Abstract
Importance
Both dietary modification and statins can lower blood cholesterol. The increase in caloric intake among general population is reported to have plateaued in the last decade, but no study has examined the relationship between the time trends of caloric intake and statin use.
Objective
To examine the difference in the temporal trends of caloric and fat intake between statin-users and non-users among US adults.
Design
A repeated cross-sectional study.
Setting
A nationally representative sample of the National Health and Nutrition Examination Survey from 1999 through 2010.
Participants
27,886 US adults aged 20 years or older.
Exposure
Statin use.
Main Outcomes and Measures
Caloric and fat intake measured through 24-hour dietary recall. Generalized linear models with interaction term between survey cycle and statin use were constructed to investigate the time trends of dietary intake for statin-users and non-users after adjustment for possible confounders. We calculated model-adjusted caloric and fat intake using these models, and examined if the time trends differ by statin use. Body mass index (BMI) changes were also compared between statin-users and non-users.
Results
In 1999-2000, the caloric intake was significantly less for statin-users compared with non-users (2,000 vs. 2,179 kcal/day, p=.007). The difference between the groups became smaller as time went by, and there was no statistical difference after 2005-2006. Among statin-users, caloric intake in 2009-2010 was 9.6% higher (95% confidence interval (CI): 1.8 to 18.1, p=.02) than that in 1999-2000. In contrast, no significant change was observed among non-users during the same study period. Statin-users also consumed significantly less fat in 1999-2000 (71.7 vs. 81.2 g/day, p=.003). Fat intake increased 14.4% in statin-users (95% CI: 3.8 to 26.1, p=.007) while not changing significantly in non-users. BMI also increased more in statin-users (1.3 kg/m2) than non-users (0.4 kg/m2) in the adjusted model (p=.02).
Conclusions and Relevance
Caloric and fat intake have increased among statin-users over time, which was not true for non-users. The increase of BMI was faster for statin-users than for non-users. Efforts aimed at dietary control among statin-users may be becoming less intensive. The importance of dietary composition may need to be reemphasized for statin-users.
INTRODUCTION
The National Cholesterol Education Program Adult Treatment Panel guideline,1-4 which was updated by 2013 ACC/AHA guideline recently,5 has consistently recommended dietary modification as a key component of antihyperlipidemic therapy. Since 2001, these guidelines also have stated that statins are more effective than other pharmacotherapies.3 Statin use has grown rapidly in the US over the past 25 years, 6,7 while caloric intake has increased overall in US adults from the 1970s to the 1990s,8 with a plateau starting in 1999-2000.9 The proportion of calories from fat in US adults decreased from the 1970s to the 1990s,8 followed by a stable trend since 1999-2000.9 No studies have examined whether the temporal trend in food intake is related to statin use, although previous studies have investigated the cross-sectional and short-term relationship between statin use and food intake.10-12 In this context, we examined whether the time trends of caloric and fat intake differ between statin-users and non-users.
METHODS
Data Sources and Study Population
This is a repeated cross-sectional study using the National Health and Nutrition Examination Survey (NHANES) data from 1999 through 2010. NHANES is conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention. NHANES uses a stratified, multistage probability sampling design, which enables samples to represent the US civilian noninstitutionalized population.13 Data are collected at their homes and mobile examination centers (MECs). Among adults in NHANES 1999-2010, the unweighted response rate for the household interview was 74.8%; that for the MEC examination was 70.8%.14
This study included data from individuals aged 20 years or older. Since pregnancy is a contraindication to statin use, we excluded pregnant women from our analyses (n = 1294), which resulted in a sample of 31,170. In the main analysis, we also excluded those with missing information on in-person dietary interview (n = 3,210), statin use (n = 13), and potential confounders of our analyses (n = 61), which produced a final sample of 27,886. Written informed consent was obtained from all participants. The NCHS Research Ethics Review Board approved the NHANES protocols.15
Food Intake
During the MEC examination, trained interviewers conducted a 24-hour dietary recall interview and obtained dietary data on the last day before the interview. For the 1999-2001 survey periods, dietary interviews were conducted using a computer-assisted automated data collection system with a multiple pass format.16 Beginning in 2002, the NHANES dietary interview began to use the US Department of Agriculture (USDA) dietary data collection instrument, the Automated Multiple-Pass Method.17 The individual foods and beverages reported in the dietary interview were assigned to USDA food codes (USDA Survey Nutrient Database for NHANES 1999-2000, USDA’s Food and Nutrient Database For Dietary Studies for NHANES 2001-201018), and their nutrient components were analyzed. For this study, we extracted data on total caloric intake and total fat intake as the primary outcome variables. We also extracted data on saturated fat intake and dietary cholesterol intake for additional analyses.
Cholesterol Levels and Body Mass Index
As secondary outcome variables, we extracted data on serum levels of total cholesterol and LDL-C and body mass index (BMI). Blood specimens were collected during the MEC examination. LDL-C level was calculated using the Friedewald equation19 (total cholesterol – high-density lipoprotein cholesterol –triglyceride/5) for participants examined in the morning in their fasting states with triglyceride levels of 400 mg/dl or less. Sample size was reduced by 54% when we used fasting values, and sampling weight for fasting blood-sampling examinees prepared by NHANES was used to estimate the entire population parameters. Height and weight were measured during the MEC examination. BMI was calculated as kilograms per meter squared.
Hyperlipidemia and Statin Use
We defined hyperlipidemia based either on self-reported diagnosis of hyperlipidemia (diagnosed and reported to the subject by a health professional) or on documentation that the subject was taking medications for hyperlipidemia (statins and others). We did not use the measurements of cholesterol or fat for our definition of hyperlipidemia in this study. That is, we grouped hyperlipidemic individuals who were undiagnosed as not having hyperlipidemia. The rationale was that those who did not know their hyperlipidemia diagnosis would not modify their diets.
Statin use was defined on the basis of interviewer-confirmed medication containers matched to a comprehensive prescription drug database (Lexicon Plus).13 We identified 7 types of statin ingredients prescribed for NHANES participants: lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, cerivastatin, and rosuvastatin. Statin use was defined regardless of whether the statin ingredient came from a separate pill or a fixed-dose combination. We divided participants into statin-users and non-users. Statin non-users included those without hyperlipidemia and those with hyperlipidemia but not receiving statins.
Potential Confounders
We extracted data on potential confounders including age, sex, race and ethnicity, educational attainment, and the diagnosis of diabetes. We categorized age into 20-39 years, 40-59 years, and 60 years and older. Race and ethnicity were classified into non-Hispanic white, non-Hispanic black, Mexican American, and others including other Hispanics and multi-racial participants. We categorized educational attainment into greater than high school, high school graduation or General Education Development (GED), and less than high school. We defined diabetes as either self-reported diagnosis of diabetes or based on use of anti-diabetic medications confirmed by interviewers.
Statistical Analysis
All statistical analyses were conducted using Stata (Version 12.1; StataCorp, College Station, TX), accounting for the complex survey design. Taylor series linearization was used for variance estimation.20 We employed an appropriate weight for each analysis selected based on the variables in the analysis. These weights accounted for unequal probabilities of selection and nonresponses, in order to make unbiased national estimates. To conduct trend analyses, we combined 6 cycles of NHANES data: from 1999-2000 through 2009-2010.21
Proportion of statin use was calculated for each survey cycle. Descriptive statistics for patients’ characteristics were calculated separately for statin-users and non-users. Linear trends over time were assessed based on chi-squared tests for categorical variables and linear regressions for continuous variables. We compared the characteristics between the groups using pooled samples across the study period. We also investigated whether time trends of cholesterol levels and BMI differed by group using models including interaction terms between survey cycle and statin use.
Next, we developed regression models to evaluate temporal time trends of caloric and fat intake separately for stain-users and non-users, and to examine whether trends for caloric and fat intake differ by statin use. We used generalized linear models (GLMs) with log-link function to take into account the right-skewed distributions of the intake. The results of the Park test22 indicated a Gamma distribution as the most appropriate distribution for our data. We included interaction terms between survey cycle (categorical) and statin use (binary), to allow non-linear time trends to be different by statin use. We also included age category, sex, racial and ethnicity, educational attainment, and diabetes diagnosis for adjustment. We applied these models to calculate model-adjusted estimates of caloric and fat intake per day for each combination of statin use and survey cycle, and tested the differences of caloric and fat intake by statin use within each survey cycle. We then calculated separately for statin-users and non-users the adjusted percent changes of caloric and fat intake in each survey cycle setting 1999-2000 as the reference cycle separately for statin-users and non-users. Linear time trend was used to approximate the change over the study period, and the significance tests of the interaction term between survey cycle (continuous) and statin use (binary) was performed to examine the difference in trends of intake between statin-users and non-users.
As additional analyses, we divided statin non-users into those with and without a diagnosis of hyperlipidemia, and compared the time trends of three groups. Moreover, we performed additional sensitivity analyses; we made trend graphs of caloric and fat intake estimates restricted to those who did not have diabetes diagnoses.
We also performed additional regression analyses to evaluate the temporal time trends of saturated fat and dietary cholesterol intake separately for stain-users and non-users.
We also created GLMs for BMI, total cholesterol level, and LDL-C level. In the models, we controlled for age category, sex, race and ethnicity, and education attainment. The results of the Park test22 indicated that a Gamma distribution is the most appropriate for BMI, whereas a Poisson distribution was the most suitable for cholesterol levels. We included interaction terms between survey cycle (categorical) and statin use (binary), and we applied these models to calculate model-adjusted estimates of BMI, total cholesterol level, and LDL-C level for each combination of statin use and survey cycle. By creating additional models with a continuous survey cycle variable, we examined the differences in trends by statin use.
RESULTS
The proportion of statin-users from the NHANES 1999-2010 study population in our sample more than doubled from 7.5% to 16.5% over the decade of observation (Table 1, eTable). We divided statin non-users into those with and without hyperlipidemia, and found that the proportion of the population who were non-users without hyperlipidemia decreased from 74.6% to 67.8% (p<.001), whereas the proportion of the population who were non-users with hyperlipidemia did not change significantly (p=.14) (eTable). We found time trends toward a smaller proportion of white race and a larger proportion of black race among statin-users, and a trend toward higher educational attainment among both groups. Diabetes diagnosis became more prevalent among statin-users. Statin-users were more likely to be older, male, white, less educated, have diagnosis of diabetes, and have higher BMI. Between 1999-2000 and 2009-2010, BMI increased by 1.3 kg/m2 among statin-users, compared with 0.5 kg/m2 in non-users (p for difference of trends =.02), while the decrease of total cholesterol between statin-users in 1999-2000 and those surveyed in 2009-10 was greater than that among non-users (from 201.9 to 178.1 mg/dl for statin-users and from 203.6 to 199.6 mg/dl for non-users (p for difference of trends <.001)). Findings were similar for LDL-C.
Table 1.
Characteristics of study samples by survey cycles and statin use, 1999-2010.a
| 1999-2000 | 2001-2002 | 2003-2004 | 2005-2006 | 2007-2008 | 2009-2010 | P for trendb | Total | P for between-group comparisonc | |
|---|---|---|---|---|---|---|---|---|---|
| Statin-Users | |||||||||
|
| |||||||||
| Unweighted sample, N | 374 | 537 | 652 | 717 | 1,105 | 1,158 | |||
|
| |||||||||
| Weighted proportion of statin-users, % | 7.5 | 9.2 | 11.1 | 13.5 | 15.4 | 16.5 | <.001 | ||
|
| |||||||||
| Age Ranged, y, % | |||||||||
| 20–39 | 0.7 | 3.8 | 1.9 | 2.6 | 3.8 | 2.5 | .31 | 2.7 | <.001 |
| 40–59 | 39.8 | 39.5 | 39.6 | 34.8 | 31.9 | 36.8 | .04 | 36.4 | <.001 |
| 60– | 59.6 | 56.7 | 58.5 | 62.7 | 64.3 | 60.7 | .10 | 60.9 | <.001 |
| Female sex, % | 47.8 | 45.5 | 47.5 | 49.0 | 48.5 | 45.8 | .89 | 47.4 | <.001 |
| Racial and ethnicityd, % | |||||||||
| Non-Hispanic white | 83.8 | 86.1 | 83.3 | 80.1 | 79.9 | 78.2 | .03 | 81.1 | <.001 |
| Non-Hispanic black | 4.9 | 6.1 | 7.4 | 10.0 | 8.7 | 9.2 | .02 | 8.2 | <.001 |
| Mexican American | 2.0 | 2.1 | 3.1 | 2.8 | 4.2 | 4.8 | .12 | 3.5 | <.001 |
| Otherse | 9.3 | 5.7 | 6.2 | 7.1 | 7.3 | 7.8 | .81 | 7.2 | <.001 |
| Educational attainment, % | |||||||||
| > High school | 41.6 | 53.0 | 45.3 | 50.2 | 48.9 | 55.2 | .03 | 49.9 | <.001 |
| High school or GEDf | 31.5 | 27.1 | 33.6 | 30.7 | 29.9 | 24.2 | .07 | 29.1 | .002 |
| < High school | 26.9 | 19.9 | 21.1 | 19.1 | 21.2 | 20.6 | .28 | 21.0 | .04 |
| Diabetes diagnosis, % | 21.5 | 23.9 | 27.7 | 29.7 | 29.8 | 29.3 | .009 | 27.9 | <.001 |
| Total cholesterol level, mean (SD), mg/dL | 201.9 (32.1) | 195.8 (35.7) | 191.1 (37.6) | 183.0 (35.1) | 177.2 (36.5) | 178.1 (33.7) | <.001 | 185.1 (36.3) | <.001 |
| LDL-Cg level, mean (SD), mg/dL | 119.3 (32.1) | 112.4 (27.7) | 100.6 (27.6) | 96.7 (30.4) | 96.4 (31.6) | 99.8 (28.7) | <.001 | 101.8 (30.5) | <.001 |
| Body mass index, mean (SD), kg/m2 | 29.2 (4.6) | 29.5 (5.3) | 29.7 (5.2) | 30.5 (5.4) | 30.4 (6.3) | 30.5 (6.0) | <.001 | 30.1 (5.7) | <.001 |
|
| |||||||||
| Statin Non-users | |||||||||
|
| |||||||||
| Unweighted sample, N | 4,220 | 4,552 | 4,154 | 3,926 | 4,768 | 4,991 | |||
|
| |||||||||
| Age Range, y, % | |||||||||
| 20–39 | 43.5 | 40.7 | 42.4 | 42.1 | 43.1 | 43.0 | .59 | 42.4 | - |
| 40–59 | 35.5 | 40.8 | 39.0 | 40.6 | 40.4 | 39.1 | .02 | 39.3 | - |
| 60– | 21.0 | 18.5 | 18.6 | 17.3 | 16.5 | 17.9 | .005 | 18.3 | - |
| Female sex, % | 51.3 | 52.0 | 51.8 | 51.2 | 51.9 | 52.3 | .53 | 51.8 | - |
| Racial and ethnicityd, % | - | ||||||||
| Non-Hispanic white | 68.7 | 70.9 | 70.6 | 70.9 | 67.9 | 66.1 | .39 | 69.2 | - |
| Non-Hispanic black | 11.2 | 11.1 | 11.6 | 11.7 | 11.7 | 11.8 | .69 | 11.5 | - |
| Mexican American | 7.6 | 7.2 | 8.2 | 8.5 | 9.0 | 9.2 | .33 | 8.3 | - |
| Otherse | 12.6 | 10.9 | 9.6 | 9.0 | 11.4 | 12.9 | .88 | 11.1 | - |
| Educational attainmentd, % | - | ||||||||
| > High school | 49.4 | 55.3 | 55.7 | 58.4 | 54.9 | 58.7 | .007 | 55.4 | - |
| High school or GEDf | 25.5 | 25.3 | 26.3 | 24.2 | 24.6 | 22.7 | .14 | 24.8 | - |
| < High school | 25.1 | 19.5 | 18.0 | 17.4 | 20.5 | 18.7 | .003 | 19.8 | - |
| Diabetes diagnosis, % | 5.5 | 5.5 | 5.8 | 4.8 | 5.7 | 5.1 | .56 | 5.4 | - |
| Diagnosed with hyperlipidemia, % | 19.3 | 18.5 | 22.0 | 21.6 | 21.7 | 18.8 | .30 | 20.3 | - |
| Total cholesterol level, mean (SD), mg/dL | 203.6 (32.3) | 203.1 (35.0) | 202.9 (33.5) | 201.1 (31.3) | 200.9 (34.2) | 199.6 (36.1) | .002 | 201.8 (33.8) | - |
| LDL-Cg level, mean (SD), mg/dL | 126.2 (30.3) | 121.7 (30.0) | 118.9 (28.7) | 118.0 (27.9) | 119.5 (30.3) | 119.8 (32.7) | <.001 | 120.6 (30.1) | - |
| Body mass index, mean (SD), kg/m2 | 27.9 (5.1) | 27.9 (5.0) | 28.0 (5.0) | 28.2 (5.2) | 28.2 (5.6) | 28.4 (5.8) | .02 | 28.1 (5.3) | - |
Sample size varies for certain characteristics. Each analysis accounted for an appropriate sample weight and the complex study design.
Trends over time were assessed using chi-squared tests for linear trends for categorical variables and linear regressions for continuous variables.
Comparisons between statin-users and non-users were made using pooled samples across the study period.
Percentages do not sum to 100% due to rounding.
The category includes other Hispanics and other races including multi-racial participants.
General Education Development.
Low-density lipoprotein cholesterol.
Table 2 and Figures 1 and 2 present model-adjusted caloric and fat intake estimates by survey cycle and the time trends. In 1999-2000, caloric intake was 179 kcal/day lower (2,000 vs. 2,179 kcal/day, p=.007) and fat intake was 9.5 g/day lower (71.7 vs. 81.2 g/day, p=.003) among statin-users than non-users. Then, the gap between the groups became smaller as cycles continued; we no longer found significant differences in caloric intake from 2005-2006 and in fat intake from 2003-2004. By 2009-2010, caloric and fat intake was insignificantly higher (54 kcal/day for caloric intake and 2.7 g/day for fat intake) among statin-users than non-users (p=.31 and .32, respectively).
Table 2.
Model-adjusteda caloric and fat intake among US adults by statin use over study period, 1999-2010.
| Model-adjusted estimate of nutrient intake (95% CI)
|
P value for group comparison within a survey cycle | |||
|---|---|---|---|---|
| Statin-user | Statin non-user | |||
| Caloric intake (kcal/day) | ||||
| 1999-2000 | 2,000 (1,870 – 2,131) | 2,179 (2,132 – 2,227) | .007 | |
| 2001-2002 | 2,034 (1,956 – 2,112) | 2,197 (2,156 – 2,237) | <.001 | |
| 2003-2004 | 2,120 (2,051 – 2,189) | 2,217 (2,178 – 2,255) | .03 | |
| 2005-2006 | 2,142 (2,080 – 2,203) | 2,183 (2,124 – 2,241) | .29 | |
| 2007-2008 | 2,088 (1,994 – 2,181) | 2,136 (2,083 – 2,191) | .28 | |
| 2009-2010 | 2,192 (2,105 – 2,280) | 2,138 (2,098 – 2,179) | .31 | |
|
| ||||
| Fat intake (g/day) | ||||
| 1999-2000 | 71.7 (65.7 – 77.7) | 81.2 (79.4 – 83.0) | .003 | |
| 2001-2002 | 73.7 (69.8 – 77.7) | 82.6 (80.7 – 84.6) | .001 | |
| 2003-2004 | 79.5 (74.5 – 84.5) | 84.3 (82.3 – 86.2) | .09 | |
| 2005-2006 | 81.9 (78.3 – 85.5) | 83.2 (80.1 – 86.3) | .52 | |
| 2007-2008 | 80.4 (76.2 – 84.5) | 81.0 (78.4 – 83.7) | .73 | |
| 2009-2010 | 82.0 (77.8 – 86.3) | 79.3 (77.1 – 81.5) | .32 | |
Adjusted for age category, sex, race and ethnicity, educational attainment, and diabetes diagnosis.
Figure 1.
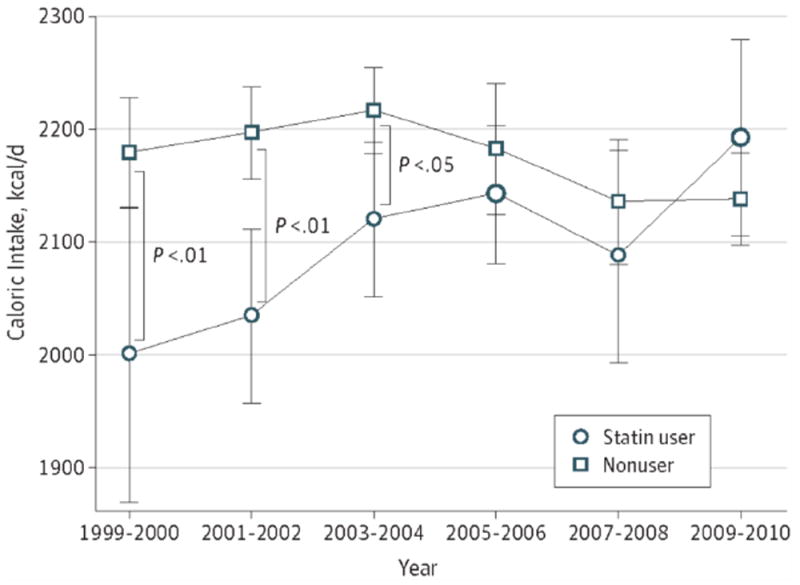
Trends of Estimates for Caloric Intake Among US Adult Statin Users and Nonusers, 1999-2010 Adjusted for age category, sex, race and ethnicity, educational attainment, and diabetes diagnosis. Error bars represent 95% CIs. Larger points represent significant changes from 1999-2000. * P < .05. ** P < .01.
Figure 2.
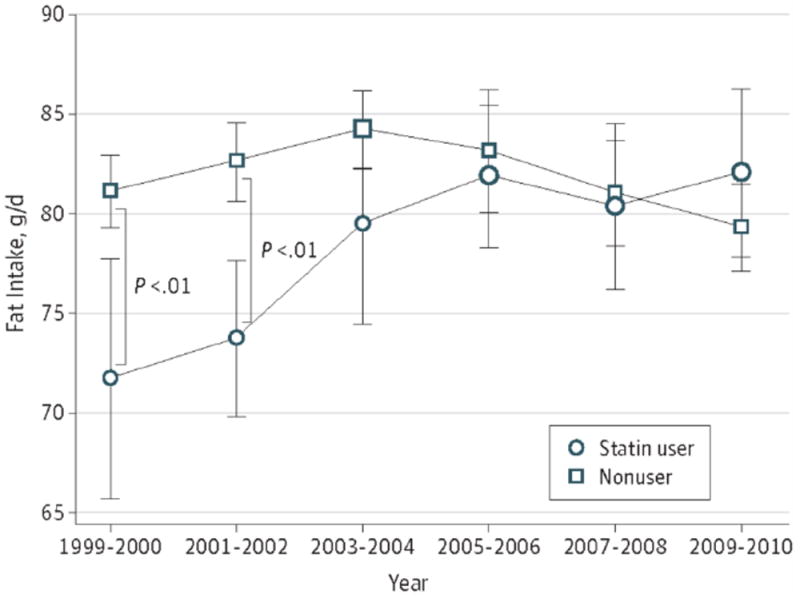
Trends of Estimates for Fat Intake Among US Adult Statin Users and Nonusers, 1999-2010 Adjusted for age category, sex, race and ethnicity, educational attainment, and diabetes diagnosis. Error bars represent 95% CIs. Larger points represent significant changes from 1999-2000. ** P < .01.
When we tested time trends of caloric intake separately for statin-users and non-users (Table 3), among statin-users, we found an increase in caloric intake during the study period; the caloric intake among statin-users in 2009-2010 was 9.6% greater (95% confidence interval (CI): 1.8 to 18.1, p=.02) than that among statin-users in 1999-2000. Among non-users, we did not observe a significant time trend. With regard to fat intake, we found similar patterns: for statin-users, fat intake in 2009-2010 was 14.4% (95% CI: 3.8 to 26.1, p=.007) greater than in 1999-2000. For non-users, fat intake increased 3.8% (95% CI: 0.5 to 7.2, p=.02) in 2003-2004 compared with 1999-2000, followed by a gradual decline to an insignificant 2.3% decrease (95% CI: -5.6 to 1.1, p=.19) in 2009-2010 compared with 1999-2000. The interactions between survey cycle and statin use were significant in the models with a continuous survey cycle variable (p=.001 for caloric intake and p<.001 for fat intake), which indicates that time trends for caloric and fat intake in the two groups were significantly different.
Table 3.
Model-adjusteda relative changes in caloric and fat intake among US adults by statin use, 1999-2010.
| Percent Change from 1999-2000 (95% CI)
|
P value for difference in trendsb | |||
|---|---|---|---|---|
| Statin-user | Statin non-user | |||
| Caloric intake | ||||
| 1999-2000 | Reference | Reference | .001 | |
| 2001-2002 | 1.7 (-5.6 to 9.5) | 0.8 (-2.0 to 3.6) | ||
| 2003-2004 | 6.0 (-1.2 to 13.7) | 1.7 (-1.0 to 4.5) | ||
| 2005-2006 | 7.1 (0.2 to 14.8) | 0.1 (-3.2 to 3.6) | ||
| 2007-2008 | 4.4 (-3.4 to 12.8) | -2.0 (-5.2 to 1.3) | ||
| 2009-2010 | 9.6 (1.8 to 18.1) | -1.9 (-4.6 to 0.9) | ||
|
| ||||
| Fat intake | ||||
| 1999-2000 | Reference | Reference | <.001 | |
| 2001-2002 | 2.8 (-6.9 to 13.6) | 1.8 (-1.4 to 5.1) | ||
| 2003-2004 | 10.9 (-0.1 to 23.0) | 3.8 (0.5 to 7.2) | ||
| 2005-2006 | 14.2 (3.9 to 25.4) | 2.5 (-1.8 to 6.9) | ||
| 2007-2008 | 12.1 (1.6 to 23.6) | -0.2 (-4.0 to 3.8) | ||
| 2009-2010 | 14.4 (3.8 to 26.1) | -2.3 (-5.6 to 1.1) | ||
Adjusted for age category, sex, race and ethnicity, educational attainment, and diabetes diagnosis.
Significance of interaction terms between survey cycle (continuous) and statin use (binary).
eFigures 1 and 2 present the results of the additional analyses stratifying statin non-users into those with and without hyperlipidemia, comparing 3 groups in total. As a result, both non-user groups (those with and without hyperlipidemia) had similar time trends of caloric intake (upward in the earlier survey cycles and downward in the later survey cycles), whereas the trend of the statin-user group was consistently upward. Difference of the trends for caloric and fat intake between statin-users and “non-users with hyperlipidemia” was significant (p = .02 for caloric intake and p = .01 for fat intake). To determine if the findings were driven by increased prevalence of diabetes, we examined time trends in caloric and fat intake in those without diabetes; time trends were similar to those for the entire sample (eFigures 3 and 4).
In the additional time trend analyses, we found similar patterns for saturated fat and dietary cholesterol intake (eFigures 5 and 6). The interaction term was significant for saturated fat intake (p<.001), and marginally significant for dietary cholesterol intake (p=.09).
The trends of model-estimated BMI revealed 1.3 kg/m2 (95% CI: 0.5 – 2.1, p=.001) increase among statin-users and 0.4 kg/m2 (95% CI: -0.1 – 1.0, p=.10) increase among non-users during the study period (eFigure 7). The test for interaction effects revealed the faster increase of BMI for statin-users (p=.03), although the increasing trends were significant for both statin-users (p<.001) and non-users (p=.02). The trends of model-estimated cholesterol levels among statin-users and non-users showed a decrease among statin-users from 193.4 mg/dl in 1999-2000 to 171.4 mg/dl in 2009-2010 and among non-users decreased from 205.1 mg/dl to 200.8 mg/dl (eFigure 8). The LDL-C level among statin-users decreased from 113.3 mg/dl in 1999-2000 to 95.8 mg/dl in 2009-2010; the level among non-users decreased from 127.3 mg/dl to 120.7 mg/dl (eFigure 9). The time trends of total cholesterol and LDL-C levels significantly differed by statin use (p<.001 for both).
DISCUSSION
In 1999-2000, statin-users consumed fewer calories and less fat than non-users, as we would expect in persons attempting to control their cholesterol level and weight. During the ensuing decade, statin use expanded rapidly, and statin-users consumed more calories and fat than earlier cohorts, which was not true for non - users. As a result, differences in intake between statin-users and non-users disappeared by 2005-2006 for caloric intake and by 2003-2004 for fat intake. This difference in the time trends for caloric and fat intake between statin-users and non-users was not explained by presence or absence of diagnoses of hyperlipidemia in non-users or higher prevalence of diabetes among statin-users.
To the best of our knowledge, this is the first study showing that time trends for caloric and fat intake differ by statin use in the US. A cross-sectional study in early 2000s in Rhode Island found that statin use was associated with an insignificant decrease in caloric intake among older adults.10 Another cross-sectional study in 2004 in Sweden found that statin-using adults were more likely to avoid food with high fat content than non-users.11 These results were consistent with our findings from earlier survey cycles that statin-users had less caloric and fat intake than non-users. A cohort study in Veterans Affairs primary care clinics followed up newly prescribed statin-users for 6 months in 2005, and observed no increase in caloric and fat intake.12 Although that longitudinal study design allowed stronger causal inference, 6 months may be too short to conclude that statin use is not associated with dietary laxity. We used cross-sectional data collected over 12 years that allowed us to see the trends of caloric and fat intake during the time when statin prescription rapidly increased.
What are the implications of the observed change in caloric intake among statin-users in terms of effect size and relationship with dietary recommendations in the guideline? Given that 7,000 kcal extra caloric imbalance is estimated to induce 1 kg weight gain in an adult,23 the estimated 192 kcal/day increase among statin-users could have contributed to the observed 1.3 kg/m2 increase in BMI (equivalent to 3-5 kg weight gain) over a decade. Since the guideline recommends that patients should prevent weight gain,3 the observed increase in caloric intake and more rapid increase in BMI among statin-users are of concern. According to the guidelines, people who receive statin therapy also should take steps to reduce fat intake,3,5 however, we did not observe a pattern of combining statin use with dietary control. The observed 14.4% increase in fat intake was greater than overall increase in caloric intake (9.6%). While the proportion of calories from fat did not exceed the upper limit of the recommended range (25-35%),3 the proportion increased from 32.3% in 1999-2000 to 33.7% in 2009-2010. The proportion of calories from saturated fat in 2009-2010 was 11.0%, whereas dietary cholesterol intake in 2009-2010 was 277.8 mg/day; both were well above 7% and 200 mg/day that are upper limits of recommended amounts in the guideline.3
Due to the self-reported information on diet and the repeated cross-sectional design of this study, the observed increase in caloric and fat intake should be interpreted carefully. First, because the information on nutrients was collected through dietary recall interview, the result was subject to social desirability bias (tendency to provide answers that convey a favorable image of the interviewee24); in the extreme, if statin-users became less likely to hesitate to reveal their true intake, our observations may not reflect true change in diet. However, the magnitude of our findings may be too large to be explained only by changes attributable to social desirability bias.
Second, our data are a serial cross-section in which participants change from one panel to the other, rather than cohort data in which same individuals are examined repeatedly. Therefore, we cannot infer that a particular individual who took statins throughout the study period consumed more calories and fat in recent years than a decade ago. However, given that the sampling weights of the NHANES allow us to make national estimates, we reasonably can conclude that an average American treated with statin in 2009-2010 consumed more calories and fat compared to an average American on statin in 1999-2000. We can only speculate mechanisms behind the observed trends; one possibility is that statin use may have undermined the perceived need to follow dietary recommendations. Patients who recognized that their LDL-C levels were lowered drastically by statins may have lost the incentive to pursue dietary modifications. Physicians might have contributed to this process by shifting the focus of consultations from diet to statin adherence, once they started statin treatment. This hypothesis is compatible with the lower cholesterol levels seen among statin-users than those among non-users in later survey cycles (Table 1). Another possible mechanism is that expanded statin use occurred in people who were likely to eat more. Some patients may have agreed to initiate statin therapy because they did not want to restrict their diet, whereas others who did not want to take medication may have declined the proposed pharmacotherapy in favor of following dietary recommendations. We adjusted for differences in characteristics among statin-users across survey cycles in the models, but did not adjust for cholesterol levels and BMI because they could have been consequences of food intake. As to physical activity (PA), NHANES measured it, but we did not include it in the main analyses due to inconsistent measurement: it changed between 2005-2006 and 2007-2008. As a sensitivity analysis, we included a PA variable in spite of the inconsistency, which did not change the results. However, other unmeasured factors may have affected the findings. For example, it is possible that those taking statins in the early survey cycles exhibited more severe hyperlipidemia, whereas those with less severe hyperlipidemia initiated statins in later cycles as their use increased. The greater decrease of cholesterol levels among statin-users over time may be partially explained by expanded therapeutic use. Our study design precluded evaluation of the extent to which these scenarios explain the findings. A cohort study with sufficiently long follow-up could address these questions. Nevertheless, whatever the mechanism, our results indicated that caloric and fat intake among statin-users in 2009-2010 was significantly greater than in 1999-2000. We may need to reemphasize the importance of dietary modification for statin-users.
At the same time, it may be appropriate to reevaluate and discuss dietary recommendations in the time of statins. The recently published ACC/AHA guideline emphasized extensive statin use for patients who are likely to experience a net benefit5; statin use is predicted to expand further if practitioners follow the guideline.25 Although the guideline articulates that lifestyle modification remains the foundation for CVD risk reduction,5 further expansion of statin use may result in more statin-users not following dietary recommendations (extrapolating from our study results). From the perspective of effectiveness, although the additional effects of low-fat diet on lowering LDL-C level among statin-users have been shown,26,27 the incremental benefit of low-fat diet on CVD prevention among those taking statins has not been fully investigated. Moreover, cost-effectiveness and ethical considerations should be taken into account. Particularly in the time when obesity and diabetes have become epidemics and US healthcare costs have been soaring, we need to consider if it is an acceptable public health strategy to encourage statin use without also taking measures to decrease the likelihood that its use will be associated with increased caloric and fat intake as well as weight gain. As with any pharmacotherapy, we believe that the goal of statin treatment should be to allow patients to decrease their CVD risks that cannot be accomplished without medication, and not to empower them to put butter on their steaks.
Supplementary Material
Acknowledgments
Funding/Support: Dr. Sugiyama was supported by National Center for Global Health and Medicine (Tokyo, Japan) and Honjo International Scholarship Foundation (Tokyo, Japan). Dr. Tsugawa was supported by Honjo International Scholarship Foundation (Tokyo, Japan).
Role of the Sponsors: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions:
Dr. Sugiyama had full access to all of data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sugiyama, Tsugawa, Shapiro.
Acquisition of data: Sugiyama.
Analysis and interpretation of data: Sugiyama, Tsugawa, Tseng.
Drafting of the manuscript: Sugiyama, Tsugawa.
Critical revision of the manuscript for important intellectual content: Tseng, Kobayashi, Shapiro.
Statistical analysis: Sugiyama, Tsugawa, Tseng, Shapiro.
Obtained funding: Sugiyama.
Administrative, technical, or material support: Kobayashi, Shapiro.
Study supervision: Kobayashi, Shapiro.
Additional Contributions: We appreciate Neil S. Wenger, MD, MPH (Division of General Internal Medicine & Health Services Research, David Geffen School of Medicine at UCLA, Los Angeles, California) for his comments on study design, and Masako Horino, RD (Department of Community Health Sciences, UCLA Fielding School of Public Health, Los Angeles, California) for her comments on grammatical issues. No compensation was given for their contributions.
Financial Disclosures: None reported.
Contributor Information
Takehiro Sugiyama, Email: tsugiyama-tky@umin.ac.jp.
Yusuke Tsugawa, Email: tsugawa@fas.harvard.edu.
Chi-Hong Tseng, Email: ctseng@mednet.ucla.edu.
Yasuki Kobayashi, Email: yasukik@m.u-tokyo.ac.jp.
Martin F. Shapiro, Email: MFShapiro@mednet.ucla.edu.
References
- 1.Goodman DW, Hulley SB, Clark LT, et al. Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. The Expert Panel. Arch Intern Med. 1988;148(1):36–69. [PubMed] [Google Scholar]
- 2.Grundy SM, Bilheimer DB, Chait A, et al. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) JAMA. 1993;269(23):3015–3023. [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 5.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Kuklina EV, Carroll MD, Shaw KM, Hirsch R. Trends in High LDL Cholesterol, Cholesterol-lowering Medication Use, and Dietary Saturated-fat Intake: United States, 1976-2010. NCHS data brief. 2013;117:1–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Capewell S. Trends in Total and Low-Density Lipoprotein Cholesterol among US Adults: Contributions of Changes in Dietary Fat Intake and Use of Cholesterol-Lowering Medications. PloS one. 2013;8(5):e65228. doi: 10.1371/journal.pone.0065228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Trends in intake of energy and macronutrients--United States, 1971-2000. MMWR Morbidity and mortality weekly report. 2004;53(4):80–82. [PubMed] [Google Scholar]
- 9.Wright JD, Wang CY. Trends in intake of energy and macronutrients in adults from 1999-2000 through 2007-2008. NCHS data brief. 2010;(49):1–8. [PubMed] [Google Scholar]
- 10.Lofgren I, Greene G, Schembre S, Delmonico MJ, Riebe D, Clark P. Comparison of diet quality, physical activity and biochemical values of older adults either reporting or not reporting use of lipid-lowering medication. The journal of nutrition, health & aging. 2010;14(2):168–172. doi: 10.1007/s12603-010-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lytsy P, Burell G, Westerling R. Cardiovascular risk factor assessments and health behaviours in patients using statins compared to a non-treated population. Int J Behav Med. 2012;19(2):134–142. doi: 10.1007/s12529-011-9157-6. [DOI] [PubMed] [Google Scholar]
- 12.Mann DM, Allegrante JP, Natarajan S, Montori VM, Halm EA, Charlson M. Dietary indiscretion and statin use. Mayo Clinic proceedings Mayo Clinic. 2007;82(8):951–957. doi: 10.4065/82.8.951. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. [December 14, 2013];2013 http://www.cdc.gov/nchs/nhanes.htm.
- 14.Centers for Disease Control and Prevention. NHANES Response Rates and CPS Totals. [December 14, 2013];2011 http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm.
- 15.Centers for Disease Control and Prevention. NCHS Research Ethics Review Board (ERB) Approval. [December 14, 2013];2012 http://www.cdc.gov/nchs/nhanes/irba98.htm.
- 16.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Dietary Interviewers Procedures Manual. [December 14, 2013];2000 http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/current_nhanes_99_00.htm.
- 17.Agricultural Research Service, United States Department of Agricultre. USDA Automated Multiple-Pass Method. [December 14, 2013];2010 http://www.ars.usda.gov/Services/docs.htm?docid=7710.
- 18.Agricultural Research Service, United States Department of Agricultre. Food and nutrient database for dietary studies. [December 14, 2013];2013 http://www.ars.usda.gov/Services/docs.htm?docid=12089.
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 20.Korn EL, Graubard BI. Analysis of health surveys. Vol. 323. John Wiley & Sons; 2011. [Google Scholar]
- 21.Centers for Disease Control and Prevention. The National Health and Nutrition Examination Surveys: analytic and reporting guidelines. [December 14, 2013];2005 http://www.cdc.gov/NCHS/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf.
- 22.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? Journal of health economics. 2001;20(4):461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 23.Schoeller DA. The energy balance equation: looking back and looking forward are two very different views. Nutrition Reviews. 2009;67(5):249–254. doi: 10.1111/j.1753-4887.2009.00197.x. [DOI] [PubMed] [Google Scholar]
- 24.Lissner L. Measuring food intake in studies of obesity. Public health nutrition. 2002;5(6A):889–892. doi: 10.1079/phn2002388. [DOI] [PubMed] [Google Scholar]
- 25.Abramson JD. The New York Times Opinions Page. Don’t Give More Patients Statins. [December 14, 2013];2013 http://www.nytimes.com/2013/11/14/opinion/dont-give-more-patients-statins.html?_r=0.
- 26.Cobb MM, Teitelbaum HS, Breslow JL. Lovastatin efficacy in reducing low-density lipoprotein cholesterol levels on high- vs low-fat diets. JAMA. 1991;265(8):997–1001. [PubMed] [Google Scholar]
- 27.Hunninghake DB, Stein EA, Dujovne CA, et al. The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. N Engl J Med. 1993;328(17):1213–1219. doi: 10.1056/NEJM199304293281701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


