhibition of luciferase activity measured at 24h post-transfection was normalized to that of cells treated with negative control RNA and untreated cells (n = 3; ***: P<0.001).
3.4. Mechanisms of siRNA delivery by DOTMA-OA LNPs
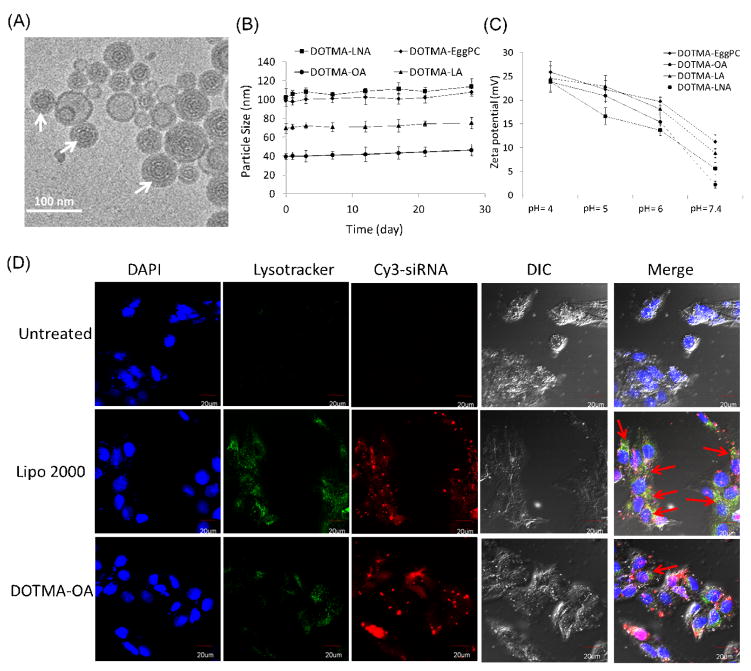
The detailed structures of the DOTMA-OA-siRNA LNPs were studied by cryo-TEM and are shown in Fig 4A. The white-arrow indicates lamellar onion-like structures that are known as the classic siRNA/lipid nanoparticles structure [25]. The onion-like structures are siRNAs sandwiched between two adjacent lipid bilayers. The lamellar layers were distinct by different contrasts of the lipids bilayer and the electron dense siRNA layer. Particle size measurements demonstrated that DOTMA based siRNA-LNPs were relatively stable by showing a gradual increase in the particle size over the first four weeks but the increase was not significant (Fig 4B).
Fig. 4. Mechanisms of siRNA delivery by DOTMA-OA LNPs.
(A) A cryo-TEM image of nanostrucure of siRNA-DOTMA based LNPs containing OA. White arrows show the onion-like structure of LNPs. (B) Stability of DOTMA based LNPs containing EggPC, OA, LA and LNA. (C) Measurement of pH-dependent zeta potential of DOTMA based LNPs. (D) Co-localization study of DOTMA-OA-Cy3-siRNA (red) with lysotracker (green). Red arrows indicate the co-localization. Scale bar=20 μm.
To investigate the ionizability of DOTMA based LNPs, we examined the zeta potential change as a function of the pH value. As shown in Fig 4C, the incorporation of unsaturated fatty acids in DOTMA based LNPs improved their pH-sensitive ionization. DOTMA-OA, LA and LNA LNPs showed stronger ionization than DOTMA-EggPC, rendering them strongly positive-charged at acidic pH but only slightly positive-charged at physiological pH. Especially, DOTMA-OA showed a low charge close to neutral at pH=7.4. The zeta potential was gradually decreased along with the increasing pH value. Since the pH of lysosome was below 5.0, the surface charge reversal from slightly positive to strongly positive would occur when siRNA loaded LNPs were taken up by cells and transported to endosome, providing a mechanism for the endosomal escape of siRNA. Furthermore, the cationic LNPs may interact with vesicular membranes leading to localized destabilization of the membrane and the escape of nanoparticles from endosome to cytoplasm. Thus, the DOTMA based LNPs containing unsaturated fatty acid provide a higher potential for endosomal escape.
To further examine the intracellular trafficking of DOTMA-OA LNPs, we studied the co-localization of Cy3-siRNA-LNPs with LysoTracker (green) using confocal imaging to determine the endosomal escape rate of siRNA. Fig 4D shows that the co-localization between the siRNA and the lysosomes was clearly observed at 4 h in cells treated with Lipo 2000, as exhibited by the significant overlap of the fluorescence signals contributed by the Cy3-siRNA (red) and the lysosomes labeled with LysoTracker® Green DND-26 (green). In contrast, the siRNA signals of DOTMA-OA LNPs were separated from the lysosomes. This could explain why the DOTMA-OA LNPs mediated siRNA delivery could provide a much greater gene silencing activity than Lipo 2000/siRNA complexes.
3.5. Delivery of miR-122 using DOTMA based LNPs
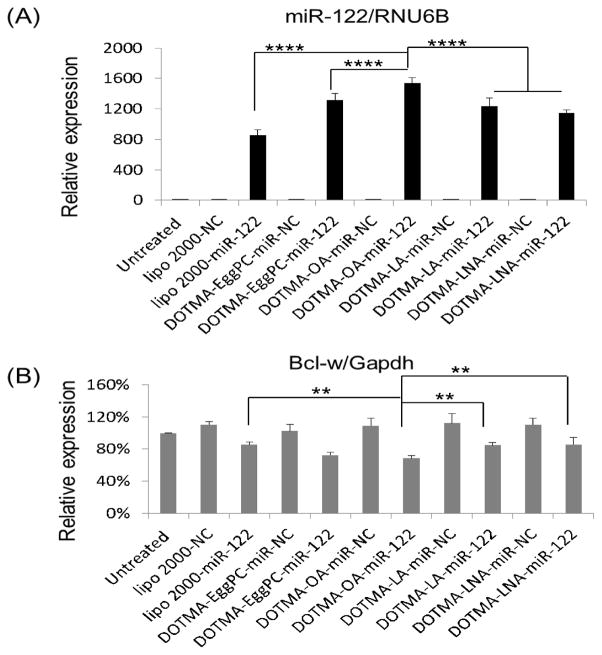
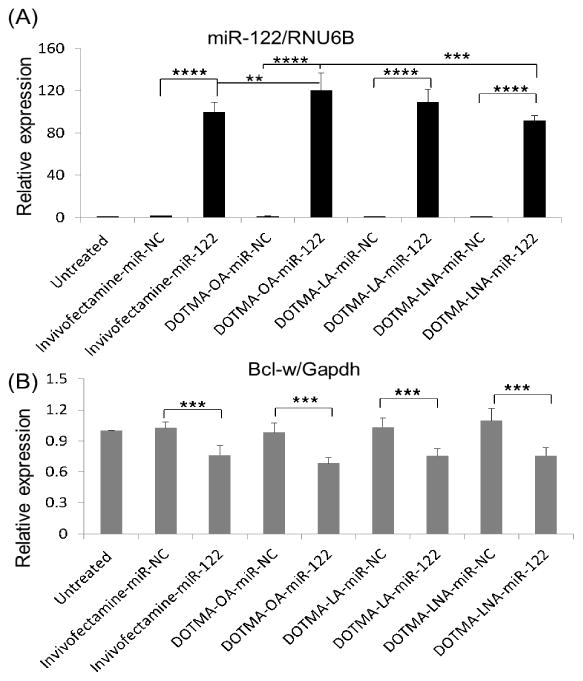
miR-122 is a liver-specific miRNA and its loss in mice has been reported to be associated with many liver diseases such as inflammation, fibrosis and cancer [10,11]. In addition, miR-122 down-regulation has been reported in murine and human HCC [10,11], suggesting that its function is associated with hepatocarcinogenesis as a tumor suppressor miRNA. We studied the exogenous delivery of miR-122 in HepG2 cells and carried out qRT-PCR to quantify the expression of miR-122 and its target gene Bcl-w [36], an anti- apoptotic Bcl-2 family member. As expected, the miR-122 level was increased by ~900 folds in cells transfected with miR-122 containing DOTMA based LNPs compared to the negative control miR (NC) (p<0.0001) (Fig. 5A). Moreover, the miR-122 expression was increased by 200~600 folds in cells transfected by DOTMA with unsaturated fatty acids LNPs compared to Lipo 2000 (p<0.0001), while that of DOTMA-OA and LNA were greater than DOTMA-EggPC LNPs (p<0.001). The miR-122 expression resulting from delivery by DOTMA-OA was significantly greater than that by DOTMA-LA or DOTMA-LNA (p<0.0001). Consistent with the increased level of miR-122, the expression of its target gene Bcl-w was inhibited up to 22.8%, 23.3%, 38.1%, 24.2% and 21.9% by Lipo 2000, DOTMA-EggPC, OA, LA and LNA LNPs, respectively (Fig. 5B). DOTMA-OA showed 16.1% more down-regulation of Bcl-w expression than DOTMA-LA (p=0.0076), LNA (p=0.005) and Lipo 2000 (p=0.0058). Although miR-122 was significantly elevated for DOTMA-LA and LNA when compared with Lipo 2000, Bcl-w expression for LA and LNA was not reduced much probably because all transfected miR-122 mimic are not loaded into RISC. These results confirmed that DOTMA-OA LNPs is a potent delivery system for siRNA/miRNA in vitro.
Fig. 5. EggPC and unsaturated fatty acids containing DOTMA LNPs mediated miR-122 delivery in HepG2 cells.
(A) Mature miR-122 and (B) Bcl-w expression 48 h after HepG2 cells were transfected with miR-122 containing DOTMA-unsaturated fatty acid LNPs, DOTMA-EggPC LNPs, Lipofectaimine 2000 LNPs as well as miR-NC containing DOTMA-OA LNPs, DOTMA-EggPC LNPs, Lipofectaimine 2000 LNPs at miRNA concentration of 100 nM. (*: P < 0.05) (n = 3). (B) Bcl-w expression at mRNA level 48 h after HepG2 were transfected with miR-122 and miR-NC (n = 3; **: P<0.01; ****: P<0.0001).
3.6. Biodistribution of LNPs in vivo
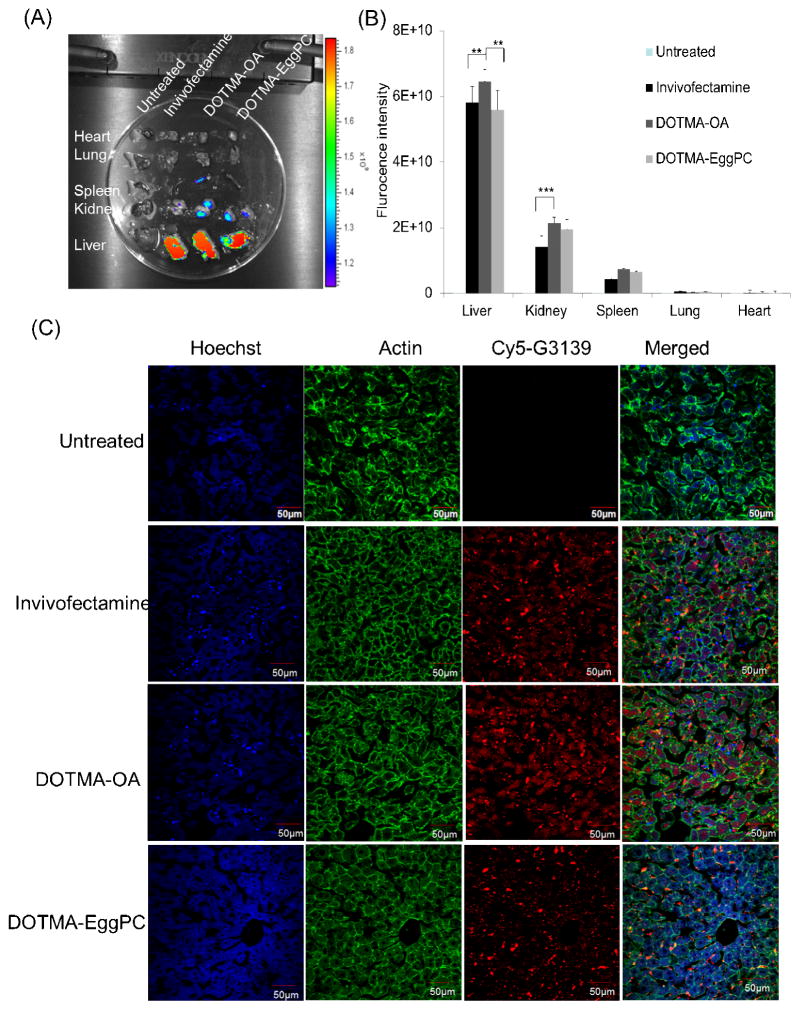
One of the major obstacles for the application of LNPs in vivo is the tissue-specific delivery. First, mice injected with Cy5-G3139 containing DOTMA-OA, DOTMA-EggPC LNPs or Invivofectmine, which was used as positive control, were examined by IVIS imaging. In the study, 200 μL of Cy5-G3139 containing LNPs were given to each mouse at 2.5 mg/kg through tail vain injection. Major organs including liver, lung, kidney, spleen and heart were harvested 4 h later. The fluorescence signal of Cy5-LNPs was analyzed by IVIS and compared with Cy5-G3139 Invivofectamine complexes and untreated mice. Figs.6A and 6B clearly show that the DOTMA-OA and EggPC LNPs facilitated preferential liver accumulation compared to other organs (p<0.0001). The DOTMA-OA formulation achieved higher liver accumulation when compared to Cy5-G3139 Invivofectamine complexes (p=0.0011) and DOTMA-EggPC LNPs (p<0.0001), accompanied by slightly greater accumulation in kidney. Confocal microscopy images of liver tissues (Fig. 6C) further confirmed that the liver accumulation of Cy5-G3139 delivered by DOTMA-OA LNPs was much higher than that delivered by Invivofectamine or DOTMA-EggPC LNPs.
Fig. 6. Tissue distribution of Cy5-G3139 containing LNPs.
(A) Tissue distribution of DOTMA-OA, DOTMA-EggPC LNPs or Invivofectamine carrying Cy5.5-labeled G3139 in normal mice. Four hours after intravenous administration of Cy5.5-labeled G3139 (2.5 mg/kg), tissues were harvested and Cy5.5 fluorescence signals were measured by IVIS imaging. The strength of the signal is shown in side bar. (B) Biodistributions of LNPs and NeoFX complexes based on the fluorescence intensity of Cy5.5 signal. (C) Confocal microscopic imaging of tissue sections was taken from heart, spleen, kidney, lung and liver. Confocal images of lung tissues in mice were treated with NeoFX complexes and LNPs (n = 3; **: P< 0.01; ***: P<0.001).
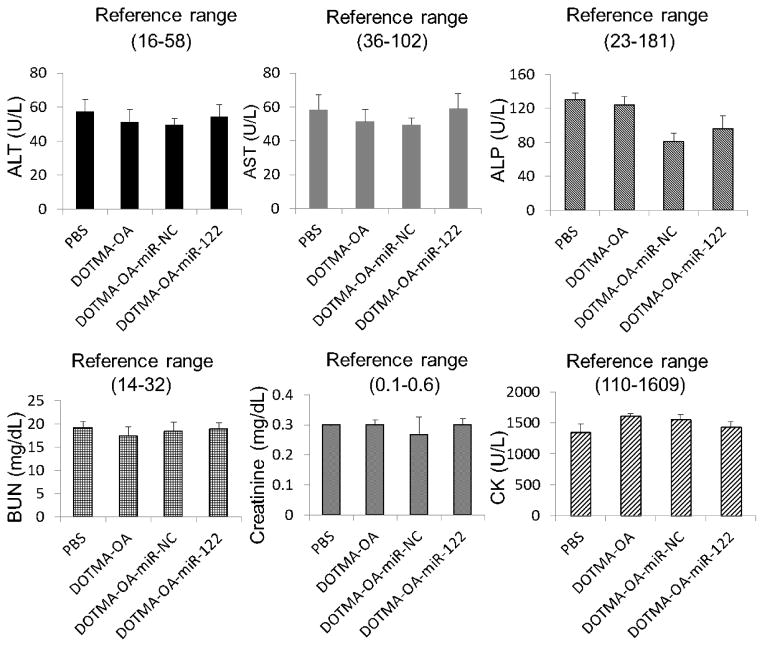
3.7. DOTMA-OA LNPs show no systemic toxicity
To assess the safety profile of systemically delivered miR-122 by the DOTMA-OA LNPs, we examined serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine, and creatine kinase (CK) from mice repeatedly treated with formulated miR-122, NC-miR, vehicle alone and PBS. Serum analysis showed no significant toxicity in mice treated with the DOTMA-OA vehicle and DOTMA-OA-miR-122 or miR-NC formulations (Fig. 7). Comparable serum ALT, AST and ALP levels were found in animals injected with DOTMA-OA vehicles and DOTMA-OA/miR-122 LNPs and miR-NC. This indicates that there was no significant liver damage caused by the LNPs. The BUN, CK and creatinine levels in the reference range elucidate that the kidney functions were normal in these mice as well. These results suggest that the miR treatment was well tolerated and DOTMA-OA LNPs is a safe delivery system for mice due to their non-immunogenic, non-pathogenic and biodegradable properties. In addition, the incorporation of LA and LNA in LNPs did not lead to any systemic toxicity (Fig. S2). In addition, the incorporation of “helper lipids” of cholesterol and Chol-PEG in the formulations may reduce the cationic liposome toxicity.
Fig. 7. DOTMA based LNPs containing oleic acid (OA) encapsulated NC miRNA and miR-122 do not exhibit systemic toxicity.
In vivo toxicity assay by measuring ALT, AST, ALP, CK, BUN and creatinine in serum of wild type mice after systemic delivery of PBS, vehicles, LNPs-miR-122 and NC-miR for 48 hours (n=3).
3.8. DOTMA-OA LNPs for hepatic delivery of miR-122
We further assessed the hepatic delivery efficacy of miR-122 encapsulated in DOTMA-OA LNPs. The DOTMA-OA LNPs and Invivofectamine containing miR-122 or NC-miR were delivered to ICR mice by a single i.v. injection (2.5 mg/kg). Mice were euthanized and liver tissues were assayed for miR-122 and its target Bcl-w expression 48 h post injection. Notably, mice treated by the DOTMA-OA-miR-122 complex and Invivofectamine had mature miR-122 levels in liver approximately 100 folds higher than that in the negative control siRNA (NC) treated liver tissue (p<0.0001). These data indicate that the observed gene silencing was a direct result of efficient miRNA delivery to liver by the DOTMA-OA LNPs (Fig 8A). Compared to NC livers, Bcl-w expression was suppressed by ~32% and ~ 24% in livers treated with DOTMA-OA-miR-122 LNPs and Invivofectamine, respectively (p<0.001) (Fig. 8B). These results showed that miR-122 could be successfully delivered to normal liver by DOTMA-OA LNPs. Although the mature miR-122 level for DOTMA-OA was greater than Invivofectamine, DOTMA-LA and LNA, there was no significant difference in Bcl-w expression. This may be due to saturated delivery of miR-122 and additional RISC are not available for miR-122 loading and further Bcl-w down-regulation.
Fig. 8. DOTMA based LNPs containing OA mediated delivery of miR-122 specifically downregulates its target gene Bcl-w in liver tissues.
(A, B) DOTMA based LNPs containing OA loaded with miR-122 or NC microRNA was delivered once to normal mice at a dose of 2.5 mg/kg by i.v. injection. After 48 hrs., mice were sacrificed and livers were processed. miR-122 and its target gene Bcl-w expression was measured using qRT-PCR. (n=3, **:P<0.01; ***:P<0.001;****:P<0.0001).
4. Discussions
RNA-based therapeutics is emerging as a treatment strategy for various human diseases including liver diseases. Small RNA molecules such as siRNA and miRNA are able to specifically silence the expression of cancer-related genes or to selectively regulate the pathways that are involved in the development and progression of malignancy [26,36]. Hepatocytes, the major parenchymal cells in liver, are a particularly attractive target cell type for siRNA/miRNA delivery. To enhance the hepatic delivery, we examined the effects of various helper lipids, including cholesterol; PCs and unsaturated fatty acids in LNP based formulations on the transfection activity both in vitro and in vivo.
The LNP formulation used in the present study was based on the commercially available cationic lipid, DOTMA, which has been demonstrated to have high transfection activity in our previous study [26]. The incorporation of PEG-lipid in LNP formulation is essential for preventing aggregation and can aid in the formation of uniform and small nanoparticles. Chol-PEG is identified as a good PEG-lipid for LNP mediated siRNA/miRNA delivery [37]. Thus, in our study, DOTMA and Chol-PEG were selected in all tested LNP formulations, while the role of other helper lipids was investigated.
Neutrally charged helper lipids such as cholesterol and PCs are often employed with cationic lipids to gain high transfection efficiency and to reduce the cytotoxicity [21]. Helper lipids play an important role during the formation of LNPs by combining cationic liposomes and nucleic acids, as they could affect the morphology of LNPs. Researchers have demonstrated that the inclusion of cholesterol in conventional PC-based liposomes reduced interactions with proteins and extended circulation time [34]. First, we examined the effect of cholesterol on intracellular uptake and transfection efficiency. It was found that the cholesterol content in DOTMA cationic LNPs did not significantly affect the particle size, intracellular uptake and transfection efficiency except when the cholesterol content reached 73 mol% (Table 1, Figs. 1A and B). However, an increase of the cholesterol/DOTMA molar ratio would significantly contribute to the liposome zeta potential from highly positive to neutral (Table 1) because of the reduction in the amount of cationic lipid DOTMA in the LNP formulation. However, the cholesterol containing DOTMA LNPs did not show good transfection efficiency compared with Lipo 2000.
Next, we tested different PCs in cholesterol containing DOTMA LNPs. There was no good correlation between cellular uptake or transfection efficiency and the saturation level or chain length of the PCs. However, we found that the choice of the PC component did affect the transfection efficacy of DOTMA based cationic LNPs. Among all the PCs studied, DOTMA-EggPC LNPs was the best and showed comparable transfection efficacy with positive control Lipo 2000, suggesting that EggPC enhanced the delivery efficiency of DOTMA LNPs (Figs. 3 and 5). Interestingly, the transfection efficiency of cholesterol containing DOTMA LNPs was significantly enhanced by replacing EggPC with unsaturated fatty acid. This was shown by greater luciferase gene silencing mediated by DOTMA-OA-siRNA LNPs. This was further confirmed by the drastic increase of miR-122 and inhibition of its down-stream target Bcl-w (Figs. 5A and B). We then investigated hepatic delivery of miR-122 by DOTMA-OA LNPs. The result demonstrated that miR-122 could be successfully delivered to liver by DOTMA-OA LNPs. Compared to in vivo positive control Invivofectamine, DOTMA-OA LNPs mediated miR-122 delivery showed a similar level of miR-122 delivery and down-stream target Bcl-w reduction (Figs. 8A and B). When the molar ratio of DOTMA, OA, cholesterol and Chol-PEG was set at 45:18:35:2, the siRNA-LNPs yielded a small positive charge which is close to neutral (Table 1) and were protected by Chol-PEG. According to the literature, the presence of PEG on the surface of LNPs has been shown to prolong half-life in blood circulation while reducing mononuclear phagocyte system uptake [38]. This may help overcome in vivo barriers and increase the accumulation in hepatocytes. As shown in Fig. S1A, a higher fluorescence signal in liver was observed in DOTMA-EggPC with PEG LNPs, and the fluorescence signal in spleen and kidney remained similar. This suggests that the DOTMA-EggPC LNPs with Chol-PEG worked better than DOTMA-EggPC LNPs without Chol-PEG. Overall, our findings suggest the importance of the helper lipids for the LNP mediated delivery of siRNA and miRNA.
Liver is an ideal tissue target for siRNAs or miRNAs based nanocarriers. The nano-sized particles may be accessible to hepatocytes by passive targeting due to the sinusoidal characteristics of the liver blood vessels. Upon reaching the liver, the nanoparticles can exit the intravascular space to directly access hepatocytes as long as the particle size is smaller than the pore size of fenestrated vasculature (100~150 nm in diameter) [39,40]. DOTMA-OA LNPs exhibited a relatively uniform particle size of 37.5 nm, which was significantly smaller than that of DOTMA-EggPC, DOTMA-LA, LNA and Invivofectamine (~90 nm; ~3.59 mV) particles. Compared to Invivofectamine, more DOTMA-OA LNPs (~37.5 nm; ~2.01 mV) accumulated in kidney, which may be due to the fenestrated endothelium (approximately 60 nm) in kidney. Yue et al [41]. also demonstrated that smaller micelles (35 nm) tended to accumulate in kidney at about 2 h post-injection. However, this slight accumulation did not cause any systemic toxicity or change in kidney functions (Fig. 7). In addition to the effect of particle size, surface charge also plays an important role in the uptake of nanoparticles by hepatocytes. ApoE, which is highly expressed in liver, is found to be responsible for the distribution of small neutral liposomes almost entirely to hepatocytes in the liver [42]. To confirm the importance of surface charge for biodistribution, IVIS study showed that DOTMA-OA-LNPs (~2.21 mV in pH=7.4 PBS) (Fig 4C) did not have any accumulation in Lung. In contrast, DOTMA-LA (~5.59 mV in pH=7.4 PBS) and LNA (~8.85 mV in pH=7.4 PBS) (Fig 4C) LNPs showed slight accumulation in lung (Fig. S1B). These results were consistent with our previous findings that highly positive charged DOTMA LNPs would facilitate the preferential accumulation in lung [26] not liver, which indicates that the surface charge is an important factor in tissue distribution.
In our study, the DOTMA-OA LNP formulation showed great advantages for siRNA/miRNA delivery both in vitro and in vivo. Although the role of OA is still not fully understood, our recent study showed that the inclusion of polyunsaturated fatty acids in LNPs could mediate pH-triggered ionization and to shield the positive charge of LNPs (Yu et al, unpublished data). The DOTMA-OA LNPs provide a means for delivering pathogenic protein-specific siRNAs/miRNA to hepatocytes. The efficacy of siRNA/miRNA delivery to liver or tumor tissue may be further increased by introducing liver tumor-targeting to the surface of LNPs. Receptors for transferrin [43], EpCAM [44] and galactose [45], are frequently over-expressed by carcinomas of liver, lung etc. Therefore, they may be included in LNPs to provide tumor the specific targeting function. Such approaches may reduce the nonspecific tissue cytotoxicity of RNA-based therapeutics.
5. Conclusions
Our study identified the importance of “helper lipid” in lipid nanoparticle formulations for the hepatic delivery of siRNA and miRNA both in vitro and in vivo. Incorporation of unsaturated fatty acids OA into DOTMA based LNPs showed potent delivery efficacy. The enhanced pH-sensitivity, smaller particle size and surface charge close to neutral could be the main reasons for the observed high delivery efficacy.
Supplementary Material
Acknowledgments
This work was supported by NSF Nanoscale Science and Engineering Center (NSEC) grant EEC-0914790 and NIH grants R01DK088076 and R21CA152969.
Footnotes
Disclosure of potential conflicts of interest
The authors declare no potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabernero J, Shapiro GI, Lorusso PM, Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR, Alsina M, Gounder MM, Falzone R, Harrop J, White AC, Toudjarska I, Bumcrot D, Meyers RE, Hinkle G, Svrzikapa N, Hutabarat RM, Clausen VA, Cehelsky J, Nochur SV, Gamba-Vitalo C, Vaishnaw AK, Sah DW, Gollob JA, Burris HA., 3rd First-in-Humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 3.Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, Ambegia E, McClintock K, MacLachlan I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119:661–673. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krugman S. The Gordon Wilson Lecture. The ABC’s of viral hepatitis. Trans Am Clin Climatol Assoc. 1992;103:145–156. [PMC free article] [PubMed] [Google Scholar]
- 5.Rahimi RS, Elliott AC, Rockey DC. Altered Mental Status in Cirrhosis: Etiologies and Outcomes. J Investig Med. 2013;61:695–700. doi: 10.2310/JIM.0b013e318289e254. [DOI] [PubMed] [Google Scholar]
- 6.Leake I. Liver: Size matters: effects of reduced paracetamol pack sizes in England and Wales. Nat Rev Gastroenterol Hepatol. 2013;10:197. doi: 10.1038/nrgastro.2013.33. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, Ghoshal K. TGFbeta-mediated upregulation of hepatic miR-181b promoteshepatocarcinogenesis by targeting TIMP3. Oncogene. 2010;29:1787–1797. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warmann SW, Frank H, Heitmann H, Ruck P, Herberts T, Seitz G, Fuchs J. Bcl-2 gene silencing in pediatric epithelial liver tumors. J Surg Res. 2008;144:43–48. doi: 10.1016/j.jss.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, Hsu MT, Wu JC, Huang HD, Shiao MS, Hsiao M, Tsou AP. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, Mao H, Wei M, Clark KR, Mendell JR, Caligiuri MA, Jacob ST, Mendell JT, Ghoshal K. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin X, Li L, Wang R, Wilcox D, Zhao X, Song J. A robust in vivo positive-readout system for monitoring siRNA delivery to xenograft tumors. RNA. 2011;17:603–612. doi: 10.1261/rna.2546011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adami RC, Seth S, Harvie P, Johns R, Fam R, Fosnaugh K, Zhu T, Farber K, McCutcheon M, Goodman TT, Liu Y, Chen Y, Kwang E, Templin MV, Severson G, Brown T, Vaish N, Chen F, Charmley P, Polisky B, Jr, Houston ME. An Amino Acid-based Amphoteric Liposomal Delivery System for Systemic Administration of siRNA. Mol Ther. 2011;19:1141–1151. doi: 10.1038/mt.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Jayaprakash KN, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhdanova RI, Podobeda OV, Vlassov VV. Cationic lipid–DNA complexes-lipoplexes-for gene transfer and therapy. Bioelectrochemistry. 2002;58:53–64. doi: 10.1016/s1567-5394(02)00132-9. [DOI] [PubMed] [Google Scholar]
- 18.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Yu B, Wu Y, Lee RJ, Lee LJ. Efficient down-regulation of CDK4 by novel lipid nanoparticle-mediated siRNA delivery. Anticancer research. 2011;31:1619–1626. [PubMed] [Google Scholar]
- 20.Koltover I, Salditt T, Rädler JO, Safinya CR. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 21.Balazs DA, Godbey W. Liposomes for use in gene delivery. J Drug Deliv. 2011:326497. doi: 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 269(199):2550–2561. [PubMed] [Google Scholar]
- 23.Mével M, Kamaly N, Carmona S, Oliver MH, Jorgensen MR, Crowther C, Salazar FH, Marion PL, Fujino M, Natori Y, Thanou M, Arbuthnot P, Yaouanc JJ, Jaffrès PA, Miller AD. DODAG; versatile new cationic lipid that mediates efficient delivery of pDNA and siRNA. J Control Release. 2010;143:222–232. doi: 10.1016/j.jconrel.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Yu B, Hsu SH, Zhou C, Wang X, Terp MC, Wu Y, Teng L, Mao Y, Wang F, Xue W, Jacob ST, Ghoshal K, Lee RJ, Lee LJ. Lipid nanoparticles for hepatic delivery of small interfering RNA. Biomaterials. 2012;33:5924–5934. doi: 10.1016/j.biomaterials.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Crawford M, Yu B, Mao Y, Nana-Sinkam SP, Lee LJ. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol Pharm. 2011;8:1381–1389. doi: 10.1021/mp2002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren T, Song YK, Zhang G, Liu D. Structural basis of DOTMA for its high intravenous transfection activity in mouse. Gene Ther. 2000;7:764–768. doi: 10.1038/sj.gt.3301153. [DOI] [PubMed] [Google Scholar]
- 28.Maitani Y, Soeda H, Junping W, Takayama K. Modified ethanol injection method for liposomes containing β-sitosterol β-D-glucoside. J Liposome Res. 2001;11:115–125. doi: 10.1081/LPR-100103174. [DOI] [PubMed] [Google Scholar]
- 29.Zhou C, Mao Y, Sugimoto Y, Zhang Y, Kanthamneni N, Yu B, Brueggemeier RW, Lee LJ, Lee RJ. SPANosomes as delivery vehicles for small interfering RNA (siRNA) Mol Pharm. 2012;9:201–210. doi: 10.1021/mp200426h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbeke G, Molenberghs G. Statistics. New York: Springer; 2000. Linear mixed models for longitudinal data; pp. 23–26. [Google Scholar]
- 31.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 32.Demel RA, De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976;457:109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- 33.Agirre A, Nir S, Nieva JL, Dijkstra J. Induction of aggregation and fusion of cholesterol-containing membrane vesicles by an anti-cholesterol monoclonal antibody. J Lipid Res. 2000;41:621–628. [PubMed] [Google Scholar]
- 34.Maruyama K, Yuda T, Okamoto A, Kojima S, Suginaka A, Iwatsuru M. Prolonged circulation time in vivo of large unilamellar liposomes composed of distearoyl phosphatidylcholine and cholesterol containing amphipathic poly(ethylene glycol) Biochim Biophys Acta. 1992;1128:44–49. doi: 10.1016/0005-2760(92)90255-t. [DOI] [PubMed] [Google Scholar]
- 35.Zelphati O, Uyechi LS, Barron LG, Szoka FC., Jr Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim Biophys Acta. 1998;1390:119–133. doi: 10.1016/s0005-2760(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 36.Lin CJ, Gong HY, Tseng HC, Wang WL, Wu JL. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2008;375:315–320. doi: 10.1016/j.bbrc.2008.07.154. [DOI] [PubMed] [Google Scholar]
- 37.Hsu SH, Yu B, Wang X, Lu Y, Schmidt CR, Lee RJ, Lee LJ, Jacob ST, Ghoshal K. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine. 2013;13:S1549–9634. doi: 10.1016/j.nano.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 39.Li LJ, Wang HY, Ong ZY, Xu KJ, Ee PLR, Zheng SS, Hedrick JL, Yang YY. Polymer- and lipid-based nanoparticle therapeutics for the treatment of liver diseases. Nano Today. 2010;5:296–312. [Google Scholar]
- 40.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue J, Liu S, Xie Z, Xing Y, Jing X. Size-dependent biodistribution and antitumor efficacy of polymer micelle drug delivery systems. J Mater Chem B. 2013;1:4273–4280. doi: 10.1039/c3tb20296h. [DOI] [PubMed] [Google Scholar]
- 42.Spanjer HH, van Galen M, Roerdink FH, Regts J, Scherphof GL. Intrahepatic distribution of small unilamellar liposomes as a function of liposomal lipid composition. Biochim Biophys Acta. 1986;863:224–230. doi: 10.1016/0005-2736(86)90262-2. [DOI] [PubMed] [Google Scholar]
- 43.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonoke S, Ueda T, Fujiwara K, Kuwabara K, Yano J. Galactose-modified cationic liposomes as a liver-targeting delivery system for small interfering RNA. Biol Pharm Bull. 2011;34:1338–1342. doi: 10.1248/bpb.34.1338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.