Abstract
Exposure to ultraviolet (UV) radiation is associated with approximately 65% of melanoma cases, and 90% of non-melanoma skin cancers (NMSC), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). While the incidence of most other malignancies has either stabilized or declined, that of NMSC has increased and is developing even in younger age groups. NMSCs account for nearly 15,000 deaths, 3.5 million new cases, and more than 3 billion dollars a year in medical costs in the United States alone, representing a major public health concern. As sun protection efforts have not been proven effective, targeted chemoprevention strategies are much needed. Skin carcinogenesis by DNA damage is considered a predominant paradigm for UV toxicity. Exposure to UV radiation can activate various oncogenes while inactivating tumor suppressor genes, resulting in inappropriate survival and proliferation of keratinocytes that harbor these damages. Moreover, increasing evidence demonstrate that inflammatory responses by the immune cells within the tumor microenvironment also contribute significantly to skin tumorigenesis. Initiation and progression of skin carcinogenesis mediated by UV radiation involve complex pathways, including those of apoptosis, proliferation, autophagy, DNA repair, checkpoint signaling, metabolism, and inflammation. In this review, we highlight the recent advances in two of these key molecular processes that result in UV-mediated skin carcinogenesis. In particular, we discuss 1) pathways that regulate DNA damage repair and 2) the regulation of the inflammatory process its crosstalk with DNA repair potentially leading to non-melanoma skin carcinogenesis.
Keywords: DNA damage, DNA repair, Inflammation, Skin cancer, Ultraviolet radiation, UV
Introduction
Exposure to ultraviolet (UV) radiation induces functional changes in keratinocytes and immune cells that lead to skin cancer, supported by strong epidemiological and molecular evidence demonstrating a strong causal link between UV exposure and all forms of skin cancer.1 In fact, it is estimated that UV exposure is associated with 65% of melanoma cases and 90% of non-melanoma skin cancers (NMSC), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).2 They account for nearly 15,000 deaths, 3.5 million new cases, and more than 3 billion dollars a year in medical costs in the United States alone, representing a major public health concern.3, 4 While the incidence of most other malignancies has either stabilized or declined, that of NMSC has increased and is developing even in younger age groups.3, 5 Physicians have long promoted the use of sunscreens and the wearing of protective apparels as primary prevention in efforts to reduce the incidence of sun-induced skin cancers. While these efforts are important, studies have shown only a modest reduction in actinic keratosis6 and SCC of the skin but no reduction in BCCs,7 thus presenting the need for targeted chemoprevention strategies.8, 9
Skin carcinogenesis by DNA damage is considered a predominant paradigm of UV toxicity. Exposure to UV radiation can activate various oncogenes while inactivating tumor suppressor genes, resulting in DNA damage and inappropriate survival and proliferation of keratinocytes that harbor these damages. Moreover, increasing evidence demonstrate that inflammatory responses by the immune cells within the tumor microenvironment also contribute significantly to skin tumorigenesis. Initiation and progression of skin carcinogenesis mediated by UV radiation involve complex pathways, including those of apoptosis, proliferation, autophagy, DNA repair, checkpoint signaling, metabolism, and inflammation. In this review, we highlight the recent advances in two of these key molecular processes that result in UV-mediated skin carcinogenesis. In particular, we will discuss 1) pathways that regulate DNA damage repair and 2) pathways that regulate the inflammatory process potentially leading to non-melanoma skin carcinogenesis.
Regulation of UV-induced DNA repair
The epidermis is constantly exposed to UV radiation and thus susceptible to DNA damage. Although contributing to less than 1–2% of UV light from the sun, UVB (280–315 nm) radiation is considered to be the major environmental carcinogen that leads to skin cancer, involved in both tumor initiation and promotion.10
Exposure to UVB results in various types of DNA damage, mainly cyclobutane pyrimidine dimers (CPD) and 6-4 photoproducts (6-4 PP), DNA strand breaks, and DNA crosslinks.11 If not repaired, this DNA damage can result in mutations in the genome, ultimately contributing to skin carcinogenesis.12 To maintain genomic stability, cells are equipped with delicate DNA repair mechanisms that allow effective response to these DNA lesions, including nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR), DNA double strand break (DSB) repair and post replication repair (PRR).13, 14, 15, 16
Among these DNA repair mechanisms, the nucleotide excision repair (NER) is critically important in the repair of CPDs and 6-4 PPs.17, 18, 19, 20 The importance of NER in cancer protection is perhaps best demonstrated in humans with xeroderma pigmentosum (XP), an autosomal recessive disorder with defects in NER.21, 22, 23 First described in the late 1800's, XP is manifested by exaggerated sun sensitivity and nearly 10,000-fold increased susceptibility to UV-induced mucocutaneous neoplasms.24 The study of XP has elucidated the mechanisms essential for efficient DNA repair in response to UV-induced DNA damages. The NER pathway involves a number of proteins that detect, unwind, and remove the damaged DNA. NER consists of two sub-pathways: global genomic repair (GG-NER), which removes DNA damage from the entire genome; and transcription-coupled repair (TC-NER), which eliminates lesions located on actively transcribed genes. During GG-NER, a specific DNA repair mechanism is activated, which involves DNA damage-binding proteins 1 and 2 (DDB1 and DDB2) and the xeroderma pigmentosum (XP) proteins (XPA, XPB, XPC, XPD, XPE, XPF, and XPG).17, 18, 19, 20 There are seven NER-deficient genetic complementation groups of XP (XP-A to -G), and the XP group C (XPC) protein has been shown to play an essential role in damage recognition for global genomic NER (GG-NER). In particular, XPC functions as a key player in damage recognition in GG-NER in protection against UV-induced cancers, and the XPC gene is deleted or mutated in human SCC.25 We know that XPC abundance is critical for efficient GG-NER and thus for preventing UVB-induced skin carcinogenesis.26, 27 The regulation of XPC is an area of active investigation and will be reviewed here. Recent studies have demonstrated that XPC abundance is regulated by multiple pathways critical for carcinogenesis. For example, the tumor suppressors p53 and ARF promote XPC expression to enhance NER.28, 29 Here we focus on some of the recent discoveries of molecular pathways that have been implicated in XPC regulation in NER and as critical targets of UVB radiation.
PTEN
Phosphatase tensin homolog (PTEN) is well established as a tumor suppressor gene that induces apoptosis and reduces cell proliferation by inhibition of the PI3K/AKT pathway. PTEN is frequently mutated, deleted, or epigenetically silenced in many human cancers. In recent years, PTEN has revealed itself to encompass even a broader role as a critical factor in the regulation DNA damage repair, including DSB repair and NER.
Shen et al demonstrated that cells deficient in PTEN have defective DNA repair, due to downregulation of Rad51 and lack of PTEN at the centromeres.30 PTEN acts on the chromatin and regulates Rad51 expression, which results in reduction of spontaneous DSBs. However, Gupta et al found that deficiency in PTEN does not lead to alterations in the initial steps of DNA damage sensing and chromatin modification associated with DNA DSBs.31 The discrepancy may suggest cell-specific function of PTEN in DSB repair and indicates the need for further investigation.
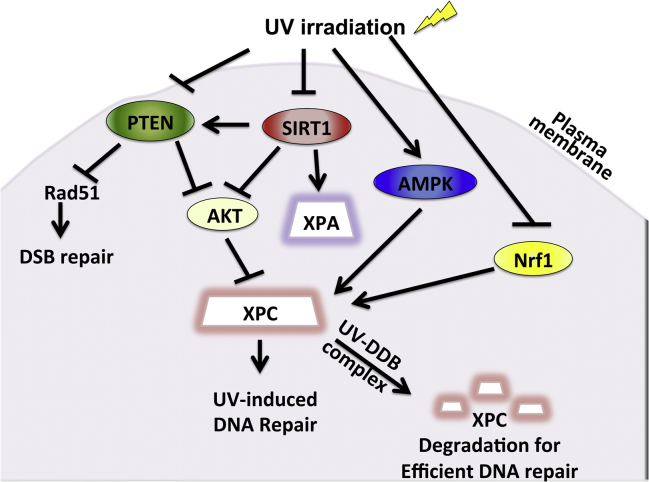
PTEN has also been shown to be essential for NER regulation in response to UV radiation (Fig. 1). Ming et al showed that mice with targeted PTEN downregulation in their epidermis are predisposed to skin tumorigenesis when exposed to low UV radiation and that PTEN was found to be significantly downregulated in both premalignant and malignant skin lesions.32 Inhibition of PTEN reduced XPC expression, which translated to significant reduction in the repair of CPDs and 6-4 PPs, indicating that PTEN is required for efficient GG-NER. Furthermore, positive regulation of XPC by PTEN was found to be via the AKT/p38 pathway.
Fig. 1.
Pathways that regulate XPC expression and activity in UV-induced DNA repair.
Nrf1
Nuclear factor erythroid 2-related factor 1 (Nrf1, also called NFE2L1) is member of the Cap'n' collar basic leucine zipper (CNC-bZIP) family of transcription factors, along with p45NF-E2, Nrf2, Nrf3, Bach1, and Bach2.33, 34, 35 Nrf1 is expressed abundantly in mouse and human tissues and upregulates expression of the antioxidant response elements (AREs) and subsequently cytoprotective genes, playing an important role in preventing oxidative stress.36, 37 Relatively little had been known about Nrf1, especially regarding its role in skin cancer and UVB response. The role of Nrf1 in UVB-induced DNA damage repair has begun to be unveiled in recent years. For the first time, Han et al demonstrated that Nrf1 functions in cell survival as well as NER upon UV damage in human keratinocytes38 (Fig. 1). Loss of Nrf1 sensitized keratinocytes to apoptosis with and without UVB-induced radiation via upregulation of Bik but not other pro-apoptotic Bcl-2 family members. A study in S. cerevisiae suggested that Nrf1 also plays a role in proteasome homeostasis, which may be a potential area of further investigation in regards to the effect of Nrf1 on cell survival upon UVB irradiation. Furthermore, Nrf1 is an essential player in the DNA repair network that responds to UVB-induced damage. Nrf1 knockdown suppressed XPC mRNA and protein levels but had no effect on other DNA repair proteins such as DDB1, DDB2, and HR23B.38 We know that CPD but not 6-4 PP is the principal culprit of skin tumorigenesis.39 Interestingly, Nrf1 was shown to be important in the repair of CPD but not 6-4 PP, suggesting that its defect may be implicated in skin cancer. Thus, Nrf1 functions as a regulator of XPC and plays a crucial role in UV-induced NER in keratinocytes. Finally, this study demonstrated a link between the role of Nrf1 in antioxidant homeostasis and DNA repair, showing that Nrf1 regulates XPC expression through maintaining GSH levels. The role of Nrf1 in DNA repair may play an important role in the pathogenesis of SCC, as human epidermis with SCC expressed less Nrf1 when compared unaffected epidermis and Nrf1 expression was downregulated by UVB irradiation. Taken together, Nrf1 demonstrates previously unrecognized functions acts as a tumor suppressor that promotes cell survival via Bik upregulation and DNA repair via enhancement of XPC transcription.
SIRT1
SIRT1 is a member of a highly conserved gene family (sirtuins) encoding nicotinamide adenine dinucleotide (NAD) (+)-dependent deacetylases, originally found to deacetylate histones leading to increased DNA stability and prolonged survival—gaining a reputation as a ‘longevity protein’.40, 41 Since then, SIRT1 has been found to deacetylate a multitude of protein targets involved in various cellular pathways, including stress responses, apoptosis, and axonal degeneration (Choi and Mostoslavsky, 2014). Increasing evidence has suggested that SIRT1 has an important role in UV signaling pathways. Cao et al found that SIRT1 is expressed in human keratinocytes and is downregulated by both UV and H2O2 in a JNK-dependent manner.42 Activation of SIRT1 resulted in reduced UV- and H2O2-induced p53 acetylation but increased AMP-activated protein kinase (AMPK) and downstream acetyl-CoA carboxylase (ACC) and phosphofructose kinase-2 (PFK-2) phosphorylation. Moreover, SIRT1 deacetylates and decreases the function of the histone acetyltransferase TIP60, such as autoacetylation and p53 acetylation, following UV radiation.43 This may explain the previously reported altered expression and function of TIP60 is altered in skin tumors.44
SIRT1 is also essential for the regulation of GG-NER (Fig. 1). Expression of SIRT1 is significantly reduced in skin neoplasms associated with chronic sun exposure, including actinic keratosis, SCC, and keratoacanthoma, SCC in situ, and invasive SCC.45 Downregulation of SIRT1 significantly sensitizes cells to UV irradiation.46 SIRT1 plays an essential role in GG-NER via its effects on XPA and XPC upon UV exposure. SIRT1 modulates the acetylation status of XPA46 and enhances the transcription of the key NER factor XPC.45 The AKT pathway was shown to be essential for the effect of SIRT1 on XPC. This is consistent with another finding that SIRT1 deacetylates the tumor suppressor PTEN, a known negative regulator for the PI3K/AKT pathway.47
Even as we outline the anti-tumor effects of SIRT1 and its important role in DNA damage in response to UV radiation here, the role of SIRT1 in cancer development is complex and still under debate.48, 49, 50 Interestingly, SIRT1 has been shown to be both a tumor suppressor and an oncogene in genetically modified mouse models. Mice with whole body SIRT1 deletion did not affect chemical carcinogen-induced skin tumorigenesis.51 A recent study by Ming et al aimed to reconcile this controversy by creating mice with keratinocyte-specific SIRT1 deletion and then monitoring UVB-induced tumor development.52 SIRT1 exerts opposite effects on skin tumorigenesis depending on the gene dose. Heterozygous deletion of SIRT1 promoted UVB tumorigenesis, whereas homozygous deletion suppressed tumorigenesis but led to increased sensitivity to chronic UVB injury and malignant conversion. Moreover, SIRT1 was haploinsufficient for UVB-induced DNA damage repair and XPC expression but only homozygous SIRT1 deletion increased p53 activation and UVB-induced apoptosis in vivo. Heterozygous deletion had no such effect.52 Thus, the opposing views on the role of SIRT1 in tumorigenesis can be explained by its gene dosage-dependent differences in function as both an oncogene and a tumor suppressor. Interestingly the SIRT1 activator resveratrol reduces skin tumorigenesis induced by either UVB or chemical carcinogens,51, 53 suggesting that activating SIRT1 reduces skin tumorigenesis.
AMPK
AMP-activated protein kinase is a well-conserved energy sensor that plays a key role in the regulation of protein and lipid metabolism in response to changes in fuel availability.54 Although AMPK is traditionally thought to play a major role in the regulation of cellular metabolism, it is now widely recognized to have antineoplastic functions. The AMPK pathway is down-regulated in human and mouse SCCs, and its activators AICAR and metformin increase UVB-induced DNA repair and XPC expression in mouse skin and in normal human epidermal keratinocytes55 (Fig. 1). Furthermore, in UVB-damaged tumor-bearing mice, both topical and systemic metformin prevented the formation of new tumors and suppressed growth of established tumors, demonstrating that AMPK acts as a tumor suppressor in the skin by promoting DNA repair and controlling cell proliferation.
DDB2/CUL4A/DDB1
The UV-damaged DNA-binding protein complex (UV-DDB) is involved in the damage recognition step of NER (Fig. 1). UV-DDB consists of two tightly associated subunits, DDB1 and DDB2.56, 57, 58 UV-DDB exhibits very strong and specific binding to UV-treated DNA and plays a role in GG-NER.59, 60 Mutations in the DDB2 gene account for the underlying defect in XP-E. Sugasawa et al demonstrated that XPC undergoes reversible ubiquitination upon UV radiation and that UV-DDB activity was necessary for this.61 XPC and UV-DDB physically interact and are polyubiquitinated by the UV-DDB-ubiquitin ligase complex, leading to efficient NER.62, 63, 64
UV-induced inflammation: regulation and crosstalk with UV-induced DNA repair
The observation that tumors arise in sites of chronic irritation and inflammation dates back to 1828 and has stimulated active research. Numerous animal models of UV-induced skin carcinogenesis have contributed to our understanding of the multistage process of carcinogenesis and shed insights in the differences between physiological inflammation in wound healing and the deregulated tumor-associated inflammation to skin cancer growth and progression. Rudolf Virchow first discovered the presence of leukocytes within tumors in 1863 and concluded that a functional connection exists between inflammation and cancer. Over the past decades, in-depth investigations of the molecular and cellular mechanisms mediating the relationship between cancer and inflammation have flourished. Classically, it was thought that the dynamic crosstalk between immune cells and tumors resulted in the eradication of tumors. However, compelling evidence against this theory has emerged, revealing the paradoxical role of immune cells in tumor survival, proliferation, and dissemination.65, 66, 67, 68, 69 UV-induced inflammatory responses lead to increased blood flow and vascular permeability, resulting in edema, erythema, hyperplastic responses, and activation of cyclooxygenase-2 (COX-2) and prostaglandin (PG) metabolites.70, 71, 72 Inflammation results in recruitment of infiltrating leukocytes secreting a variety of proinflammatory cytokines at the UV-irradiated sites, and is therefore considered an early event in tumor promotion. Chronic inflammation plays a crucial role in all three stages of tumor development: initiation, promotion, and progression.71 Various animal models and use of anti-inflammatory agents as chemotherapy highlight the importance of UV-induced inflammation in the development and progression of skin tumors.73, 74, 75, 76
Nuclear factor-kappa B
Nuclear factor kappa B (NF-κB) is a dimeric transcription factor composed of p50 and p65 Rel A family proteins that is found in almost all cell types and plays a critical role in the cellular stress response.77 In particular, NF-κB plays a key role inflammation, cellular proliferation, and induction of cancers. Constitutive upregulation of NF-κB has been demonstrated in a variety of tumor cells.78, 79, 80 UVB radiation results in the activation of IKKα, which phosphorylates and degrades IκBα in epidermal keratinocytes.81 Interestingly, UVB sequentially regulates the activities of different subunits of NF-κB82 (Fig. 2). While NF-κB/p50 is downregulated in the early stage (6 h), NF-κB/p65 is upregulated in the later stage (12 h). NF-κB is activated by UVB radiation and contributes to UVB-induced skin carcinogenesis. Interestingly, the early activation phase following UVB radiation has been shown to occur through a non-canonical eIF-2 pathway. UVB radiation activates the uncoupling of constitutive nitric oxide synthase (cNOS) in an eIF-2-dependent manner.83 Recently, Tong and Wu reported that cNOS activation results in the activation of NF-κB following UVB radiation. Continuous, but not acute, inhibition of cNOS with a specific inhibitor led to suppression of IκB reduction and subsequent NF-κB activation.84 Consequently, the NF-κB signaling pathway has been a major target for the development of alternative anti-cancer agents. Several groups have demonstrated that epigallocatechin-3-gallate (EGCG), the major constituent of green tea with significant anti-inflammatory and cancer chemopreventive properties, inhibits UV-induced activation of NF-κB.85, 86, 87 Activation of and nuclear translocation of NF-κB leads to induction of pro-inflammatory cytokines, such as IL-6 and TNF-α, both of which are thought to play a role in the response to UVB damage leading to apoptosis and inflammation.88, 89, 90
Fig. 2.
UV-induced inflammation: regulation and crosstalk with UV-induced DNA repair.
Cyclooxygenase
Accumulating evidence demonstrate that cyclooxygenases, enzymes involved in prostaglandin synthesis, may be involved in the pathogenesis of NMSC. There are two major isoforms of cyclooxygenase, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). While COX-1 is constitutively expressed in most cell types, COX-2 expression is induced by a variety of stimuli. UV radiation is known to augment COX-2 expression in the human skin, and COX-2 is overexpressed in chronically UVB-irradiated skin and UVB-induced SCCs91, 92 (Fig. 2). COX-2, but not COX-1, is found to be involved in increased susceptibility to SCCs.93, 94 On the other hand, both COX-1 and COX-2 appear to participate in the development of BCCs. Multiple pathways have been demonstrated to mediate UVB-induced COX-2 expression, including AKT, p38, AMPK and SIRT6.95, 96, 97 This is further supported by the findings that inhibiting p38α,98 AKT,99, 100, 101 or SIRT6,97 or activating AMPK55, 102 reduces skin tumorigenesis. Animals that have been treated with pharmacological inhibitors of COX-2 develop significantly fewer tumors upon UV radiation compared with control mice. Recent studies suggest that drugs that block COX-2 expression may prevent the development of NMSCs and can be used as effective chemopreventive agents for NMSCs. Animal models also have shown that both selective COX-2 inhibitor celecoxib and nonselective COX-1 and COX-2 inhibitors, including naproxen, indomethacin, and sulindac, can be used as effective chemopreventive agents for UV-induced NMSCs.73, 94, 103, 104 Moreover, phytochemicals such as proanthocyanidins have shown association with reduced UV-induced skin tumors in mice.105 Moreover, epidemiologic studies support that the use of COX inhibitors (non-steroidal anti-inflammatory drugs) is associated with decreased of NMSC.106, 107 A case–control study in Australia reported that people who used NSAIDs more than two times per week for at least a year had a statistically significantly lower incidence of SCCs and lower AK counts than those who had never used them or used them infrequently.106 Another case–control study in Demark reported that the use of aspirin, nonselective NSAIDs, and COX-2 inhibitors was associated with decreased risk for SCC and melanoma.107
IL-12
Interleukin-12 (IL-12) is a pleiotropic cytokine involved in inflammation composed of two subunits p35 and p40 and has been shown to have antitumor activity in a wide variety of tumor models.108, 109 IL-12 possesses antitumor activity, enhances antitumor immunity, and can repair ultraviolet (UV)-induced DNA damage in the form of cyclobutane pyrimidine dimers.110, 111, 112, 113 The demonstration of its anti-tumor effects in animal models has led to significant interest in the therapeutic use of IL-12. A number of studies have shown that IL-12p40-deficiency enhances skin carcinogenesis in mice in tumor incidence, tumor multiplicity, and the extent of tumor angiogenesis114 (Fig. 2). Maeda et al111 and Meeran et. al114 have shown that mice deficient in IL-12 are at higher risk for UV radiation-induced skin tumors than wild-type mice. Also, long-term exposure to UVB radiation stimulates the phosphorylation of MAPK proteins in IL-12-deficient mice to a greater extent than long-term UVB exposure of WT mouse skin.115 Furthermore, Meeran et al116 further elucidated the mechanism underlying the antitumor effects of IL-12. Deficiency of IL-12 leads to enhanced COX-2 expression and thus PGE2 production as well as increased inflammatory cytokines such as IL-1β, TNF-α, and IL-6. Reconstituting with recombinant IL-12 prior to UV exposure leads to decreased UVB-induced leukocyte infiltration, reduced NF-κB activation, and cyclin D expression, further substantiating the role of IL-12 in the inhibition of UV-induced skin tumorigenesis.115
HMGB1
Exposure to UV light induces inflammation independent of microbial recognition, in which case endogenous signals are activated to alert the body of the danger. These signals are termed alarmins and represent a new class of inflammatory mediators beginning to be recognized for their importance in cutaneous inflammation. Classically, alarmins are intracellular molecules that become released into the extracellular milieu upon damage. When released, alarmins bind pattern recognition receptors (PRRs) or other surface receptors, resulting in NF-κB activation and subsequent pro-inflammatory cytokine production. High mobility group box-1 (HMGB1) is an alarmin that has been extensively studied. Normally localized to the nucleus, HMGB1 is released into the cytoplasm and subsequently extracellularly to stimulate inflammation by binding to toll-like receptors (TLRs) or receptor of advanced glycation end products (RAGE).117 A recent study by Johnson and Wulff demonstrated that UV radiation stimulated HMGB1 release in keratinocytes in vitro and that HMGB1 is expressed in skin tumors after chronic radiation118 (Fig. 2). This was supported by Bald et al who showed that UV-induced inflammation promotes metastatic progression of melanoma and that this is initiated by the release of HMGB1 from UV-damaged keratinocytes and driven by TLR4.119 Together, these studies suggest that HMGB1 plays a contributory role in UV-mediated inflammation and tumor formation in both melanoma and non-melanoma skin cancers.
Toll-like receptors 2, 3, 4, 7, 8, 9
Toll-like receptors (TLRs) are a class of membrane-spanning, non-catalytic receptors that recognize structurally conserved microbial molecules and play a key role in the inflammatory innate immune system. TLRs have emerged as a major class of pattern recognition receptors that initiate cutaneous immune responses and are expressed on many different cell types in the skin, including keratinocytes and Langerhans cells in the epidermis. Not only do TLRs mediate responses to pathogens but also sterile inflammation triggered by endogenous molecules. TLRs have achieved an extraordinary amount of interest in cancer research due to their role in tumor progression. Recent literature show that activation of TLRs not only leads to the upregulation of host defense mechanisms, but also results in upregulation of DNA repair genes and increased functional DNA repair, thus providing a link between inflammation and DNA damage.120 Several studies have suggest that TLR2, 3, 4, 7, 8, and 9 may be involved in DNA repair mechanisms induced by UVB radiation (Fig. 2).
In 2008, Zheng et al121 observed that activating TLR9 by its ligand CpG DNA exhibited increased DNA repair rates after irradiation in CD4+ T cells. Thereafter, a global gene expression analysis revealed an increase in mouse splenic mRNAs that code for proteins involved in DNA repair after activation of TLR9 by CpGs.122 This was paralleled by another study that showed upregulation of genes that encode DNA repair molecules after intraperitoneal injection of CpG in mice.123
TLRs 7 and 8 are located in the endosome membrane and recognize single-stranded RNAs.124 Imiquimod, TLR7 and TLR8 agonist, is being widely used now as a topical therapeutic agent to treat dermatologic diseases such as HPV infection, NMSC, and actinic keratosis.125, 126, 127, 128 Animal studies suggest that the mechanism by which Imiquimod exerts its effects is by enhancing DNA repair gene expression as well as functional repair of DNA damage mechanisms.129 Moreover, in vitro treatment of bone marrow–derived cell lines with Imiquimod leads to increased NER gene expression and nuclear localization of the repair molecule, XPA.
While TLR4 was first recognized as a receptor for lipopolysaccharide from the cell wall of Gram-negative bacteria, various non-microbial agonists such as taxol, fibronectin, and heat shock proteins can bind and signal through TLR4.130, 131, 132, 133 Depending on the type of tumor, polymorphisms in the TLR4 allele have been associated with both susceptibility and resistance to cancer.134, 135, 136, 137 Lewis et al had suggested that TLR4 is required for UV-induced immune suppression, showing that TLR4−/− mice, when compared with TLR4+/+ mice, are resistant to UVB-induced immunosuppression.138 To further substantiate the basis of this finding, Ahmad et al investigated whether DNA damage repair mechanism was involved. Specifically, XPA mRNA levels were significantly increased and less DNA damage measured as CPD+ cells was evident in TLR4-deficient mice after acute UVB exposure compared to wild-type mice.139 Moreover, increased IL-12 and IL-23 production was observed in bone marrow-derived dendritic cells (BMDC) of UVB-exposed TLR4-deficient mice compared to wild-type mice. Pre-UVB treatment of BMDCs with anti-IL-12 and anti-IL-23 antibodies inhibited repair of CPDs, with a concomitant decrease in XPA expression. Thus, inhibiting TLR4 may be an approach to managing UVB-induced cutaneous DNA damage and skin cancer.
A recent study revealed for the first time that a role for TLR2 in mediating UVB-induced responses. When TLR2-deficient mice were treated with repetitive UVB radiation, they demonstrated less severe inflammatory responses compared to the wild-type mice. UVB-induced expression of heat-shock protein 70, an endogenous ligand of TLR2, as well as UVB-induced IL-1β, IL6, and MMP-13 were lower in TLR2-deficient mice.140 Further investigation is warranted to delineate the specific mechanism underlying this finding.
TLR3 senses self-RNA released from necrotic keratinocytes following UV damage, and its activation in the skin induces expression of the pro-inflammatory cytokines TNF-α and IL-6.141 Bernard et al demonstrated that UV radiation damages self noncoding RNA (U1 RNA), which is detected by TLR3. Moreover, TLR3 was necessary for UVB radiation-induced immune suppression using TLR3-deficient mice.142 This study provides a novel framework to understand the UVB damage response, revealing the potential role for RNAs as biomarkers of UVB-induced injury. This study can serve as a platform to establish the role of TLR3 in UVB-induced skin carcinogenesis.
ERBB2
UV radiation activates epidermal growth factors receptor (EGFR) family members, including Erbb2 (human epithelial growth factor receptor 2 (HER2)/neu). Erbb2 is a proto-oncogene that is activated in many types of cancer and is associated with aggressive and therapy-resistant disease. Erbb2 is expressed in both follicular and epidermal keratinocytes within the skin and plays many important functions, including regulating inflammation, angiogenesis, cell division, apoptosis, cell adhesion, and migration following UV irradiation143 (Fig. 2). Inhibition or knockdown of Erbb2 suppresses UV-induced cell proliferation, cell survival, and inflammation. In addition, Erbb2 was necessary for the expression of NF-κB-regulated proinflammatory genes, including IL-1β and COX-2. More recently, Rao et al demonstrated that Erbb2 accelerates skin cancer progression via upregulation of ADAM12 (A Disintegrin And Metalloproteinase 12), suggesting a novel mechanism by which Erbb2 contributes to the malignant progression of UV-induced skin cancer. Inhibition of Erbb2 reduced tumor burden, increased tumor regression, and delayed the progression of malignant SCC in UV-exposed mice.144
Cannabinoid receptor
Manipulation of the cannabinoid receptors has been useful in pain management and treatment of cancer, and two cannabinoid receptors from mammalian tissues have been cloned and characterized.145, 146 Cannabinoid receptor 1 (CB1) is highly expressed in the brain, whereas the CB2 receptor is found mainly in the immune system. Until recently, the function of CB1 and CB2 in cancer has remained controversial. A study by Zheng et al demonstrated that UV radiation directly activates CB1 and CB2 and that mice lacking both CB1 and CB2 are resistant to UV-induced skin inflammation (Fig. 2). UVB induced CB1/2 phosphorylation and internalization, activating TNF-α-mediated NF-κB activation. This was mediated by the activation of ERK and JNK by UV radiation, which was markedly reduced in CB1/2−/− mouse embryonic fibroblasts (MEF). Using the two-stage skin carcinogenesis mouse model in which skin papilloma are induced by 7,12-dimethyl benz(a)anthracene (DMBA) and UVB radiation, CB1/2−/− mice developed smaller and less number of skin papilloma compared to wild-type mice. This is the first study to show that the CB1/2 receptors may enhance the effect of DMBA/UVB-induced skin tumorigenesis, demonstrating a novel function of these receptors in UV-induced inflammation and cancer development.147
Conclusion and perspective
Excess exposure to solar UVB radiation is a key risk factor for skin cancer, resulting in DNA damage and inflammation. Although skin carcinogenesis by DNA damage is considered a predominant paradigm of UV toxicity, the role of inflammation in UV-induced carcinogenesis has become an established principle. In light of this, herein we highlight the recent advances in two of these key molecular processes that result in UV-mediated skin carcinogenesis: DNA damage repair and inflammation. Understanding these mechanisms will contribute to the development of more effective chemopreventive tools in the control of UV-induced skin cancer.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgment
We apologize to those investigators whose work could not be directly referenced owing to space limitations. Work in the authors' laboratory was supported by NIH/NIEHS grants ES016936 (YYH), the American Cancer Society (ACS) grant RSG-13-078-01 (YYH), the University of Chicago Cancer Research Center (P30 CA014599), the CTSA (NIH UL1RR024999), and the University of Chicago Friends of Dermatology Endowment Fund.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Linos E., Swetter S.M., Cockburn M.G., Colditz G.A., Clarke C.A. Increasing burden of melanoma in the United States. J Invest Dermatol. Jul 2009;129(7):1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pleasance E.D., Cheetham R.K., Stephens P.J. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. Jan 14 2010;463(7278):191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers H.W., Weinstock M.A., Harris A.R. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. Mar 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 4.Chen J.G., Fleischer A.B., Jr., Smith E.D. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg. Dec 2001;27(12):1035–1038. doi: 10.1046/j.1524-4725.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 5.Christenson L.J., Borrowman T.A., Vachon C.M. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. Aug 10 2005;294(6):681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 6.Thompson S.C., Jolley D., Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med. Oct 14 1993;329(16):1147–1151. doi: 10.1056/NEJM199310143291602. [DOI] [PubMed] [Google Scholar]
- 7.Green A., Williams G., Neale R. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. Aug 28 1999;354(9180):723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- 8.Harvey I., Frankel S., Marks R., Shalom D., Nolan-Farrell M. Non-melanoma skin cancer and solar keratoses II analytical results of the South Wales Skin Cancer Study. Br J Cancer. Oct 1996;74(8):1308–1312. doi: 10.1038/bjc.1996.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naylor M.F., Boyd A., Smith D.W., Cameron G.S., Hubbard D., Neldner K.H. High sun protection factor sunscreens in the suppression of actinic neoplasia. Arch Dermatol. Feb 1995;131(2):170–175. [PubMed] [Google Scholar]
- 10.Setlow R.B. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci USA. Sep 1974;71(9):3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brash D.E. Sunlight and the onset of skin cancer. Trends Genet. Oct 1997;13(10):410–414. doi: 10.1016/s0168-9525(97)01246-8. [DOI] [PubMed] [Google Scholar]
- 12.Brash D.E., Rudolph J.A., Simon J.A. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. Nov 15 1991;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeijmakers J.H. Genome maintenance mechanisms for preventing cancer. Nature. May 17 2001;411(6835):366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 14.Friedberg E.C. DNA damage and repair. Nature. Jan 23 2003;421(6921):436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 15.Cline S.D., Hanawalt P.C. Who's on first in the cellular response to DNA damage? Nat Rev Mol Cell Biol. May 2003;4(5):361–372. doi: 10.1038/nrm1101. [DOI] [PubMed] [Google Scholar]
- 16.Branzei D., Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. Apr 2008;9(4):297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 17.Kraemer K.H., Lee M.M., Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. Apr 1984;5(4):511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer K.H., Lee M.M., Andrews A.D., Lambert W.C. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. Aug 1994;130(8):1018–1021. [PubMed] [Google Scholar]
- 19.Sugasawa K., Ng J.M., Masutani C. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell. Aug 1998;2(2):223–232. doi: 10.1016/s1097-2765(00)80132-x. [DOI] [PubMed] [Google Scholar]
- 20.Sugasawa K. UV-induced ubiquitylation of XPC complex, the UV-DDB-ubiquitin ligase complex, and DNA repair. J Mol Histol. Sep 2006;37(5-7):189–202. doi: 10.1007/s10735-006-9044-7. [DOI] [PubMed] [Google Scholar]
- 21.Cleaver J.E. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nat Rev Cancer. Jul 2005;5(7):564–573. doi: 10.1038/nrc1652. [DOI] [PubMed] [Google Scholar]
- 22.Cleaver J.E., Lam E.T., Revet I. Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nat Rev Genet. Nov 2009;10(11):756–768. doi: 10.1038/nrg2663. [DOI] [PubMed] [Google Scholar]
- 23.DiGiovanna J.J., Kraemer K.H. Shining a light on xeroderma pigmentosum. J Invest Dermatol. Mar 2012;132(3 Pt 2):785–796. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford P.T., Goldstein A.M., Tamura D. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. Mar 2011;48(3):168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Feraudy S., Ridd K., Richards L.M. The DNA damage-binding protein XPC is a frequent target for inactivation in squamous cell carcinomas. Am J Pathol. Aug 2010;177(2):555–562. doi: 10.2353/ajpath.2010.090925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugasawa K. Xeroderma pigmentosum genes: functions inside and outside DNA repair. Carcinogenesis. Mar 2008;29(3):455–465. doi: 10.1093/carcin/bgm282. [DOI] [PubMed] [Google Scholar]
- 27.Berg R.J., Ruven H.J., Sands A.T., de Gruijl F.R., Mullenders L.H. Defective global genome repair in XPC mice is associated with skin cancer susceptibility but not with sensitivity to UVB induced erythema and edema. J Invest Dermatol. Apr 1998;110(4):405–409. doi: 10.1111/j.1523-1747.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- 28.Adimoolam S., Ford J.M. p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc Natl Acad Sci USA. Oct 1 2002;99(20):12985–12990. doi: 10.1073/pnas.202485699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominguez-Brauer C., Chen Y.J., Brauer P.M., Pimkina J., Raychaudhuri P. ARF stimulates XPC to trigger nucleotide excision repair by regulating the repressor complex of E2F4. EMBO Rep. Sep 2009;10(9):1036–1042. doi: 10.1038/embor.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen W.H., Balajee A.S., Wang J. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. Jan 12 2007;128(1):157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 31.Gupta A., Yang Q., Pandita R.K. Cell cycle checkpoint defects contribute to genomic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle. Jul 15 2009;8(14):2198–2210. doi: 10.4161/cc.8.14.8947. [DOI] [PubMed] [Google Scholar]
- 32.Ming M., Feng L., Shea C.R. PTEN positively regulates UVB-induced DNA damage repair. Cancer Res. Aug 1 2011;71(15):5287–5295. doi: 10.1158/0008-5472.CAN-10-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan J.Y., Han X.L., Kan Y.W. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc Natl Acad Sci USA. Dec 1 1993;90(23):11371–11375. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan J.Y., Han X.L., Kan Y.W. Isolation of cDNA encoding the human NF-E2 protein. Proc Natl Acad Sci USA. Dec 1 1993;90(23):11366–11370. doi: 10.1073/pnas.90.23.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews N.C., Erdjument-Bromage H., Davidson M.B., Tempst P., Orkin S.H. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. Apr 22 1993;362(6422):722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- 36.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. Dec 10 1996;93(25):14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L., Kwong M., Lu R. Nrf1 is critical for redox balance and survival of liver cells during development. Mol Cell Biol. Jul 2003;23(13):4673–4686. doi: 10.1128/MCB.23.13.4673-4686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han W., Ming M., Zhao R., Pi J., Wu C., He Y.Y. Nrf1 CNC-bZIP protein promotes cell survival and nucleotide excision repair through maintaining glutathione homeostasis. J Biol Chem. May 25 2012;287(22):18788–18795. doi: 10.1074/jbc.M112.363614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jans J., Schul W., Sert Y.G. Powerful skin cancer protection by a CPD-photolyase transgene. Curr Biol. Jan 26 2005;15(2):105–115. doi: 10.1016/j.cub.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Dali-Youcef N., Lagouge M., Froelich S., Koehl C., Schoonjans K., Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39(5):335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 41.Choi J.E., Mostoslavsky R. Sirtuins, metabolism, and DNA repair. Curr Opin Genet Dev. Jul 5 2014;26C:24–32. doi: 10.1016/j.gde.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao C., Lu S., Kivlin R. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. Sep 2009;13(9B):3632–3643. doi: 10.1111/j.1582-4934.2008.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Chen J. SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. J Biol Chem. Apr 9 2010;285(15):11458–11464. doi: 10.1074/jbc.M109.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hobbs C.A., Wei G., DeFeo K., Paul B., Hayes C.S., Gilmour S.K. Tip60 protein isoforms and altered function in skin and tumors that overexpress ornithine decarboxylase. Cancer Res. Aug 15 2006;66(16):8116–8122. doi: 10.1158/0008-5472.CAN-06-0359. [DOI] [PubMed] [Google Scholar]
- 45.Ming M., Shea C.R., Guo X. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci USA. Dec 28 2010;107(52):22623–22628. doi: 10.1073/pnas.1010377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan W., Luo J. SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol Cell. Jul 30 2010;39(2):247–258. doi: 10.1016/j.molcel.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Ikenoue T., Inoki K., Zhao B., Guan K.L. PTEN acetylation modulates its interaction with PDZ domain. Cancer Res. Sep 1 2008;68(17):6908–6912. doi: 10.1158/0008-5472.CAN-08-1107. [DOI] [PubMed] [Google Scholar]
- 48.Haigis M.C., Sinclair D.A. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks C.L., Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. Feb 2009;9(2):123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng C.X. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5(2):147–152. doi: 10.7150/ijbs.5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boily G., He X.H., Pearce B., Jardine K., McBurney M.W. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. Aug 13 2009;28(32):2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- 52.Ming M., Soltani K., Shea C.R., Li X., He Y.Y. Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene. Jan 20 2014 doi: 10.1038/onc.2013.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aziz M.H., Reagan-Shaw S., Wu J., Longley B.J., Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. Jul 2005;19(9):1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 54.Sanli T., Steinberg G.R., Singh G., Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther. Feb 2014;15(2):156–169. doi: 10.4161/cbt.26726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu C.L., Qiang L., Han W., Ming M., Viollet B., He Y.Y. Role of AMPK in UVB-induced DNA damage repair and growth control. Oncogene. May 23 2013;32(21):2682–2689. doi: 10.1038/onc.2012.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takao M., Abramic M., Moos M., Jr. A 127 kDa component of a UV-damaged DNA-binding complex, which is defective in some xeroderma pigmentosum group E patients, is homologous to a slime mold protein. Nucleic Acids Res. Aug 25 1993;21(17):4111–4118. doi: 10.1093/nar/21.17.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dualan R., Brody T., Keeney S., Nichols A.F., Admon A., Linn S. Chromosomal localization and cDNA cloning of the genes (DDB1 and DDB2) for the p127 and p48 subunits of a human damage-specific DNA binding protein. Genomics. Sep 1 1995;29(1):62–69. doi: 10.1006/geno.1995.1215. [DOI] [PubMed] [Google Scholar]
- 58.Hwang B.J., Toering S., Francke U., Chu G. p48 Activates a UV-damaged-DNA binding factor and is defective in xeroderma pigmentosum group E cells that lack binding activity. Mol Cell Biol. Jul 1998;18(7):4391–4399. doi: 10.1128/mcb.18.7.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. Oct 28 1988;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- 60.Keeney S., Chang G.J., Linn S. Characterization of a human DNA damage binding protein implicated in xeroderma pigmentosum E. J Biol Chem. Oct 5 1993;268(28):21293–21300. [PubMed] [Google Scholar]
- 61.Sugasawa K., Okuda Y., Saijo M. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell. May 6 2005;121(3):387–400. doi: 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q.E., Zhu Q., Wani G., El-Mahdy M.A., Li J., Wani A.A. DNA repair factor XPC is modified by SUMO-1 and ubiquitin following UV irradiation. Nucleic Acids Res. 2005;33(13):4023–4034. doi: 10.1093/nar/gki684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J., Wang Q.E., Zhu Q. DNA damage binding protein component DDB1 participates in nucleotide excision repair through DDB2 DNA-binding and cullin 4A ubiquitin ligase activity. Cancer Res. Sep 1 2006;66(17):8590–8597. doi: 10.1158/0008-5472.CAN-06-1115. [DOI] [PubMed] [Google Scholar]
- 64.Wang Q.E., Praetorius-Ibba M., Zhu Q. Ubiquitylation-independent degradation of Xeroderma pigmentosum group C protein is required for efficient nucleotide excision repair. Nucleic Acids Res. 2007;35(16):5338–5350. doi: 10.1093/nar/gkm550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeNardo D.G., Andreu P., Coussens L.M. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. Jun 2010;29(2):309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. Mar 19 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grivennikov S.I., Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. Feb 2010;20(1):65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. Apr 2 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. Jul 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 70.Rivas J.M., Ullrich S.E. The role of IL-4, IL-10, and TNF-alpha in the immune suppression induced by ultraviolet radiation. J Leukoc Biol. Dec 1994;56(6):769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 71.Mukhtar H., Elmets C.A. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. Apr 1996;63(4):356–357. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 72.Katiyar S.K., Meeran S.M. Obesity increases the risk of UV radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling. Free Radic Biol Med. Jan 15 2007;42(2):299–310. doi: 10.1016/j.freeradbiomed.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fischer S.M., Lo H.H., Gordon G.B. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. Aug 1999;25(4):231–240. [PubMed] [Google Scholar]
- 74.Pentland A.P., Schoggins J.W., Scott G.A., Khan K.N., Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. Oct 1999;20(10):1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 75.Schafer M., Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. Aug 2008;9(8):628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 76.Wilgus T.A., Koki A.T., Zweifel B.S., Kusewitt D.F., Rubal P.A., Oberyszyn T.M. Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Mol Carcinog. Oct 2003;38(2):49–58. doi: 10.1002/mc.10141. [DOI] [PubMed] [Google Scholar]
- 77.Gilmore T.D. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. Oct 30 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 78.Bargou R.C., Emmerich F., Krappmann D. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest. Dec 15 1997;100(12):2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duffey D.C., Chen Z., Dong G. Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res. Jul 15 1999;59(14):3468–3474. [PubMed] [Google Scholar]
- 80.Poppelmann B., Klimmek K., Strozyk E., Voss R., Schwarz T., Kulms D. NF{kappa}B-dependent down-regulation of tumor necrosis factor receptor-associated proteins contributes to interleukin-1-mediated enhancement of ultraviolet B-induced apoptosis. J Biol Chem. Apr 22 2005;280(16):15635–15643. doi: 10.1074/jbc.M413006200. [DOI] [PubMed] [Google Scholar]
- 81.Syed D.N., Afaq F., Mukhtar H. Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem Photobiol. Sep-Oct 2012;88(5):1184–1190. doi: 10.1111/j.1751-1097.2012.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu C.S., Lan C.C., Kuo H.Y., Chai C.Y., Chen W.T., Chen G.S. Differential regulation of nuclear factor-kappa B subunits on epidermal keratinocytes by ultraviolet B and tacrolimus. Kaohsiung J Med Sci. Nov 2012;28(11):577–585. doi: 10.1016/j.kjms.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 83.Lu W., Laszlo C.F., Miao Z., Chen H., Wu S. The role of nitric-oxide synthase in the regulation of UVB light-induced phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Biol Chem. Sep 4 2009;284(36):24281–24288. doi: 10.1074/jbc.M109.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tong L., Wu S. The role of constitutive nitric oxide synthase in ultraviolet B light-induced nuclear factor kappa B activity. J Biol Chem. Aug 11 2014 doi: 10.1074/jbc.M114.600023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Afaq F., Adhami V.M., Ahmad N., Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea constituent (-)-epigallocatechin-3-gallate. Oncogene. Feb 20 2003;22(7):1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 86.Xia J., Song X., Bi Z., Chu W., Wan Y. UV-induced NF-kappaB activation and expression of IL-6 is attenuated by (-)-epigallocatechin-3-gallate in cultured human keratinocytes in vitro. Int J Mol Med. Nov 2005;16:943–950. [PubMed] [Google Scholar]
- 87.Ahmad N., Gupta S., Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys. Apr 15 2000;376(2):338–346. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 88.Walsh L.J. Ultraviolet B irradiation of skin induces mast cell degranulation and release of tumour necrosis factor-alpha. Immunol Cell Biol. Jun 1995;73(3):226–233. doi: 10.1038/icb.1995.37. [DOI] [PubMed] [Google Scholar]
- 89.Chen G., Goeddel D.V. TNF-R1 signaling: a beautiful pathway. Science. May 31 2002;296(5573):1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 90.Vincek V., Kurimoto I., Medema J.P., Prieto E., Streilein J.W. Tumor necrosis factor alpha polymorphism correlates with deleterious effects of ultraviolet B light on cutaneous immunity. Cancer Res. Feb 15 1993;53(4):728–732. [PubMed] [Google Scholar]
- 91.Buckman S.Y., Gresham A., Hale P. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. May 1998;19(5):723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 92.Athar M., An K.P., Morel K.D. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. Feb 2 2001;280(4):1042–1047. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- 93.Tiano H.F., Loftin C.D., Akunda J. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. Jun 15 2002;62(12):3395–3401. [PubMed] [Google Scholar]
- 94.Rundhaug J.E., Mikulec C., Pavone A., Fischer S.M. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol Carcinog. Aug 2007;46(8):692–698. doi: 10.1002/mc.20329. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J., Bowden G.T. UVB irradiation regulates Cox-2 mRNA stability through AMPK and HuR in human keratinocytes. Mol Carcinog. Dec 2008;47(12):974–983. doi: 10.1002/mc.20450. [DOI] [PubMed] [Google Scholar]
- 96.Bowden G.T. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. Jan 2004;4(1):23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 97.Ming M., Han W., Zhao B. SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-14-1308. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu K., Yu D., Cho Y.Y. Sunlight UV-induced skin cancer relies upon activation of the p38alpha signaling pathway. Cancer Res. Apr 1 2013;73(7):2181–2188. doi: 10.1158/0008-5472.CAN-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Segrelles C., Ruiz S., Perez P. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. Jan 3 2002;21(1):53–64. doi: 10.1038/sj.onc.1205032. [DOI] [PubMed] [Google Scholar]
- 100.Segrelles C., Lu J., Hammann B. Deregulated activity of Akt in epithelial basal cells induces spontaneous tumors and heightened sensitivity to skin carcinogenesis. Cancer Res. Nov 15 2007;67(22):10879–10888. doi: 10.1158/0008-5472.CAN-07-2564. [DOI] [PubMed] [Google Scholar]
- 101.Han W., Soltani K., Ming M., He Y.Y. Deregulation of XPC and CypA by cyclosporin A: an immunosuppression-independent mechanism of skin carcinogenesis. Cancer Prev Res. Sep 2012;5(9):1155–1162. doi: 10.1158/1940-6207.CAPR-12-0185-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Checkley L.A., Rho O., Angel J.M. Metformin inhibits skin tumor promotion in overweight and obese mice. Cancer Prev Res. Jan 2014;7(1):54–64. doi: 10.1158/1940-6207.CAPR-13-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilgus T.A., Koki A.T., Zweifel B.S., Rubal P.A., Oberyszyn T.M. Chemotherapeutic efficacy of topical celecoxib in a murine model of ultraviolet light B-induced skin cancer. Mol Carcinog. Sep 2003;38(1):33–39. doi: 10.1002/mc.10142. [DOI] [PubMed] [Google Scholar]
- 104.Mikulec C.D., Rundhaug J.E., Simper M.S., Lubet R.A., Fischer S.M. The chemopreventive efficacies of nonsteroidal anti-inflammatory drugs: the relationship of short-term biomarkers to long-term skin tumor outcome. Cancer Prev Res. Jul 2013;6(7):675–685. doi: 10.1158/1940-6207.CAPR-13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharma S.D., Meeran S.M., Katiyar S.K. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E(2) and prostaglandin E(2) receptors. Mol Cancer Ther. Mar 2010;9(3):569–580. doi: 10.1158/1535-7163.MCT-09-0638. [DOI] [PubMed] [Google Scholar]
- 106.Butler G.J., Neale R., Green A.C., Pandeya N., Whiteman D.C. Nonsteroidal anti-inflammatory drugs and the risk of actinic keratoses and squamous cell cancers of the skin. J Am Acad Dermatol. Dec 2005;53(6):966–972. doi: 10.1016/j.jaad.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 107.Johannesdottir S.A., Chang E.T., Mehnert F., Schmidt M., Olesen A.B., Sorensen H.T. Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study. Cancer. Oct 1 2012;118(19):4768–4776. doi: 10.1002/cncr.27406. [DOI] [PubMed] [Google Scholar]
- 108.Brunda M.J., Luistro L., Warrier R.R. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. Oct 1 1993;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Robertson M.J., Ritz J. Interleukin 12: basic biology and potential applications in cancer treatment. Oncologist. 1996;1(1 & 2):88–97. [PubMed] [Google Scholar]
- 110.Meeran S.M., Katiyar S., Elmets C.A., Katiyar S.K. Interleukin-12 deficiency is permissive for angiogenesis in UV radiation-induced skin tumors. Cancer Res. Apr 15 2007;67(8):3785–3793. doi: 10.1158/0008-5472.CAN-06-3134. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Maeda A., Schneider S.W., Kojima M., Beissert S., Schwarz T., Schwarz A. Enhanced photocarcinogenesis in interleukin-12-deficient mice. Cancer Res. Mar 15 2006;66(6):2962–2969. doi: 10.1158/0008-5472.CAN-05-3614. [DOI] [PubMed] [Google Scholar]
- 112.Katiyar S.K. Interleukin-12 and photocarcinogenesis. Toxicol Appl Pharmacol. Nov 1 2007;224(3):220–227. doi: 10.1016/j.taap.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwarz A., Stander S., Berneburg M. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol. Jan 2002;4(1):26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 114.Meeran S.M., Mantena S.K., Meleth S., Elmets C.A., Katiyar S.K. Interleukin-12-deficient mice are at greater risk of UV radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol Cancer Ther. Apr 2006;5(4):825–832. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- 115.Meeran S.M., Katiyar N., Singh T., Katiyar S.K. Loss of endogenous interleukin-12 activates survival signals in ultraviolet-exposed mouse skin and skin tumors. Neoplasia. Sep 2009;11(9):846–855. doi: 10.1593/neo.09528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meeran S.M., Punathil T., Katiyar S.K. IL-12 deficiency exacerbates inflammatory responses in UV-irradiated skin and skin tumors. J Invest Dermatol. Nov 2008;128(11):2716–2727. doi: 10.1038/jid.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mazarati A., Maroso M., Iori V., Vezzani A., Carli M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and receptor for advanced glycation end products. Exp Neurol. Dec 2011;232(2):143–148. doi: 10.1016/j.expneurol.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson K.E., Wulff B.C., Oberyszyn T.M., Wilgus T.A. Ultraviolet light exposure stimulates HMGB1 release by keratinocytes. Arch Dermatol Res. Nov 2013;305(9):805–815. doi: 10.1007/s00403-013-1401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bald T., Quast T., Landsberg J. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. Mar 6 2014;507(7490):109–113. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 120.Harberts E., Gaspari A.A. TLR signaling and DNA repair: are they associated? J Invest Dermatol. Feb 2013;133(2):296–302. doi: 10.1038/jid.2012.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng L., Asprodites N., Keene A.H., Rodriguez P., Brown K.D., Davila E. TLR9 engagement on CD4 T lymphocytes represses gamma-radiation-induced apoptosis through activation of checkpoint kinase response elements. Blood. Mar 1 2008;111(5):2704–2713. doi: 10.1182/blood-2007-07-104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klaschik S., Tross D., Shirota H., Klinman D.M. Short- and long-term changes in gene expression mediated by the activation of TLR9. Mol Immunol. Mar 2010;47(6):1317–1324. doi: 10.1016/j.molimm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sommariva M., De Cecco L., De Cesare M. TLR9 agonists oppositely modulate DNA repair genes in tumor versus immune cells and enhance chemotherapy effects. Cancer Res. Oct 15 2011;71(20):6382–6390. doi: 10.1158/0008-5472.CAN-11-1285. [DOI] [PubMed] [Google Scholar]
- 124.Larange A., Antonios D., Pallardy M., Kerdine-Romer S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J Leukoc Biol. Apr 2009;85(4):673–683. doi: 10.1189/jlb.0808504. [DOI] [PubMed] [Google Scholar]
- 125.Edwards L., Ferenczy A., Eron L. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group. Human PapillomaVirus. Arch Dermatol. Jan 1998;134(1):25–30. doi: 10.1001/archderm.134.1.25. [DOI] [PubMed] [Google Scholar]
- 126.Oster-Schmidt C. Two cases of squamous cell carcinoma treated with topical imiquimod 5% J Eur Acad Dermatol Venereol. Jan 2004;18(1):93–95. doi: 10.1111/j.1468-3083.2004.00852.x. [DOI] [PubMed] [Google Scholar]
- 127.Hanke C.W., Beer K.R., Stockfleth E., Wu J., Rosen T., Levy S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 3-week cycles. J Am Acad Dermatol. Apr 2010;62(4):573–581. doi: 10.1016/j.jaad.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 128.Swanson N., Abramovits W., Berman B., Kulp J., Rigel D.S., Levy S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. Apr 2010;62(4):582–590. doi: 10.1016/j.jaad.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 129.Fishelevich R., Zhao Y., Tuchinda P. Imiquimod-induced TLR7 signaling enhances repair of DNA damage induced by ultraviolet light in bone marrow-derived cells. J Immunol. Aug 15 2011;187(4):1664–1673. doi: 10.4049/jimmunol.1100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Byrd-Leifer C.A., Block E.F., Takeda K., Akira S., Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. Aug 2001;31(8):2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 131.Okamura Y., Watari M., Jerud E.S. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. Mar 30 2001;276(13):10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 132.Termeer C., Benedix F., Sleeman J. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. Jan 7 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ohashi K., Burkart V., Flohe S., Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. Jan 15 2000;164(2):558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 134.Schroder N.W., Schumann R.R. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect Dis. Mar 2005;5(3):156–164. doi: 10.1016/S1473-3099(05)01308-3. [DOI] [PubMed] [Google Scholar]
- 135.Apetoh L., Ghiringhelli F., Tesniere A. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. Sep 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 136.Hold G.L., Rabkin C.S., Chow W.H. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. Mar 2007;132(3):905–912. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 137.Szczepanski M.J., Czystowska M., Szajnik M. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. Apr 1 2009;69(7):3105–3113. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lewis W., Simanyi E., Li H. Regulation of ultraviolet radiation induced cutaneous photoimmunosuppression by toll-like receptor-4. Arch Biochem Biophys. Apr 15 2011;508(2):171–177. doi: 10.1016/j.abb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ahmad I., Simanyi E., Guroji P. Toll-like receptor-4 deficiency enhances repair of UVR-induced cutaneous DNA damage by nucleotide excision repair mechanism. J Invest Dermatol. Jun 2014;134(6):1710–1717. doi: 10.1038/jid.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park H.S., Jin S.P., Lee Y. Toll-like receptor 2 mediates a cutaneous reaction induced by repetitive ultraviolet B irradiation in C57/BL6 mice in vivo. Exp Dermatol. Aug 2014;23(8):591–595. doi: 10.1111/exd.12477. [DOI] [PubMed] [Google Scholar]
- 141.Borkowski A.W., Gallo R.L. UVB radiation illuminates the role of TLR3 in the epidermis. J Invest Dermatol. May 1 2014 doi: 10.1038/jid.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bernard J.J., Cowing-Zitron C., Nakatsuji T. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. Aug 2012;18(8):1286–1290. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Madson J.G., Hansen L.A. Multiple mechanisms of Erbb2 action after ultraviolet irradiation of the skin. Mol Carcinog. Aug 2007;46(8):624–628. doi: 10.1002/mc.20335. [DOI] [PubMed] [Google Scholar]
- 144.Rao V.H., Vogel K., Yanagida J.K. Erbb2 up-regulation of ADAM12 expression accelerates skin cancer progression. Mol Carcinog. May 5 2014 doi: 10.1002/mc.22171. [DOI] [PubMed] [Google Scholar]
- 145.Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. Aug 9 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 146.Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. Sep 2 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 147.Zheng D., Bode A.M., Zhao Q. The cannabinoid receptors are required for ultraviolet-induced inflammation and skin cancer development. Cancer Res. May 15 2008;68(10):3992–3998. doi: 10.1158/0008-5472.CAN-07-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]