Abstract
Objective
To assess trends and outcomes of assisted hatching among assisted reproductive technology (ART) cycles.
Design
Retrospective cohort analysis using National ART Surveillance System (NASS) data.
Setting
U.S. fertility centers reporting to NASS.
Patient(s)
Fresh autologous noncanceled ART cycles conducted from 2000–2010.
Intervention(s)
None.
Main Outcome Measure(s)
Implantation, clinical pregnancy, live-birth, miscarriage, multiple gestation.
Result(s)
Assisted hatching use statistically significantly increased in absolute number (from 25,724 to 35,518 cycles), percentages of day-3 (from 50.7% to 56.3%) and day-5 transfers (from 15.9% to 22.8%), and percentage of transfers among women ≥38 years (from 17.8% to 21.8%) or women with ≥2 prior ART cycles and no live birth(s) (from 4.3% to 7.4%). Both day-3 and day-5 cycles involving assisted hatching were associated with lower odds of implantation (adjusted odds ratios [aOR] 0.7 and 0.6, respectively), clinical pregnancy (aOR 0.8 and 0.7, respectively), live birth (aOR 0.8 and 0.7, respectively), and increased odds of miscarriage (aOR 1.4 and 1.4, respectively), as compared with cycles without assisted hatching. Assisted hatching was associated with lower odds of multiple gestation in day-5 cycles (aOR 0.8). In cycles for women with a “poor prognosis,” the association of assisted hatching with pregnancy outcomes was not statistically significant.
Conclusion(s)
Assisted hatching use had an increasing trend but was not associated with improved pregnancy outcomes, even in poor-prognosis patients. Prospective studies are needed to identify the patients who may benefit from assisted hatching.
Keywords: Assisted hatching, assisted reproductive technology (ART), in vitro fertilization (IVF), live birth rate, pregnancy outcome
Since its inception in the late 1970s, the field of assisted reproductive technology (ART) has grown exponentially. Over the past 35 years, technological advances in ART, including advances in protocols for ovarian stimulation, oocyte retrieval, fertilization, and embryo culture and transfer, have resulted in more efficient, though still imperfect, approaches for treating infertility. Ideally, adoption of new technology should be preceded by a proven favorable risk-benefit ratio, but the rate of scientific progress and adoption of new techniques often supersedes the field’s ability to validate their safety and efficacy.
Assisted hatching, the purposeful disruption of an embryo’s zona pellucida by laser, mechanical, or chemical means, is often performed in an effort to improve implantation rates among patients with a poor prognosis or on embryos noted to have a thick zona pellucida (1–3). The definition of poor prognosis varies from one clinic to another, which makes comparison of existing studies challenging, but the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) suggest that assisted hatching may be clinically useful among women who have failed at least two ART cycles, are 38 years of age or older, or have poor-quality embryos (2). A recent Cochrane review that included 31 randomized controlled trials found marginal statistical significance in the clinical pregnancy rate among women for whom assisted hatching was used compared with controls (odds ratio [OR] 1.13, 95% confidence interval [CI] 1.01–1.27), although a wide variation in the results among the trials was noted (1). The same review found no statistically significant differences in the odds of live birth (9 randomized controlled trials) or miscarriage (14 randomized controlled trials), but identified a statistically significant increase in the multiple birth rate (14 randomized controlled trials) among cycles using assisted hatching (1). The subgroup analyses of poor-prognosis patients—defined by increased age, prior ART failure, high follicle-stimulating hormone (FSH) concentration, use of frozen embryos, or use of a “poor prognosis protocol”—showed similar results (1). The existing evidence is insufficient to justify the universal use of assisted hatching. There is also limited evidence of the effect of assisted hatching on outcomes other than clinical pregnancy—namely, miscarriage and live birth—among poor-prognosis patients. Furthermore, assisted hatching is not without risk; the procedure may increase the risk of monozygotic twinning (1, 4–8).
Our study quantified the assisted hatching trends in the United States from 2000 to 2010 using data from the Centers for Disease Control and Prevention (CDC) National ART Surveillance System (NASS). We evaluated the association between use of assisted hatching and cycle outcomes, including implantation, clinical pregnancy, live birth, miscarriage, and multiple gestation rates, among fresh autologous in vitro fertilization (IVF) cycles.
MATERIALS AND METHODS
Data used in this study were obtained from the NASS, a federally mandated reporting system that collects information about ART cycles involving the laboratory handling of gametes performed in the United States (Fertility Clinic Success Rate and Certification Act of 1992 [FCSRCA], Public Law No. 102–493, October 24, 1992) (9). The NASS data include patient demographics, medical and obstetric history, infertility diagnoses, detailed parameters of each ART treatment cycle, and, if applicable, the resultant pregnancy outcome. Although 6% to 12% of ART clinics did not report data to the CDC in any given year between 2000 and 2010, we estimate that NASS includes data from more than 95% of all ART cycles performed in the United States (10). Additionally, for each of the study years, approximately 7% to 10% of reporting clinics were randomly selected for full validation, where selected ART data reported by the clinics are compared with information recorded in medical records. Validated variables include (if applicable) patient date of birth, cycle intention, number of embryos transferred, cycle outcome, number of fetal hearts on ultrasound, pregnancy outcome, and patient diagnosis. Overall, the discrepancy rates for the variables evaluated in our study were less than 5%; however, the diagnosis of infertility had higher discrepancy rates (up to 18%), mostly due to the report of “other” or “unexplained” infertility in NASS instead of a specific cause recorded in the medical record (11).
An initial analysis to explore trends in use of assisted hatching included all fresh autologous noncanceled IVF cycles performed in the United States between 2000 and 2010 not involving a gestational carrier (n = 835,067). Clinicians indicated whether hatching by any method was performed when submitting cycle data. In the trend analysis, we report the absolute number and percentage of fresh autologous non-canceled cycles for which hatching was performed among the following subgroups: [1] cycles involving a day-3 transfer, [2] cycles involving a day-5 transfer, [3] cycles for which the patient was 38 years of age or older at time of retrieval, [4] cycles preceded by two or more failed ART cycles (characterized by ≥2 prior ART cycles and no prior history of live birth), [5] cycles meeting either of these latter two criteria (patient age ≥38 years, ≥2 prior ART cycles, and no prior history of live birth), and [6] “unindicated” cycles meeting neither of these two criteria (resulting in a subgroup in which the patient age was <38 years and the number of failed ART cycles was <2 or the patient had a history of live birth). We performed an analysis of trends for each of these groups by calculating linear regression over the years 2000 to 2010.
For all subsequent analyses, the cycles were limited to fresh autologous cycles from 2000 to 2010 for which a transfer was performed on either day 3 or day 5 (n = 751,879 cycles). We first examined differences in the distribution of the following patient and treatment characteristics among cycles with and without assisted hatching: maternal age, maternal race/ethnicity, infertility diagnosis, number of prior preterm and full term births, number of prior ART cycles, number of oocytes retrieved, use of intracytoplasmic sperm injection (ICSI), embryo stage at transfer, number of embryos transferred, number of extra embryos cryopreserved, number of fetal hearts at first trimester ultrasound, and number of live-born infants. The Pearson chi-square test was used to assess the statistical significance of differences.
We then performed analyses of outcomes, assessing associations with use of assisted hatching. Our outcomes of interest were implantation, clinical pregnancy, live birth, miscarriage, and multiple gestation. Implantation was calculated as the number of embryos resulting in implantation (defined as the larger of either the number of maximum fetal hearts by ultrasound or maximum infants born including live births and stillbirths) out of the total number of embryos transferred. Cycles were considered to result in pregnancy if clinical intrauterine gestation or heterotopic pregnancy was reported; cycles that had no indication of clinical pregnancy or were biochemical or ectopic pregnancies were considered to not result in clinical pregnancy. The NASS definition for a clinical intrauterine gestation is ultrasound confirmation of gestational sac(s) within the uterus, regardless of whether a heartbeat(s) is/ are observed or fetal pole(s) established. Without ultrasound data, confirmation is achieved through documented birth, spontaneous miscarriage, or induced abortion. Live birth was defined as a birth of one or more live infant(s) at a gestation age ≥20 weeks. A cycle was classified as a miscarriage if the patient was reported to have had a spontaneous miscarriage and the gestational age was <20 weeks. Multiple gestation was defined by >1 fetal heartbeats at first trimester ultrasound. If the fetal heartbeat value was missing, gestation was set to equal the number of infants born.
Cycles were stratified by day of transfer (day 3 and day 5) for bivariable and multivariable analyses of the associations between assisted hatching and the five outcomes of interest to account for possible effect modification; assisted hatching may have different associations with birth outcomes depending on the duration of embryo culture. Unadjusted odds ratios (OR), adjusted OR (aOR), and 95% confidence intervals (CI) were generated using mixed effects logistic regression models with the ART clinic as the random effect. Multivariable analyses were conducted to explore the relationship between the use of assisted hatching and the outcomes of interest, while adjusting for reporting year and important patient and treatment characteristics. Stepwise regression was used for all multivariable analyses to assess the significance of independent variables and all potential interactions that the model would support, using a statistical significance level of 0.05. Separate multivariable models were constructed for each outcome of interest for cycles in each of the following patient groups: [1] all patients, [2] patients ≥38 years of age or for whom the cycle was preceded by at least two ART cycles with no history of live birth, and [3] patients with “unindicated” cycles meeting neither of those criteria. Independent variables that were determined to be significant for all outcomes in a given patient group were included as covariates in the final models for each outcome in that group (except number of embryos transferred was not included in the models with implantation as the outcome). We found that the number of embryos transferred modified the effect of assisted hatching on multiple births in cycles involving day-3 transfers among all patients and patients with unindicated cycles. Therefore, we stratified the results for these outcomes by the number of embryos transferred. In addition, we calculated OR and aOR for poor-prognosis patients defined as those in which patient age is ≥38 years, the maximum serum follicle-stimulating hormone concentration is ≥10 mIU/mL, the infertility diagnosis is diminished ovarian reserve, the patient has a history of two or more ART cycles and no prior live births, and no embryos were available for cryopreservation at time of transfer. Although race/ethnicity was excluded from primary models due to a very high percentage of missing values (55.3%), it was added to the final logistic models in a supplemental analysis as race is thought to have an association with obstetric outcome. For the analyses including race, missing race was treated as a single “missing” category. All statistical tests were two-sided, and statistical significance was determined using an alpha of 0.05. All analyses were conducted using SAS v. 9.3 (SAS Institute) or SUDAAN v. 11.0 (RTI International). This study was reviewed and approved by the institutional review board of the Centers for Disease Control and Prevention.
RESULTS
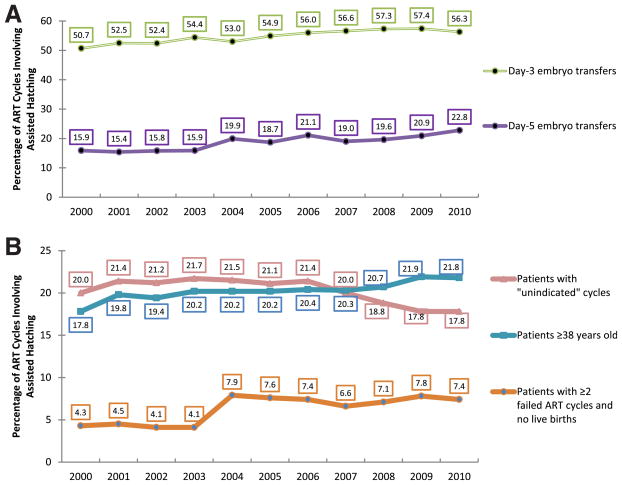
The absolute number of ART cycles in the United States involving assisted hatching increased significantly from 25,724 to 35,518 from 2000 to 2010 (P=.002). The percentage of fresh autologous cycles with assisted hatching increased over the 11-year period for cycles involving either a day-3 (from 50.7% to 56.3%, P<.0001) or day-5 transfer (from 15.9% to 22.8%, P=.0002) (Fig. 1A). An increasing linear trend in use of assisted hatching was also noted for cycles for which the patient was ≥38 years old (17.8% to 21.8%, P=.0002), for cycles preceded by two or more failed ART cycles (4.3% to 7.4%, P=.005), and for cycles meeting either of the above two criteria (20.1% to 25.3%, P=.0003) (see Fig. 1B). A decreasing linear trend (20.0% to 17.8%, P=.01) was noted in use of assisted hatching for “unindicated” cycles that met neither of the above criteria (i.e., patient age <38 and prior history of live birth or <2 prior ART cycles).
FIGURE 1.
Assisted hatching trends by embryo stage at transfer and patient prognosis, fresh autologous ART cycles, United States, 2000–2010. Trends of assisted hatching are shown for (A) 3-day and 5-day embryo transfer cycles and for (B) the following groups of patient prognosis: patients with “unindicated” cycles (defined as cycles in which patient age <38 years, number of failed ART cycles is <2, or the patient has a history of live birth), patients ≥38 years of age, and patients with ≥2 failed ART cycles and no live births.
Among all fresh autologous IVF cycles involving a day-3 or day-5 embryo transfer performed in the United States from 2000 to 2010, assisted hatching was used in 337,109 (44.8%) of 751,879 cycles (Table 1). Women in the assisted hatching group were more likely to be ≥38 years old, have a diagnosis of diminished ovarian reserve, and have undergone two or more ART cycles compared with women who did not use assisted hatching (P<.0001 for all comparisons). Cycles that involved assisted hatching had fewer oocytes retrieved, were more likely to use intracytoplasmic sperm injection, to involve transfer of day-3 rather than day-5 embryos, transfer a higher number of embryos, and to have no extra embryos available for cryopreservation than cycles without assisted hatching (P<.0001 for all comparisons).
TABLE 1.
Characteristics of day-3 and day-5 embryo transfers among ART patients by assisted hatching use, fresh autologous ART cycles, United States, 2000–2010.
| Characteristica,b | Assisted hatching | |||
|---|---|---|---|---|
| Used | Not used | |||
| n | % | n | % | |
| Total | 337,109 | 44.8 | 414,770 | 55.2 |
| Maternal age (y) | ||||
| <30 | 25,883 | 7.7 | 69,645 | 16.8 |
| 30–34 | 82,515 | 24.5 | 164,733 | 39.7 |
| 35–37 | 75,493 | 22.4 | 95,833 | 23.1 |
| 38 or older | 153,218 | 45.4 | 84,559 | 20.4 |
| Race or ethnicityc | ||||
| Non-Hispanic white | 115,344 | 44.8 | 135,238 | 42.0 |
| Non-Hispanic black | 10,436 | 4.1 | 11,822 | 3.7 |
| Asian or Pacific Islander | 17,970 | 7.0 | 17,171 | 5.3 |
| Hispanic | 12,492 | 4.8 | 14,765 | 4.6 |
| Other | 293 | 0.1 | 365 | 0.1 |
| Unknown/Missing | 100,899 | 39.2 | 142,527 | 44.3 |
| Infertility diagnosis | ||||
| Tubal factor | 66,152 | 19.6 | 88,815 | 21.4 |
| Endometriosis | 45,197 | 13.4 | 59,218 | 14.3 |
| Uterine factor | 18,667 | 5.5 | 19,407 | 4.7 |
| Ovulatory dysfunction | 42,289 | 12.5 | 66,745 | 16.1 |
| Diminished ovarian reserve | 75,002 | 22.3 | 46,985 | 11.3 |
| Male factor | 125,197 | 37.1 | 158,803 | 38.3 |
| Unexplained | 41,158 | 12.2 | 55,103 | 13.3 |
| No. of prior preterm births | ||||
| 0 | 277,562 | 82.3 | 340,483 | 82.1 |
| 1 or more | 7,580 | 2.3 | 8,616 | 2.1 |
| Unknown/missing | 51,967 | 15.4 | 65,671 | 15.8 |
| No. of prior full-term births | ||||
| 0 | 195,070 | 57.9 | 244,897 | 59.0 |
| 1 | 69,838 | 20.7 | 77,569 | 18.7 |
| 2 or more | 21,137 | 6.3 | 27,415 | 6.6 |
| Unknown/missing | 51,064 | 15.1 | 64,889 | 15.7 |
| No. of prior ART cycles | ||||
| 0 | 166,079 | 49.3 | 259,465 | 62.5 |
| 1 | 73,837 | 21.9 | 76,574 | 18.5 |
| 2 or more | 97,098 | 28.8 | 78,353 | 18.9 |
| No. of oocytes retrieved | ||||
| 0–10 | 183,474 | 54.4 | 157,561 | 38.0 |
| 11–20 | 120,942 | 35.9 | 185,510 | 44.7 |
| ≥21 | 32,693 | 9.7 | 71,699 | 17.3 |
| Use of ICSI | ||||
| Used | 246,720 | 73.2 | 261,256 | 63.0 |
| Did not use | 90,337 | 26.8 | 153,235 | 36.9 |
| Embryo stage at transfer | ||||
| Day 3 | 296,015 | 87.8 | 245,578 | 59.2 |
| Day 5 | 41,094 | 12.2 | 169,192 | 40.8 |
| Number of embryos transferred | ||||
| 1 | 30,940 | 9.2 | 38,640 | 9.3 |
| 2 | 100,168 | 29.7 | 219,057 | 52.8 |
| 3 | 111,370 | 33.0 | 106,383 | 25.7 |
| 4 or more | 94,631 | 28.1 | 50,690 | 12.2 |
| Extra embryos cryopreserved | ||||
| No | 257,697 | 76.4 | 238,377 | 57.5 |
| Yes | 78,737 | 23.4 | 173,494 | 41.8 |
| No. of fetal heartbeats at first trimester ultrasound (for transfers resulting in pregnancy) | ||||
| 1 | 78,203 | 62.1 | 119,512 | 59.0 |
| 2 | 31,429 | 24.9 | 63,665 | 31.5 |
| ≥3 | 6,904 | 5.5 | 8,371 | 4.1 |
| Unknown/missing | 9,469 | 7.5 | 10,936 | 5.4 |
| No. of live-born infants (for transfers resulting in live birth) | ||||
| 0 | 722 | 0.7 | 1,351 | 0.8 |
| 1 | 69,616 | 69.7 | 112,438 | 65.4 |
| 2 | 26,721 | 26.8 | 54,243 | 31.6 |
| ≥3 | 2,782 | 2.8 | 3,752 | 2.2 |
Note: ART = assisted reproductive technology; ICSI = intracytoplasmic sperm injection.
“Unknown/missing” category is shown if >1% of values are unknown or missing.
Comparing characteristics by assisted hatching use, all are P<.05.
Excludes years 2000, 2001, and 2002 because race/ethnicity data were not available.
Kissin. Assisted hatching trends and outcomes. Fertil Steril 2014.
Day-3 Embryo Transfer
From 2000 to 2010, 54.8% of a total of 536,852 fresh autologous IVF cycles involving a day-3 transfer used assisted hatching (Table 2). Of the cycles involving women who were either ≥38 years old or who had a history of two or more prior ART cycles with no history of live birth (227,372 cycles), 70.7% used assisted hatching. For cycles meeting neither of those two criteria (297,972 cycles), 42.5% used assisted hatching. In multivariable analyses, the results were similar in the three groups: cycles involving assisted hatching were associated with a lower odds of implantation, clinical pregnancy, and live birth, and with an increased odds of miscarriage, as compared with cycles not involving assisted hatching. Although the chance of multiple birth after single-embryo transfer is low, the odds of twinning (spontaneous splitting of transferred embryo) were higher with assisted hatching (compared with no hatching) among all patients and patients for whom assisted hatching was not indicated. The odds of multiple gestation were lower with assisted hatching among patients ≥38 years or for whom the cycle was preceded by at least two ART cycles with no history of live birth, and among all patients when two or more embryos were transferred.
TABLE 2.
Assisted hatching and cycle outcomes, day-3 embryo transfers, fresh autologous ART cycles, United States, 2000–2010.
| Patient groups and cycle outcomesa | No. and percentage of cycle outcomes | Association between assisted hatching and cycle outcomes | ||||
|---|---|---|---|---|---|---|
| Used assisted hatching | Did not use assisted hatching | |||||
| n | % | n | % | OR (95% CI) | aOR (95% CI)c | |
| All patients (N = 536,852) | ||||||
| Implantation | 136,631 | 15.8 | 141,166 | 22.1 | 0.66 (0.61, 0.71) | 0.72 (0.67, 0.77) |
| Clinical pregnancy | 106,399 | 36.2 | 106,257 | 43.8 | 0.73 (0.67, 0.79) | 0.80 (0.74, 0.86) |
| Live birth | 83,125 | 28.3 | 88,521 | 36.5 | 0.69 (0.64, 0.74) | 0.75 (0.70, 0.81) |
| Miscarriage | 20,017 | 18.9 | 14,228 | 13.5 | 1.49 (1.40, 1.60) | 1.43 (1.34, 1.52) |
| Multiple gestation | ||||||
| 1 embryo transferred | 74 | 2.0 | 47 | 1.3 | 1.59 (1.17, 2.16) | 1.77 (1.31, 2.38) |
| ≥2 embryos transferred | 31,887 | 31.1 | 35,281 | 34.4 | 0.86 (0.82, 0.89) | 0.90 (0.87, 0.94) |
| Patients ≥38 y, or ≥2 prior ART cycles and no live birth (N = 227,372) | ||||||
| Implantation | 59,452 | 11.3 | 28,892 | 13.5 | 0.81 (0.76, 0.86) | 0.84 (0.79, 0.90) |
| Clinical pregnancy | 47,375 | 29.5 | 21,816 | 32.7 | 0.86 (0.79, 0.93) | 0.89 (0.82, 0.97) |
| Live birth | 33,279 | 20.7 | 16,054 | 24.1 | 0.82 (0.77, 0.88) | 0.86 (0.80, 0.92) |
| Miscarriage | 12,459 | 26.4 | 4,924 | 22.7 | 1.22 (1.13, 1.32) | 1.19 (1.12, 1.28) |
| Multiple gestation | 11,425 | 24.1 | 5,680 | 26.0 | 0.90 (0.86, 0.95) | 0.93 (0.89, 0.98) |
| Patients <38 y, and <2 prior ART cycles or live birth (N = 297,972) | ||||||
| Implantation | 77,035 | 22.8 | 112,181 | 26.5 | 0.82 (0.76, 0.88) | 0.86 (0.80, 0.92) |
| Clinical pregnancy | 56,557 | 44.6 | 82,695 | 48.3 | 0.86 (0.80, 0.93) | 0.91 (0.84, 0.98) |
| Live birth | 47,747 | 37.7 | 70,956 | 41.4 | 0.85 (0.79, 0.92) | 0.90 (0.83, 0.97) |
| Miscarriage | 7,248 | 12.9 | 9,122 | 11.1 | 1.18 (1.13, 1.24) | 1.16 (1.11, 1.22) |
| Multiple gestation | ||||||
| 1 embryo transferred | 43 | 2.2 | 32 | 1.1 | 2.05 (1.34, 3.14) | 2.20 (1.45, 3.32) |
| ≥2 embryos transferred | 19,587 | 35.9 | 28,966 | 36.3 | 0.98 (0.94, 1.02) | 1.01 (0.96, 1.05) |
| Poor-prognosis patients (N = 6,511)b | ||||||
| Implantation | 1,142 | 7.5 | 230 | 7.5 | 1.01 (0.84, 1.21) | 0.98 (0.83, 1.17) |
| Clinical pregnancy | 1,125 | 20.9 | 222 | 19.6 | 1.08 (0.82, 1.43) | 1.05 (0.81, 1.35) |
| Live birth | 691 | 12.9 | 128 | 11.3 | 1.16 (0.90, 1.49) | 1.12 (0.89, 1.40) |
| Miscarriage | 391 | 34.9 | 85 | 38.6 | 0.85 (0.63, 1.15) | 0.86 (0.64, 1.17) |
| Multiple gestation | 160 | 14.2 | 38 | 17.1 | 0.80 (0.56, 1.14) | 0.78 (0.55, 1.10) |
Note: aOR = adjusted odds ratio; ART = assisted reproductive technology; CI = confidence interval; OR = odds ratio.
Outcomes are not mutually exclusive, and numbers/percentages may not add up due to missing values. Percentages of implantation are calculated per embryo transferred; clinical pregnancy and live birth are calculated per embryo transfer; percentages of miscarriage and multiple gestation are calculated per clinical pregnancy.
Poor-prognosis patients include women who are ≥38 years of age, have had ≥2 prior ART cycles (fresh or frozen) and no prior live birth(s), have FSH ≥10 mIU/mL, have a diagnosis of diminished ovarian reserve, and have no extra embryos available for cryopreservation.
Multivariable analysis considered via reporting year and all patient and treatment characteristics shown in Table 1, excluding race/ethnicity. Final models in the first three patient groups included extra embryos cryopreserved (yes, no), number of oocytes retrieved (0–10, 11–20, 21+) for implantation, and number of embryos transferred (1, 2+) and interaction between assisted hatching and the number of embryos transferred for other outcomes; final models in poor-prognosis group included reporting year and ovulatory dysfunction (yes, no) for implantation, and number of embryos transferred (1, 2+) for other outcomes. In supplemental analyses, magnitude and direction of effect did not change significantly when race/ethnicity was included in the final models.
Kissin. Assisted hatching trends and outcomes. Fertil Steril 2014.
For cycles performed on poor-prognosis patients (n = 6,511 cycles), 82.6% used assisted hatching. In multivariable analyses, the pregnancy outcomes were not statistically significantly different by assisted hatching status.
Day-5 Embryo Transfer
From 2000 to 2010, 19.4% of a total of 207,155 fresh autologous IVF cycles involving a day-5 transfer used assisted hatching (Table 3). Of the cycles involving women who were either ≥38 years old or who had a history of two or more prior ART cycles with no history of live birth (58,610 cycles), 27.5% used assisted hatching. For cycles meeting neither of the above two criteria (146,719 cycles), 16.1% used assisted hatching. In multivariable analyses, the results were similar for each of these groups to those with day-3 transfers: cycles involving assisted hatching were associated with lower odds of implantation, clinical pregnancy, live birth, and multiple gestation and with increased odds of miscarriage when compared to cycles without assisted hatching.
TABLE 3.
Assisted hatching and cycle outcomes, day-5 embryo transfers, fresh autologous ART cycles, United States, 2000–2010.
| Patient groups and cycle outcomesa | No. and percentage of cycle outcomes | Association between assisted hatching and cycle outcomes | ||||
|---|---|---|---|---|---|---|
| Used assisted hatching | Did not use assisted hatching | |||||
| n | % | n | % | OR (95% CI) | aOR (95% CI)c | |
| All patients (N = 207,155) | ||||||
| Implantation | 23,821 | 25.5 | 126,612 | 36.8 | 0.59 (0.54, 0.64) | 0.63 (0.59, 0.69) |
| Clinical pregnancy | 18,414 | 45.8 | 93,858 | 56.2 | 0.66 (0.61, 0.72) | 0.70 (0.65, 0.76) |
| Live birth | 14,839 | 36.9 | 79,317 | 47.5 | 0.65 (0.60, 0.70) | 0.69 (0.64, 0.74) |
| Miscarriage | 2,961 | 16.2 | 11,477 | 12.3 | 1.38 (1.26, 1.51) | 1.35 (1.23, 1.48) |
| Multiple gestation | 6,108 | 33.2 | 36,194 | 38.6 | 0.79 (0.73, 0.85) | 0.79 (0.75, 0.83) |
| Patients ≥38 y, or ≥2 prior ART cycles and no live birth (N = 58,610) | ||||||
| Implantation | 7,827 | 18.4 | 25,391 | 24.9 | 0.68 (0.60, 0.77) | 0.72 (0.64, 0.80) |
| Clinical pregnancy | 6,245 | 38.7 | 19,296 | 45.4 | 0.76 (0.66, 0.87) | 0.79 (0.71, 0.89) |
| Live birth | 4,605 | 28.6 | 14,825 | 34.9 | 0.75 (0.66, 0.84) | 0.78 (0.71, 0.86) |
| Miscarriage | 1,419 | 22.9 | 3,749 | 19.5 | 1.22 (1.11, 1.35) | 1.20 (1.09, 1.33) |
| Multiple gestation | 1,765 | 28.3 | 6,089 | 31.6 | 0.85 (0.78, 0.94) | 0.88 (0.81, 0.95) |
| Patients <38 y, and <2 prior ART cycles or live birth (N = 146,719) | ||||||
| Implantation | 15,983 | 31.3 | 101,194 | 41.9 | 0.63 (0.58, 0.70) | 0.67 (0.62, 0.73) |
| Clinical pregnancy | 12,038 | 50.9 | 73,863 | 60.0 | 0.69 (0.63, 0.75) | 0.72 (0.66, 0.78) |
| Live birth | 10,121 | 42.8 | 63,883 | 51.9 | 0.69 (0.64, 0.75) | 0.72 (0.66, 0.78) |
| Miscarriage | 1,527 | 12.8 | 7,649 | 10.4 | 1.26 (1.13, 1.40) | 1.25 (1.12, 1.40) |
| Multiple gestation | 4,293 | 35.7 | 29,824 | 40.4 | 0.82 (0.75, 0.89) | 0.80 (0.75, 0.86) |
| Poor-prognosis patients (N = 708)b | ||||||
| Implantation | 71 | 10.8 | 149 | 14.2 | 0.73 (0.49, 1.07) | 0.69 (0.48, 0.99) |
| Clinical pregnancy | 74 | 25.8 | 131 | 31.1 | 0.77 (0.56, 1.05) | 0.84 (0.61, 1.16) |
| Live birth | 51 | 17.8 | 98 | 23.3 | 0.71 (0.52, 0.99) | 0.78 (0.56, 1.09) |
| Miscarriage | 20 | 27.8 | 29 | 22.1 | 1.35 (0.77, 2.37) | – |
| Multiple gestation | 8 | 10.8 | 29 | 22.1 | 0.43 (0.20, 0.91) | – |
Note: aOR = adjusted odds ratio; ART = assisted reproductive technology; CI = confidence interval; OR = odds ratio.
Outcomes are not mutually exclusive, and numbers/percentages may not add up due to missing values. Percentages of implantation are calculated per embryo transferred; clinical pregnancy and live birth are calculated per embryo transfer; percentages of miscarriage and multiple gestation are calculated per clinical pregnancy.
Poor-prognosis patients include women who are ≥38 years of age, have had ≥2 prior ART cycles (fresh or frozen) and no prior live birth(s), have FSH ≥10 mIU/mL, a diagnosis of diminished ovarian reserve, and no extra embryos available for cryopreservation.
Multivariable analysis considered reporting year and all patient and treatment characteristics shown in Table 1, excluding race/ethnicity. Final models in the first three patient groups included extra embryos cryopreserved (yes, no) for implantation as well as the number of embryos transferred (1, 2+) and interaction between assisted hatching and number of embryos transferred for other outcomes; final models in the poor-prognosis group included number of oocytes retrieved (0–10, 11–20, 21+) and uterine factor (yes, no) for implantation, and number of embryos transferred (1, 2+) for other outcomes. In the poor-prognosis group, models could not be constructed for miscarriage and multiple gestation because of the small sample size. In supplemental analyses, magnitude and direction of effect did not change significantly when race/ethnicity was included in the final models.
Kissin. Assisted hatching trends and outcomes. Fertil Steril 2014.
For cycles performed on poor-prognosis patients (n =708 cycles), 40.5% used assisted hatching. In multivariable analyses, assisted hatching was associated with lower odds of implantation, but no statistically significant associations were detected between use of assisted hatching and other outcomes (clinical pregnancy and live birth).
DISCUSSION
In the United States from 2000 to 2010, assisted hatching use statistically significantly increased in absolute number, percentage of day-3 and day-5 embryo transfers, and cycles for women meeting SART/ASRM suggested criteria for using assisted hatching. Regardless of embryo stage at transfer, use of assisted hatching was associated with lower odds of implantation, clinical pregnancy, and live birth, and with increased odds of miscarriage, except in poor-prognosis patients where no statistically significant differences in outcomes were observed between cycles with and without assisted hatching.
The increasing trend of assisted hatching use during the last 11 years was especially notable among patients for whom assisted hatching has been shown to be beneficial in some studies. Several systematic reviews and SART/ASRM committee opinions supported the use of assisted hatching “in patients with a poor prognosis, including those with ≥2 failed IVF cycles and poor embryo quality and older women (≥38 years of age)” (1, 2, 7). Our study shows that practice patterns appear to reflect the recommendations of SART/ ASRM practice committees; assisted hatching use has increased among patients for whom it is indicated, based on the guidelines, and decreased among those for whom it is not indicated (2).
Although we found that cycles in which assisted hatching was used were less likely to result in pregnancy or live births and more likely to result in miscarriage, the association with poor pregnancy and birth outcomes may be partially explained by the fact that assisted hatching procedure is often chosen for patients with poor prognosis. However, we also did not find a statistically significant association between assisted hatching and pregnancy outcomes for cycles among women with poor prognosis. Our findings are consistent with the majority of published data, which suggest any potential benefit of assisted hatching is either marginal or unproven. A beneficial effect, if present, may not be fully ascertainable without a purposefully designed prospective study, such as a randomized controlled trial, which would be less prone to biases and allow better collection of data on, and adjustment for, known and potential confounding factors. Our finding of an association between assisted hatching and monozygotic twinning with single day-3 embryo transfer among patients for whom assisted hatching was not indicated is consistent with a recent study that showed assisted hatching of cleavage-stage embryos to be associated with a higher risk of monozygosity (12). Our ability to detect monozygotic twinning with transfer of two or more embryos was limited because splitting of one embryo may be compensated by inability of another embryo to survive.
Our primary limitation is the lack of embryo quality data that may have contributed to selection bias if poorer quality embryos were more often selected for assisted hatching. In addition, embryo quality may be an important predictor of assisted hatching success (13). We attempted to minimize such bias by including the criterion of zero additional embryos available for cryopreservation in our definition of the poor-prognosis group, a group that would likely have poor quality embryos regardless of assisted hatching use. The number of embryos available for cryopreservation has been shown to correlate well with embryo quality (14–16). Moreover, we were unable to control for additional patient medical and social history or additional laboratory or clinical factors that may have influenced the decision to perform assisted hatching or may affect the observed outcomes but are not included within the surveillance system such as presence or absence of hypertensive disorder or diabetes, patient body mass index, or tobacco-use status. Another limitation of the study is the lack of information on the type of assisted hatching (mechanical, chemical, or laser), which may have varied over time or from one clinic to another. Adjustments for reporting year and clustering helped to overcome this limitation. Our study is strengthened by the high compliance of clinics with nationally mandated fertility clinic reporting, the large sample size, and the ability to perform subgroup analysis of poor-prognosis patients. Furthermore, it is one of few studies to provide data not only on early pregnancy outcomes but also on live births.
Assisted hatching use has increased in the United States since 2000. Although we observed a decreasing trend of using assisted hatching among women for whom it is not recommended, it is still used in a relatively large number of cycles among women <38 years of age and those with <2 prior ART cycles or a history of live births. In addition, there is an increasing trend of assisted hatching among blastocyst (day-5) transfers despite the lack of convincing evidence of its effectiveness in that population. Although population-based surveillance data are limited in their ability to answer some clinical questions, they can be used to monitor the use of ART and its subtypes and serve to generate hypotheses. The assessment of assisted hatching use in the United States could be improved by including data on the types of assisted hatching as well as data on embryo quality in the National ART Surveillance System. Although limited by a lack of embryo quality data, this observational study did not find assisted hatching to be associated with improved pregnancy outcomes among fresh autologous IVF cycles even in poor-prognosis patients. The association between assisted hatching and pregnancy outcomes may differ depending on patient prognosis, embryo stage, and embryo quality, and this warrants further investigation. A well-designed prospective study may help clinicians identify patients who may benefit from assisted hatching.
Footnotes
D.M.K. has nothing to disclose. J.F.K. has nothing to disclose. M.M. has nothing to disclose. S.L.B. has nothing to disclose. D.R.S. has nothing to disclose. D.J.J. has nothing to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Carney SK, Das S, Blake D, Farquhar C, Seif MM, Nelson L. Assisted hatching on assisted conception in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) Cochrane Database Syst Rev. 2012:CD001894. doi: 10.1002/14651858.CD001894.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The role of assisted hatching in in vitro fertilization: a review of the literature. A Committee opinion. Fertil Steril. 2008;90(Suppl):S196–8. doi: 10.1016/j.fertnstert.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J, Malter H, Fehilly C, Wright G, Elsner C, Kort H, et al. Implantation of embryos after partial opening of oocyte zona pellucida to facilitate sperm penetration. Lancet. 1988;2:162. doi: 10.1016/s0140-6736(88)90710-6. [DOI] [PubMed] [Google Scholar]
- 4.Hagemann AR, Lanzendorf SE, Jungheim ES, Chang AS, Ratts VS, Odem RR. A prospective, randomized, double-blinded study of assisted hatching in women younger than 38 years undergoing in vitro fertilization. Fertil Steril. 2010;93:586–91. doi: 10.1016/j.fertnstert.2009.01.116. [DOI] [PubMed] [Google Scholar]
- 5.Hurst BS, Tucker KE, Awoniyi CA, Schlaff WD. Assisted hatching does not enhance IVF success in good-prognosis patients. J Assist Reprod Genet. 1998;15:62–4. doi: 10.1007/BF02766826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparks AE. Culture systems: embryo culture and monozygotic twinning. Methods Mol Biol. 2012;912:387–97. doi: 10.1007/978-1-61779-971-6_22. [DOI] [PubMed] [Google Scholar]
- 7.Martins WP, Rocha IA, Ferriani RA, Nastri CO. Assisted hatching of human embryos: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2011;17:438–53. doi: 10.1093/humupd/dmr012. [DOI] [PubMed] [Google Scholar]
- 8.Schieve LA, Meikle SF, Peterson HB, Jeng G, Burnett NM, Wilcox LS. Does assisted hatching pose a risk for monozygotic twinning in pregnancies conceived through in vitro fertilization? Fertil Steril. 2000;74:288–94. doi: 10.1016/s0015-0282(00)00602-6. [DOI] [PubMed] [Google Scholar]
- 9.Adashi EY, Wyden R. Public reporting of clinical outcomes of assisted reproductive technology programs: implications for other medical and surgical procedures. JAMA. 2011;306:1135–6. doi: 10.1001/jama.2011.1249. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2011 Assisted reproductive technology fertility clinic success rates report. Atlanta: U.S. Department of Health and Human Services; 2013. [Google Scholar]
- 11.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2010 Assisted reproductive technology national summary report. Atlanta: U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 12.Luke B, Brown MB, Wantman E, Stern JE. Factors associated with monozygosity in assisted reproductive technology pregnancies and the risk of recurrence using linked cycles. Fertil Steril. 2014;101:683–9. doi: 10.1016/j.fertnstert.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grace J, Bolton V, Braude P, Khalaf Y. Assisted hatching is more effective when embryo quality was optimal in previous failed IVF/ICSI cycles. J Obstet Gynaecol. 2007;27:56–60. doi: 10.1080/01443610601056335. [DOI] [PubMed] [Google Scholar]
- 14.Wang JG, Douglas NC, Dicken C, Nakhuda GS, Guarnaccia MM, Sauer MV. Cryopreservation of supernumerary high quality embryos predicts favorable outcomes for patients undergoing repeated cycles of in vitro fertilization. Fertil Steril. 2008;89:368–74. doi: 10.1016/j.fertnstert.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Hill MJ, Richter KS, Heitmann RJ, Lewis TD, DeCherney AH, Graham JR, et al. Number of supernumerary vitrified blastocysts is positively correlated with implantation and live birth in single-blastocyst embryo transfers. Fertil Steril. 2013;99:1631–6. doi: 10.1016/j.fertnstert.2013.01.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern JE, Lieberman ES, Macaluso M, Racowsky C. Is cryopreservation of embryos a legitimate surrogate marker of embryo quality in studies of assisted reproductive technology conducted using national databases? Fertil Steril. 2012;97:890–3. doi: 10.1016/j.fertnstert.2011.12.050. [DOI] [PubMed] [Google Scholar]