Abstract
Objectives/Hypotheses
The Superior Laryngeal Nerve (SLN) is the major sensory nerve for the upper larynx. Damage to this nerve impacts on successful swallowing. The first aim of the study was to assess the effect of unilateral SLN lesion on the threshold volume sufficient to elicit swallowing in an intact pig model; this volume was defined radiographically as the maximum bolus area visible in lateral view. The second aim was to determine if a difference existed between ipsi-lateral and contra-lateral function as a result of unilateral sensory loss, measured as the radiologic density of fluid seen in the valleculae. Finally, we determined if there was a relationship between the threshold volume and the occurrence of aspiration after a unilateral SLN lesion.
Study Design
Repeated measures animal study.
Methods
Four female infant pigs underwent unilateral SLN lesion surgery. The maximum vallecular bolus area in lateral view and the relative vallecular density on each side in the dorsoventral view were obtained from videofluoroscopic recordings in both the pre-lesion control and post lesion experimental states.
Results
In lateral view, the lesioned group had a bigger maximum bolus area than the control group (p<0.001). Although occasional left-right asymmetry in the dorsoventral view was observed,, the vallecular densities were, on average, equal on both the left (intact) and right (lesioned) sides (p>0.05). A bigger maximum bolus area did not predict aspiration in the lesioned group (p>0.05).
Conclusion
Unilateral SLN lesions increased the swallowing threshold volume symmetrically in right and left valleculae but the increased threshold may not be the main mechanism for the occurrence of aspiration. The Effect of Unilateral SLN Lesion on Swallowing Threshold Volume
Keywords: superior laryngeal nerve, swallowing threshold, bolus area, aspiration, videofluoroscopy, deglutition/deglutition disorders, suck-swallow ratio, animal models, nerve lesion
INTRODUCTION
The internal branch of the superior laryngeal nerve (SLN) is a sensory nerve that supplies the hypopharyngeal and supraglottic areas. Sensory deficits in these areas correlate with the occurrence of swallowing dysfunction and the development of aspiration pneumonia in selected patient populations1. Using an animal model we have previously shown that a unilateral SLN lesion results in an increased incidence of aspiration (Ding et al - submitted), however, the mechanism for this aspiration is unclear. Studies of the threshold bolus volume, required to elicit aerodigestive reflexes, show that a normal swallowing threshold is an important factor in preventing aspiration2–4. The threshold liquid volume required to trigger the reflexive pharyngeal swallow in man is less than the maximum volume of liquid that can be contained within the hypopharynx without it spilling into the airway4. When the local reflexes are abolished with pharyngeal anesthesia3,4, this threshold volume can be exceeded and resulting in aspiration. However, whether a unilateral SLN lesion results in an increased threshold and whether a higher threshold volume in the valleculae is responsible for the occurrence of aspiration have not been investigated.
Various methods can be used to study the swallowing threshold. In both human and animal studies, the threshold volume of liquid required to trigger the pharyngeal swallow is a standard parameter used in many studies2–5. Where the fluid is delivered by a syringe or an infusion pump, the threshold volume is easily measured. However, for a solid bolus in human trials, the number of chewing cycles preceding the swallow has been widely used as an indirect measure of the swallowing threshold 6. Although it is not possible to measure bolus volume directly in a free-feeding subject, the bolus area of barium liquid in the valleculae is a potential parameter to express threshold volume. Bolus area is easily measured and steadily expands between swallows during feeding in the lateral view of the videofluoroscopic swallowing study (VFSS) in infant pigs 7. Infant pigs store milk in their valleculae over several transport/suck cycles prior to a swallow. The volume stored in the valleculae by the rhythmic tongue movements of suckling is considered the primary factor to initiate pharyngeal swallow8. In the present study, we used this videofluoroscopic value, at the onset of a swallow, as a measure of the volume threshold for swallowing liquid containing barium; this is referred to as the maximal bolus area. In dorsoventral view, the video uoroscopically determined relative densities of the left and right valleculae were used as an index of the equality of filling of the two sides.
The purpose of this investigation was to evaluate the association between a unilateral SLN lesion and the swallowing threshold as expressed by maximum bolus area. We asked if unilateral SLN lesion has the potential to change the swallowing threshold. If this lesion does modify the threshold of bolus volume in the valleculae, is bolus accumulation symmetric? That is, does a unilateral lesion produce changes only on one side, or is it a change that impacts on both sides. Finally, if there is an increase in bolus threshold in the valleculae, does this increase cause aspiration? To answer all these questions, we tested three hypotheses in this study. H1: after a unilateral SLN lesion, the maximum bolus area is larger than that of the control group. H2: the unilateral SLN lesion doesn’t affect the symmetrical bolus dispersal in the vallecula i.e. both left and right sides contain equal volume when measured by vallecular radiographic density. H3: a higher maximum bolus area correlates with a higher incidence of aspiration in the unilateral SLN lesioned group (Fig. 1).
Figure 1.
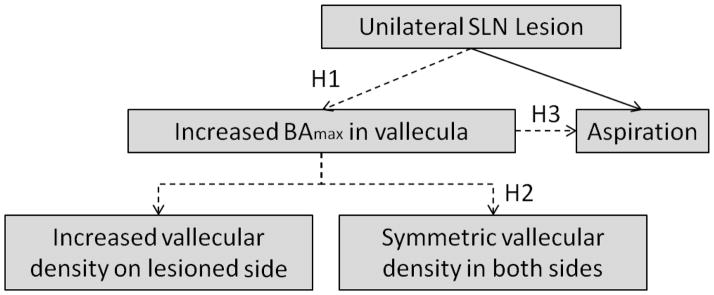
The schematics of the three hypotheses. BAmax: the maximum bolus area in valleculae.
MATERIALS AND METHODS
Four female pigs, 2–3 weeks old, were obtained from Tom Morris Farms (Reisterstown, MD). They weighed 4–5 kg at the time of arrival at the vivarium of the Johns Hopkins University. All pigs were fed on milk replacement formula (Land O’Lakes Solustart pig milk replacer, St. Paul, MN). Each pig was examined and determined to be healthy by a veterinarian prior to surgery. All procedures on living animals were approved by the Johns Hopkins IACUC (Protocol Number: SW10M212) and have been described in detail in a previous paper (Ding et al, pers comm.)
During the first two days at the facility, we trained the pigs to suckle milk from a bottle fitted with a commercial pig nipple (Nasco Inc., Fort Atkinson, WI). On day three, the first surgery was performed to mark the right side SLN and implant markers into the epiglottis and tongue. At this time recording bipolar EMG electrodes were placed in the hyoid musculature, but the results from those measurements will be addressed in a separate paper. After animals were entirely alert and awake, approximately 3–4 hours post-surgery, we collected control data using videofluoroscopy, as described below. A second surgery was done on day 4 to cut the marked SLN. After this procedure, data were collected on the lesioned animals. Fluoroscopy was performed using a Toshiba Infinix C-Arm imaging system (Infinix -I, Toshiba Corp., Tokyo, Japan). The video images were recorded at a rate of 30 frames per second and exported in AVI format for later analysis in ImageJ (National Institutes of Health, Bethesda, MD, USA). All subjects were fed with milk mixed with barium powder in a fixed ratio of 8 oz milk with 1/3 cup powdered barium (E-Z-HD, E-Z-EM, Inc., Westbury, NY, USA). During feeding, each pig was contained in a plexiglass feeding box, where it naturally held a steady position on the teat. We collected the VFSS imaging data separately in both dorsoventral and lateral projections, both before and after a unilateral SLN lesion.
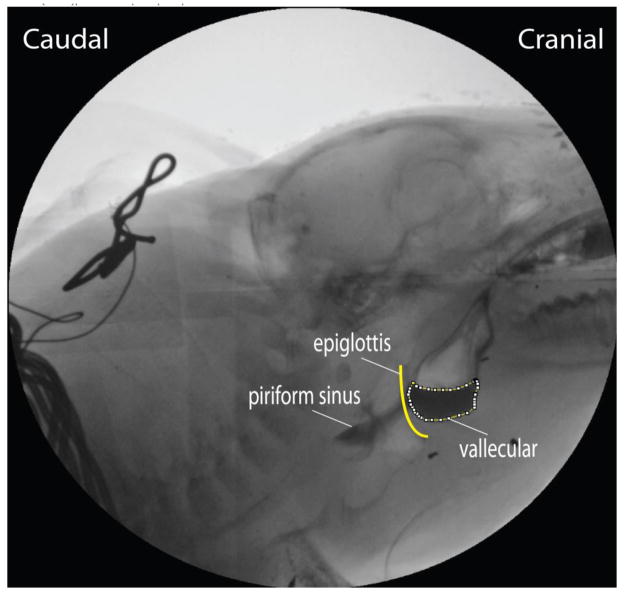
In this study, we validated the new video uoroscopic measure of swallowing threshold referred to as the maximum bolus area. This was defined as the maximum area of the liquid containing barium in the valleculae in the lateral view of the VFSS, measured in the video frame recorded immediately before the epiglottis flipped during the swallow 8. A large maximum bolus area would suggest an increased swallowing threshold volume which may predict the likelihood of aspiration. To establish the utility of the maximum bolus area as a parameter representing the threshold volume, we randomly sampled 30 swallows from the control feedings and 30 from the lesioned feedings. These were matched by individual.
There were 1–5 suck cycles prior to a swallow to transport milk from the nipple into valleculae. At the end of each suck cycle, we measured the vallecular bolus area by the “Polygon Selections” tool in ImageJ. This tool enables the user to outline an area on an image. The program then calculates the area inside the outline. The end of a suck cycle was defined as one video frame before the jaw started to close. Then we performed linear regression analysis using SYSTAT 13 (Systat Software, Inc., Chicago, IL, USA). If the bolus area in the valleculae has a positive relationship with the accumulation of bolus volume by each suck, we can say that the bolus area increases with and reflects the increase of bolus volume.
All videos selected for movement analysis were chosen on the basis of minimum rotation or yaw of the head as determined by the overlap of the images of the teeth from the two sides of the head. To examine hypothesis 1 and 3, we selected 86 swallows from the control feedings (24, 22, 27, 13 swallows in each subject) and 89 swallows from the lesioned feedings (25, 13, 20, 31 swallows in each subject) from the four animals, recorded in the lateral view. The reduction in numbers from the initial 30 randomly chosen swallows reflect swallows with head rotation. In these cases we used the “Polygon Selections” tool to digitize the maximum bolus area, the area of the bolus contained in the valleculae, one frame before the epiglottis moved posteriorly/inferiorly (the epiglottal “flip”) (Fig 2). We used this measure as a two-dimensional approximation of the volume of the barium milk contained in the valleculae. The level of radiological image magnification was established from the known nipple diameter and its uncompressed diameter from videos when suckling was not occurring. Aspiration was identified following frame by frame examination of each swallow. In this paper any barium milk passing the vocal folds into the trachea was considered aspiration (IMPAS score 7) (Holman et al - submitted).
Figure 2.
The dotted line showed the area of the bolus in the valleculae as measured in the lateral VFSS image.
In dorsoventral view, with the delivery of milk containing barium from oral cavity to the valleculae, the optical density of the accumulated liquid in the valleculae changed more obviously than the area. Therefore, for the dorsoventral data, we used the vallecular density on each side to express the relative amounts of barium milk contained in each vallecula. To test hypothesis 2, we included 58 swallows from the three animal control group (no dorsoventral control data was available for animal C) i.e. respectively 11, 20 and 27 swallows in the three subjects and 90 swallows from the lesioned group of 4 animals i.e. respectively 21, 17, 25 and 27 swallows from the subjects. These were examined in the dorsoventral plane and the vallecular density of each side was measured one frame before the epiglottis inversion in the swallow. Within each polygon outlining the valleculae, We used the “Mean Gray Value” tool in ImageJ to calculate the density of barium in that space. in We used the same-sized area of the vallecula of each side (Fig 3). The Mean Gray Value was the average gray value within the selection. This was the sum of the gray values of all the pixels in the selection divided by the number of pixels. Because we were interested in comparing left to right, variation from frame to frame or session to session in density was not a problem.
Figure 3.
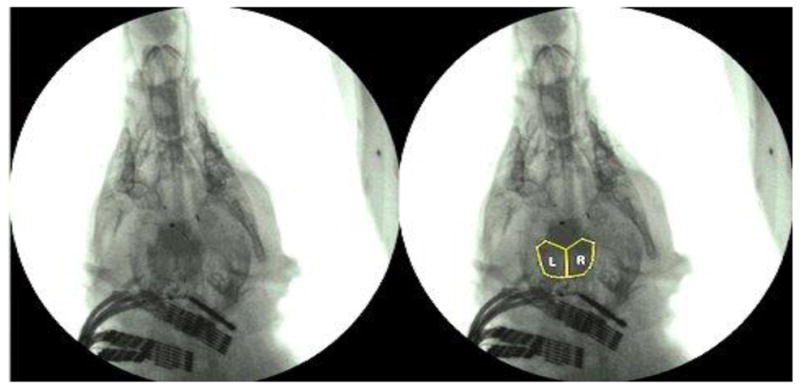
The yellow line in the right picture showed the areas in which the vallecular density on each side was measured in the dorsoventral VFSS image. The left picture is the original image.
For hypothesis 1, we used the linear mixed-model (with individual included as a random factor) to test whether the maximum bolus area increased after a unilateral SLN lesion. To test hypothesis 2, concerning vallecular density differences between the two sides, we used the ANOVA repeated measures to compare the radiographic vallecular density of the left and right sides in dorsoventral image data. Finally we used binary logistic regression to analyze the relationship between the maximum bolus area and aspiration incidence with aspiration as a dependent variable, separately in the control group and in the lesioned group9. For all of these analyses, the unit of analysis was a swallow.
RESULTS
1. Validation for using the maximum bolus area
There was a total of 75 sucks proceeding the 30 swallow cycles in the control group and 89 sucks proceeding the 30 swallow cycles in the lesioned group. Of the 60 swallows, the bolus areas in 59 increased monotonically over the sucks proceeding the swallow. The one that decreased in volume did so by only 2%. In this case, aspiration did not occur but more liquid was transported into the piriform sinuses. Bolus area and the number of sucks per unit time were positively correlated in both the control and lesioned groups.
2. Results for hypothesis 1
After correction for image magnification, the mean maximum bolus area was 1.76 cm2 (SD=0.65) in the control group and 2.43 cm2 (SD=0.75) in the lesioned group. The maximum bolus area was significantly larger in the lesioned group than in the control group (p< 0.001). There was variation among the individuals but the area in the lesioned state was larger (Fig 4).
Figure 4.
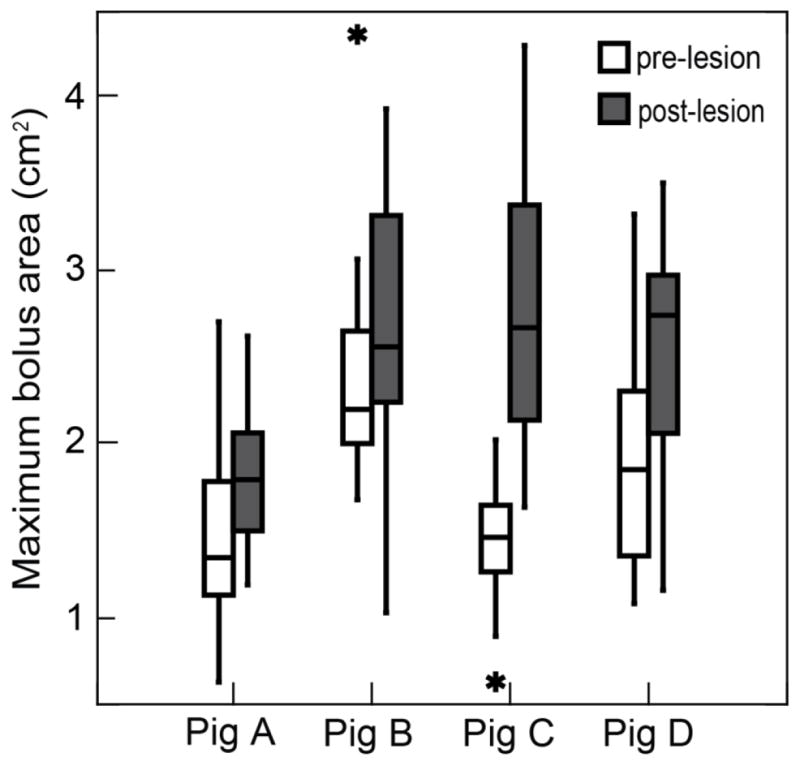
The difference in maximum bolus area between the control and the unilateral SLN lesioned groups. Data from the lateral VFSS images in 4 subjects (p< 0.001, cases=175, linear mixed-model).
3. Results for hypothesis 2
However, in the lesioned group viewed dorsoventrally, the differences in left to right vallecular densities were not significant (p=0.224) (Fig 5), except for Pig C (p=0.002) where the right side was denser. Similarly, in the control group, the vallecular densities were also not significantly different between the two sides (p=0.171).
Figure 5.
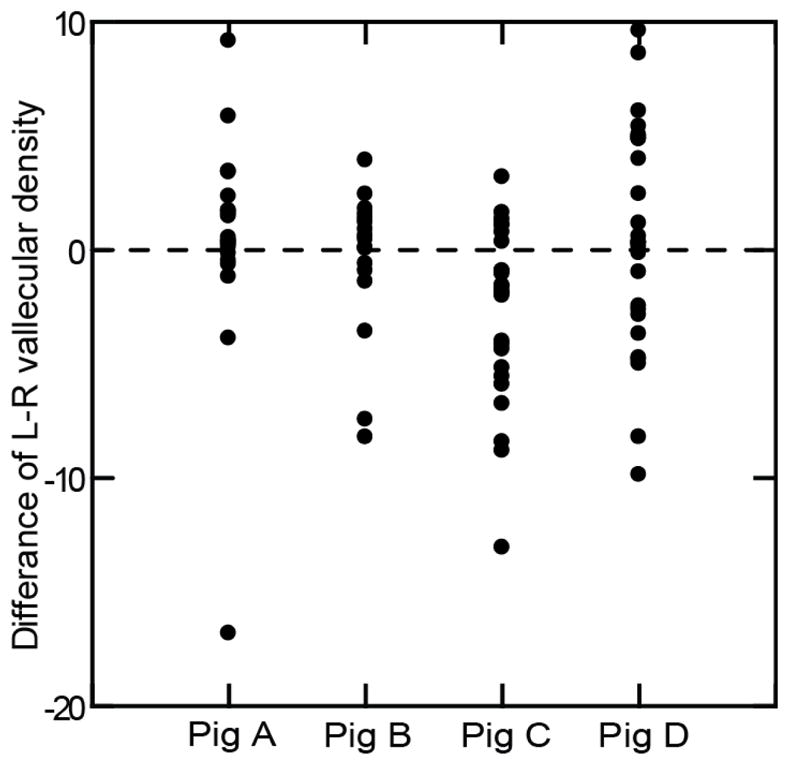
The difference of vallecular density between left and right side in the dorsoventral VFSS images of the unilateral SLN lesioned group among four subjects (p=0.224, cases=90, ANOVA repeated measures).
4. Results for hypothesis 3
Among the 86 swallows in the control group, viewed laterally, three involved aspiration i.e. an incidence of 3.5%. The difference in the maximum bolus area between the cases with aspiration and those without aspiration was not significant in the control group (p= 0.619) (Fig 6). In contrast, the incidence of aspiration in the lesioned group was 67.4% (60/89). There was no significant difference in the maximum bolus area between cases with aspiration and without aspiration in the lesioned group (p=0.602) (Fig 6). All values for maximum bolus area in the control and the lesioned groups are shown in Table 1.
Figure 6.
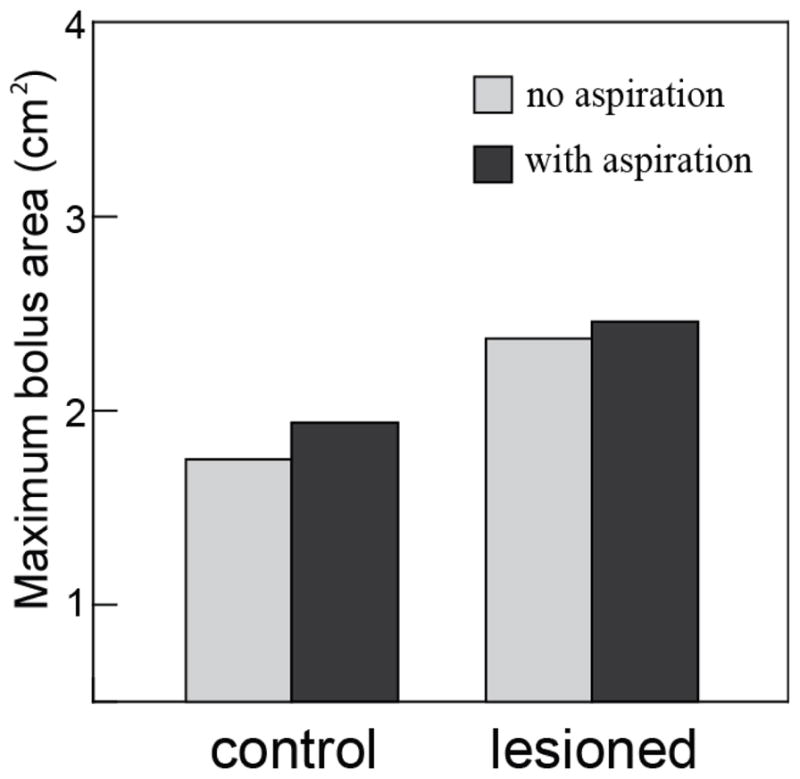
The comparison of mean maximum bolus area between non-aspirating and aspirating cases in the control group (p= 0.619, cases=86, binary logistic regression test) and the unilateral SLN lesioned group (p=0.602, cases=89, binary logistic regression test). Data from lateral VFSS image in four subjects.
Table 1.
The values of the maximum bolus area in the control and lesioned groups.
| Swallow number | * BAmax (cm2) | SD (cm2) | ||
|---|---|---|---|---|
| SLN | intact-control | 86 | 1.76 | 0.65 |
| lesioned | 89 | 2.43 | 0.75 | |
| Control group | no aspiration | 83 | 1.75 | 0.66 |
| with aspiration | 3 | 1.94 | 0.79 | |
| Lesioned group | no aspiration | 29 | 2.37 | 0.73 |
| with aspiration | 60 | 2.46 | 0.77 |
BAmax: the maximum bolus area in valleculae
DISCUSSION
Swallowing is a reflex that is critical for the protection of the respiratory tract9–11. Many pathological lesions can result in swallowing disorders by depressing the areodigestive reflexes so that there is a higher swallow threshold volume12–15. We have previously reported that, using a pig model, unilateral SLN lesions result in a higher risk of aspiration (Ding et al, Holman et al - submitted). In the present study we further demonstrate that there is an increased maximum bolus area after a unilateral SLN lesion. This implied that additional sensory input, arising from a larger accumulation of fluid, was required to reach the reflex threshold before a swallow could be elicited. This finding also correlated with the anatomy of sensory innervation by the SLN. The highest density of sensory receptors is located in the posterior tonsillar pillars, the laryngeal surface of the epiglottis and the postcricoid and arytenoid regions 16. These areas of the SLN innervations are the same areas that are known to be the most sensitive for triggering reflexive swallowing or glottal protection 16. Hence, even a unilateral SLN lesion could result in an increased threshold volume for swallowing.
As hypothesized, there was no asymmetry when vallecular density was compared between two sides in the dorsoventral plane after a unilateral SLN lesion. Both ipsilateral and contralateral valleculae held equal amounts of milk. A possible explanation is that the pharyngeal phase is relatively isolated from the oral phase which is responsible for the transport of milk into the vallecula; this, is consistent with Lang’s view that the oral, pharyngeal, and esophageal phases of swallowing can be independent of each other 17. Consequently, the lack of sensory feedback from one side of the hypopharyngeal and supraglottic areas would not affect the milk delivery into the vallecula; in the present experimental situation, milk delivery to the valleculae was a function of the central pattern for sucking, preceding any pharyngeal swallow. In humans, unilateral vallecular pooling is seldom seen at all. This could be that, unlike the pyriform sinuses, a liquid bolus is unlikely to occupy one side of the vallecula even in the presence of a significant unilateral motor or sensory impairment. The median glossoepiglottic fold separates the valleculae into right and left sides. It may provide an insufficient barrier to contain a bolus of liquid in one “side” of the vallecula or the other. In infant pig anatomy, however, the median glossoepiglottic fold separates the valleculae into two sides based on our observation by the direct laryngoscope and the vallecular impression made on the fresh pig cadavers.
In the lesioned group, a higher maximum bolus area did not predict the occurrence of aspiration. We found no relationship between bolus volume and incidence of aspiration although a unilateral SLN lesion did, however, increase the maximum bolus area. While the volume of the bolus may be a factor in aspiration, it is clearly not the major one and the SLN plays a critical role on the airway protection by eliciting the laryngeal adductor reflex. The main reason for aspiration could be related to incomplete closure of the larynx during the pharyngeal phase of swallowing which has been reported after bilateral SLN lesions 18. In a diverse cohort of patients with dysphagia, absence of the laryngeal adductor reflex was significantly associated with laryngeal penetration and aspiration during swallowing 19. Hence, even one side sensory loss of the SLN could compromise the protective function. In the control group, cases of small amounts of aspiration may contribute to the non-significant statistic result. Hence we can’t exclude the possibility that a higher bolus volume could be a risk for aspiration in normal swallows.
In this study, a positive relationship was found between the number of sucks and the results, radiographically defined, area of the bolus in the valleculae (seen in the lateral view VFSS). This demonstrated firstly that the maximum bolus area was an appropriate surrogate for the volume of liquid in the valleculae and secondly that the area recorded immediately prior to epiglottal flexion was an expression of the threshold volume required to elicit the swallow. In conscious active infants, it is rarely feasible to test the liquid threshold volume for the pharyngeal swallow by direct liquid infusion into the pharynx. Therefore the method of this study may be of particular benefit in the study of dysphagic infants.
CONCLUSION
Our study demonstrated that the unilateral SLN lesion increases the threshold volume of liquid for swallowing using a new videofluoroscopic parameter for vallecular volume, the maximum bolus area. The unilateral sensory loss following SLN lesion does not affect the symmetry of bolus delivery into the valleculae which is a function of the central pattern generator for sucking. However, an increased threshold volume is not the main factor contributing to aspiration in the unilateral SLN lesioned group.
Acknowledgments
We appreciate Laurie Pipitone for her support in the radiologic data collection. We also thank Takako Fukuhara and Wallace Feng for their assistance in data collection. This study was funded by the National Institutes of Health, DC9980 to RZG.
Footnotes
The work was done in the Johns Hopkins University School of Medicine.
Conflict of Interest: None.
Level of Evidence: N/A
Financial disclosure: This study was funded by NIH DC9980 to RZG.
BIBLIOGRAPHY
- 1.Sulica L. The superior laryngeal nerve: function and dysfunction. Otolaryngol Clin North Am. 2004;37:183–201. doi: 10.1016/S0030-6665(03)00175-0. [DOI] [PubMed] [Google Scholar]
- 2.Shaker R, Ren J, Zamir Z, Sarna A, Liu J, Sui Z. Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology. 1994;107:396–402. doi: 10.1016/0016-5085(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 3.Shaker R, Medda BK, Ren J, Jaradeh S, Xie P, Lang IM. Pharyngoglottal closure reflex: identification and characterization in a feline model. Am J Physiol. 1998;275:G521–525. doi: 10.1152/ajpgi.1998.275.3.G521. [DOI] [PubMed] [Google Scholar]
- 4.Dua K, Surapaneni SN, Kuribayashi S, Hafeezullah M, Shaker R. Pharyngeal airway protective reflexes are triggered before the maximum volume of fluid that the hypopharynx can safely hold is exceeded. Am J Physiol Gastrointest Liver Physiol. 2011;301:G197–202. doi: 10.1152/ajpgi.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumbley F, Huckabee ML, Doeltgen SH, Witte U, Moran C. Effects of bolus volume on pharyngeal contact pressure during normal swallowing. Dysphagia. 2008;23:280–285. doi: 10.1007/s00455-007-9137-9. [DOI] [PubMed] [Google Scholar]
- 6.Fontijn-Tekamp FA, van der Bilt A, Abbink JH, Bosman F. Swallowing threshold and masticatory performance in dentate adults. Physiol Behav. 2004;83:431–436. doi: 10.1016/j.physbeh.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool. 1998;280:327–343. doi: 10.1002/(sici)1097-010x(19980401)280:5<327::aid-jez2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and Rate of Milk Delivery as Determinants of Swallowing in an Infant Model Animal (Sus scrofia) Dysphagia. 2004;19:147–154. doi: 10.1007/s00455-004-0001-x. [DOI] [PubMed] [Google Scholar]
- 9.Selvin S. Statistical Tools for Epidemiologic Research. USA: Oxford University Press; 2011. [Google Scholar]
- 10.Nishino T. Swallowing as a protective reflex for the upper respiratory tract. Anesthesiology. 1993;79:588–601. doi: 10.1097/00000542-199309000-00024. [DOI] [PubMed] [Google Scholar]
- 11.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102:1017–1025. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aviv JE, Sacco RL, Mohr JP, et al. Laryngopharyngeal sensory testing with modified barium swallow as predictors of aspiration pneumonia after stroke. Laryngoscope. 1997;107:1254–1260. doi: 10.1097/00005537-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Ku PK, Vlantis AC, Leung SF, et al. Laryngopharyngeal sensory deficits and impaired pharyngeal motor function predict aspiration in patients irradiated for nasopharyngeal carcinoma. Laryngoscope. 2010;120:223–228. doi: 10.1002/lary.20701. [DOI] [PubMed] [Google Scholar]
- 14.Shaker R, Ren J, Bardan E, et al. Pharyngoglottal closure reflex: characterization in healthy young, elderly and dysphagic patients with predeglutitive aspiration. Gerontology. 2003;49:12–20. doi: 10.1159/000066504. [DOI] [PubMed] [Google Scholar]
- 15.Dua KS, Surapaneni SN, Santharam R, Knuff D, Hofmann C, Shaker R. Effect of systemic alcohol and nicotine on airway protective reflexes. Am J Gastroenterol. 2009;104:2431–2438. doi: 10.1038/ajg.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu L, Sanders I. Sensory nerve supply of the human oro- and laryngopharynx: a preliminary study. Anat Rec. 2000;258:406–420. doi: 10.1002/(SICI)1097-0185(20000401)258:4<406::AID-AR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Lang IM. Brain stem control of the phases of swallowing. Dysphagia. 2009;24:333–348. doi: 10.1007/s00455-009-9211-6. [DOI] [PubMed] [Google Scholar]
- 18.Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550:287–304. doi: 10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112:338–341. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]