Abstract
We demonstrate that the cation-selective channel formed by gramicidin A can be used as a reliable sensor for studying the multivalent ion accumulation at the surfaces of charged lipid membranes and the “charge inversion” phenomenon. In asymmetrically charged membranes with the individual leaflets formed from pure negative and positive lipids bathed by 0.1 M CsCl solutions the channel exhibits current rectification which is comparable to that of a typical n/p semiconductor diode. We show that even at these highly asymmetrical conditions the channel conductance can be satisfactorily described by the electrodiffusion equation in the constant field approximation but, due to predictable limitations, only when the applied voltages do not exceed 50 mV. Analysis of the changes in the voltage-dependent channel conductance upon addition of trivalent cations allows us to gauge their interactions with the membrane surface. The inversion of the sign of the effective surface charge takes place at the concentrations which correlate with the cation size. Specifically, these concentrations are close to 0.05 mM for lanthanum, 0.25 mM for hexaamminecobalt, and 4 mM for spermidine.
Keywords: molecular diode, nano-sensing, current rectification, bilayer lipid membrane, gramicidin A
1. INTRODUCTION
Long-range electrostatic forces are crucially involved in many interactions within and between biomolecules. Charged groups play the well-recognized roles in functioning of proteins, nucleic acids, phospholipids and their supra-molecular assemblies. Phospholipid molecules, building blocks of cellular membranes, mostly contain zwitterionic or negatively charged headgroups exposed on the membrane/water interface. Membrane surface potential is crucially involved in regulation of membrane transport, cell-cell recognition, and membrane-bound enzymes1. In the presence of multivalent cations the surface charge screening may be accompanied by its overcompensation, or the so-called “charge inversion” at the membrane surface. This phenomenon has been observed experimentally 2–12, and discussed in a number of theoretical studies, e.g. 13–18. Charge inversion by polyvalent ions is thought to be highly relevant for the number of biological processes, including action of drugs 19, gene delivery 20, DNA condensation7, and viral packing 21, 22. However, despite the extensive experimental and theoretical work, problems regarding electrostatics at the charged membrane interfaces, counter-ion screening, and charge inversion phenomenon remain a subject of intense discussions, e.g. 11, 23.
The motivation for this study is two-fold. First, in addition to the biological processes mentioned above, charge inversion could also be involved in regulation of channel function during membrane fusion, which is shown to require the presence of highly charged polypeptide chains 24. Second, most of the approaches for studying charge inversion published so far deal with different modifications of electrokinetic measurements, which involve the notion of a sliding plane. The present study is different in this respect as there is no need to postulate the position of the sliding plane, though we have to use other adjustable parameters as described below. We study the accumulation of multivalent ions at the charged surfaces by using the conductance of an ion channel as a sensor of the potential at the surface of the membrane hosting the channel. With the planar lipid bilayer membranes as established models of cell membranes, the technique of lipid monolayer opposition 25 allows obtaining artificial bilayers with asymmetrical distribution of lipids between the two monolayers. The charge state of the bilayer surface may be probed by measuring ion currents in the presence of lipophilic ions 26, ion transporters 27, 28, or ion pores induced by short peptides 29–34 and channel-forming proteins 35–39.
The conductive pore of a benchmark channel formed by pentadecapeptide gramicidin A is composed of two β-helical 15 amino acid monomers and is exclusively permeable for monovalent cations 40. As shown in a number of publications, the ion conductance of the gramicidin pore reports well on the charged state of surface bilayer groups. Indeed, charges on the membrane surface attract counter-ions and reduce the concentration of co-ions near the entrance of the pore, thus increasing or decreasing the number of cations, depending on the sign of the surface charge. The effects of the surface charge are particularly strong in electrolytes of low ionic strength.
In the present work we measure the ion conductance of the gramicidin A channel to assess changes in the charge state of phospholipid bilayers as a function of concentration of trivalent ions in the bulk. With 0.1 M cesium as the current-mediating ion we first study the channel conductance in asymmetric bilayers where one monolayer is formed from negatively charged phosphatidylserine (PS) and the other from either neutral phosphatidylcholine (PC) or positively charged trimethylammonium propane (TAP). We demonstrate that at small voltages the gramicidin A channel in asymmetrically charged membranes displays current rectification which is close to that of a typical solid-state diode. We also show that even at these highly asymmetrical conditions, our measurements are reasonably well described by a simple theoretical model of biased diffusion with only two adjustable parameters.
We then investigate the effects of three cations: lanthanum chloride, hexaamminecobalt chloride, and spermidine chloride on negatively charged bilayers. At asymmetrical, one side addition of these trivalent cations the channel conductance becomes asymmetric in applied voltage. Most pronounced reduction of the channel conductance is observed at the voltages that are positive from the side of trivalent cation addition. To estimate the bilayer surface charge in the presence of trivalent cations, we compare the corresponding conductance-voltage relationships with those obtained in control experiments with asymmetrical bilayers formed from lipid monolayers of different charge. All these cations are able to overcompensate the effective surface charge of the PS monolayer at the concentrations that correlate with their size. The most potent is lanthanum, which neutralizes the surface charge felt by the channel at 0.05 mM concentration, followed by hexaamminecobalt with about five-fold higher concentration and then by spermidine with about hundred-fold higher concentration of charge inversion.
2. EXPERIMENTAL METHODS
Bilayer lipid membranes were prepared from diphytanoylphosphoserine (PS), diphytanoylphosphocholine (PC), and dioleoyl trimethylammonium propane (TAP), Avanti Polar Lipids, Alabaster, AL, using monolayer-opposition technique by Montal and Mueller 25. The Teflon chamber, with two (cis and trans) compartments of 1.7 ml, was divided by a 15-μm-thick Teflon partition with a 60–70-μm diameter aperture. PS, PC and TAP monolayers were made from 2 mg/ml aliquots of lipids in pentane. After bilayer formation gramicidin A (a generous gift from O. S. Andersen, Weill Cornell University Medical College) was added from 1–10 nM ethanol stock solutions to both aqueous compartments at the amount sufficient to produce a single channel activity. Buffer solutions contained 100 mM CsCl, 5 mM HEPES at pH 7. Lanthanum chloride (Sigma), hexaamminecobalt chloride (Fluka), and spermidine chloride (Fluka) were admixed from the aliquots to the cis compartment of the membranes, made from PS monolayers. The choice of CsCl over KCl or NaCl was dictated by the fact that the gramicidin channel conductance in CsCl is about 1.8 higher than in KCl and 2.9 higher than in NaCl of the same molarity41.
The membrane potential was maintained using Ag/AgCl electrodes with 2 M KCl and 15 % (w/v) agarose bridges. The membrane chamber and headstage were isolated from external noise sources with a double metal screen (Amuneal Manufacturing Corp., Philadelphia, PA). Conductance measurements were performed using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA) in the voltage clamp mode. Data were filtered by a low-pass 8-pole Butterworth filter (Model 9002, Frequency Devices, Inc., Haverhill, MA) at 5 kHz, directly saved into the computer memory with a sampling frequency of 10 kHz, and analyzed using pClamp 10 software. All measurements were made at room temperature, T = (23 ± 1) °C. Gramicidin A amplitudes at a given transmembrane voltage were collected from individual single-channel events and calculated by Gaussian fitting of a histogram of ~50 single events.
Liposomes were prepared by sonication according to the Morrissey Laboratory protocol (Morrissey, J. H. 2001. Morrissey laboratory protocol for preparing phospholipid vesicles (SUV) by sonication. http://tf7.org/suv.pdf). Liposome buffer was the same as for bilayer measurements. Liposomes diameter (75–120 nm) was checked with light scattering. Measurement of the liposome ζ-potential and sizing of the liposomes were performed with Zeta-Plus ζ-potential analyzer (Brookhaven Instruments Corporation, Holtsville, NY). Diluted (1:10) PS or TAP liposome solutions in 1.5 ml cuvettes were injected with appropriate amounts of trivalent cations and transferred into the analyzer. PC liposomes were used as a standard of neutrality of the liposome surface in ζ-potential measurements. The measurements were performed at room temperature, T = (23 ± 1) °C.
3. RESULTS AND DISCUSSION
The results of a control experiment with gramicidin A channels incorporated into asymmetric planar lipid bilayers made of the negatively charged lipid (PS, the cis monolayer) and the positively charged lipid (TAP, the trans monolayer) are shown in Figure 1. Raw data in panels A and B and the corresponding current-voltage curves in panel C demonstrate that in asymmetric membranes the channel exhibits a highly non-linear behavior with a significant asymmetry resembling that of a solid-state diode rectifier. The current at +150 mV applied from the side of the negative monolayer is more than an order of magnitude higher than the current at the opposite polarity. Non-linear current-voltage dependencies were first reported for gramicidin A channel in membranes with only one lipid leaflet charged in a classical study by Frohlich 29. Here, in Fig. 1B, C we present experiments with the membranes where the leaflets are oppositely charged.
Figure 1.
A: Currents through single cationic channels formed by gramicidin A in a symmetrically charged membrane do not show rectification. The magnitude of the current steps is independent of the applied voltage polarity. (B) In an asymmetrically charged membrane the current becomes highly sensitive to voltage polarity. At 150 mV, positive from the side of the positive leaflet, the currents are much smaller than those at the opposite polarity. C: The current-voltage characteristic of the channel in an asymmetrically charged membrane (filled diamonds) shows strong rectification, which, within the voltage range of −50 mV to +50 mV, is very close to that of a typical n/p diode. Symmetric membranes were formed from the negatively charged PS; asymmetric membranes were from the positively charged TAP (the cis leaflet) and negatively charged PS (the trans leaflet) in 0.1 M CsCl.
It is instructive to compare the current-voltage characteristic of the gramicidin channel presented in Fig. 1C (filled diamonds) with the typical characteristics of semiconductor n/p diodes. The expression for the diode current Id as a function of applied voltage V can be written in the form:
| (1) |
where e is the elementary (proton) charge, kB and T have their standard meaning of the Boltzmann constant and absolute temperature, and n is the so-called “ideality factor” which reflects recombination enhancement by defects. Typically, for semiconductor diodes the value of n is between 1.0 and 2.0 42. Fitting Eq. (1) to the data for small voltages, |V|< 50 mV, in Fig. 1C, we obtain n ⊔ 1.8. Thus, the ionic diode represented by the symmetric gramicidin A channel incorporated into asymmetrically charged bilayer is close by this parameter to its solid state counterparts. However, as both experiment and analytical considerations (see below) demonstrate, at larger voltages the rectification of this “ionic diode” starts to saturate. At voltages higher than ±100 mV the ratio of the currents tends to a constant value.
Rectification in protein channels and synthetic nanopores has been reported in a number of earlier studies. The asymmetry was either an inherent property of the channel 43–49 or was introduced by a gradient of acidity across the membrane 50, 51. The rectification factor calculated as the ratio of the current in the higher conductance branch of the I/V characteristic to the current at the opposite polarity at ±100 mV reaches about 5.0 or smaller values 50, 51. For example, the rectification factor for the gramicidin channel in the membrane with only one leaflet negatively charged was found to increase with the decreasing salt concentration, but did not exceed 3.0 even when the concentration was reduced to 10 mM (see Fig. 5 of Ref. 29). The rectification factor calculated for ±100 mV from the data in Fig. 1 is close to 7.0, thus representing the so far most efficient ionic diode.
The physics of rectification in a cation-selective channel incorporated in asymmetrically charged membrane is similar to that of a semiconductor diode. In both cases the origin of rectification is the asymmetry in concentration of available charge carriers. However, analytical description as well as the final expressions and their limiting behavior are quite different.
The profile of electric potential across an asymmetrically charged lipid bilayer is schematically shown in Figure 2. In this particular example the cis side monolayer is positively charged, and the trans monolayer is negatively charged. If the potential of the trans side of the membrane-bathing solution at x → ∞ is considered to be zero, the potential at distance z from the trans surface can be calculated using the Gouy-Chapman expression1
Figure 2.
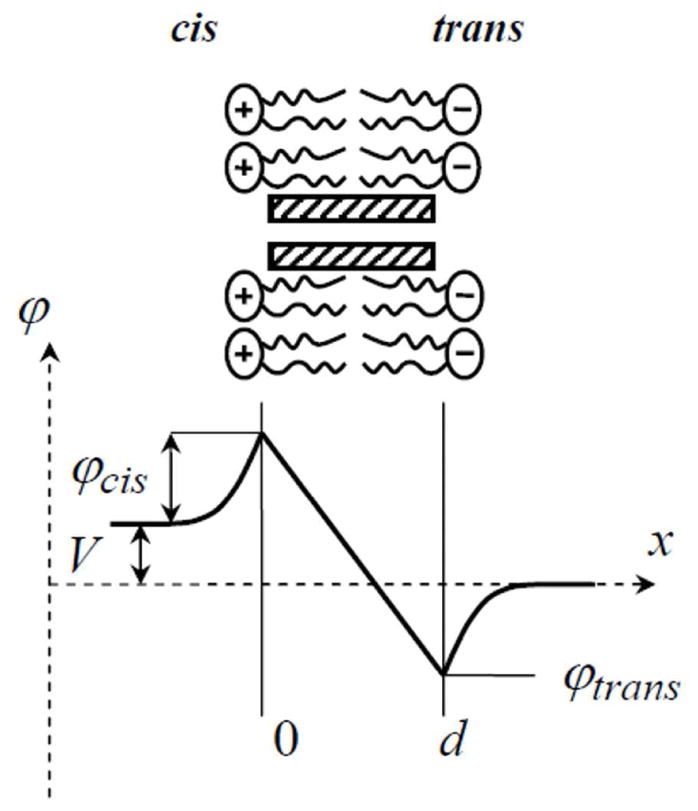
A schematic illustration of potential distribution along the channel axis shows that the total potential difference includes contributions from the surface charge on both sides of the membrane, thus imposing a pronounced asymmetry in the case of oppositely charged leaflets.
| (2) |
Here γ is defined through the Debye length, λD = (εε0kBT/8πe2c(b))1/2, and the Gouy-Chapman length, λGC = εε0kBT / 2πσe, as , where ε is the dielectric constant of the aqueous phase, ε0 is the permittivity of free space, c(b) is electrolyte number concentration in the bulk, and σ is the surface charge density. The potential at the cis surface of the membrane is a combination of the applied voltage V and the potential given by Eq. (2) taken with the minus sign to account for the positively charged lipids of the cis side.
Approximation given by Eqs. (2) and (3) for monovalent strong electrolytes after a self-consistent correction 52 to relate intrinsic binding constants for protons and ions with their apparent binding constants gives a surprisingly accurate description of the surface potential. This was confirmed in numerous experiments 1. One of the most recent ones is a surface-sensitive synchrotron X-ray scattering study 53 of Cs distribution in the vicinity of the surface with charge density as high as one elementary charge per 41 Å2. It was shown that for 0.1 M solutions of CsI at neutral pH the corrections to Eq. (3) due to the protonation of lipid headgroup charges are negligible. The protonation turned out to be significant only at much smaller salt concentration.
Another correction described by Ninham and Parsegian 52 is due to Cs+ binding to the lipid headgroups. This correction seems to be out of reach of the synchrotron X-ray scattering because of the finite resolution function on these experiments 53. Though small, it is readily measurable by other, more traditional methods. For example, it was shown 54 that in 0.1 M cesium chloride the ζ-potential of phosphatidylserine vesicles is changed from the “ideal” value of about −90 mV to the experimentally obtained −80 mV, thus giving the intrinsic association constant of cesium cation with this lipid of about 0.05 M−1.
Yet the most significant correction for the potential at the entrance of the channel comes from the finite size of the channel-forming molecule. As a result, the channel entrance feels only a fraction of the potential at the surface given by Eq. (2) for z = 0. This is a well-known fact. To account for this correction researchers introduce the so-called effective distance between the nearest charge and channel entrance 30, 34–38, 55. However, as it was discussed elsewhere 33 there is no straightforward analytical procedure to calculate the drop in potential because of the generally complex geometry of the system. We will follow one of the ad hoc approaches discussed in a previous study of gramicidin channel in a symmetrically charged lipid bilayer 33. Specifically, we assume that potentials at the channel entrance can be described by Eq. (2) at some value of z > 0 treated as an adjustable parameter.
The cationic current through a cylindrical channel can be calculated using the following one-dimensional diffusion equation
| (3) |
where c(x) and φ(x) are position-dependent cation concentration and potential, correspondingly, Aeff is the effective cross section of the channel and D is the diffusion coefficient of cations in the channel. Potential along the channel axis is distributed as shown in Fig. 2. At x = 0 it is φcis + V, at x = d it is φtrans. In this approach we assume that the flux through the channel is small enough not to disturb equilibrium counter-ion concentrations at the channel entrances. This assumption was repeatedly used to describe conductance properties of lipid bilayers in the presence of charged surface active agents, e.g. 56, 57 and synthetic nanopores with fixed charges 58. Integrating Eq. (3) between x = 0 and x = d in the constant field approximation, and taking into account the following relations between the bulk concentrations, and , and cation concentrations at the channel entrances and , we have
| (4) |
This result can also be obtained from the classical analysis of Neumcke 56 in the limit of small surface potentials 32.
Eq. (4) contains only two adjustable parameters. One is the effective cross section of the channel multiplied by the diffusion coefficient of cations in the channel, AeffD. The other one is the effective distance z in Eq. (2). The rest of the parameters in Eqs. (2) – (4) are given by structural data (the length of the gramicidin channel 59, d = 2.2 nm) and by the surface charge density σ, which, in principle, can be measured independently. However, different studies give significantly different estimates for its value. Depending on the study, σ varies from as high as 0.32 C/m2 37 to as low as 0.2 C/m2 60, assuming one elementary charge per lipid headgroup). Even for the structurally close lipids estimates for the surface charge can differ significantly, with the carrier conductance method 27 giving higher charge densities 33 than it may follow from the lipid packing densities obtained with X-ray methods 61, 62. The reasons for these discrepancies are not clear, but could be tentatively attributed to the membrane dipole potential influencing carrier conductance. Because of this uncertainty we assume that σ = 0.25 C/m2, keeping in mind that the error in the surface charge density could be compensated by the adjustable parameter z in Eq. (2) used to calculate the potentials φcis and φtrans at the channel entrances. Indeed, if the actual σ is smaller than the assumed, it could be corrected by the choice of a smaller z, and vice versa.
It is seen that Eq. (4) differs significantly from Eq. (1) for the current-voltage relationship for a n/p semiconductor diode. In particular, analysis of Eq. (4) at the high applied voltages predicts linear, Ohmic behavior with the current rectification ratio at |V| →∞ tending to a constant value of
| (5) |
Figure 3 illustrates the best fit of Eq. (4) to the data for PS/TAP and PS/PC bilayers. The fitting parameters are AeffD = 7.3×10−29 m4/s and z = 0.55 nm. The agreement between the experiment and the model is good for the applied voltages in the range |V| ≤ 50 mV. The systematic deviations at higher voltages are expected consequences of the model limitations. At large negative biases the conductance tends to saturate because the channel approaches the states of the maximum ion occupancy 40; at high positive biases the diffusion-limited access starts to dominate 63. Nevertheless, the correspondence bonetween the model and experiment at small voltages suggests that the origin of the rectification is in enrichment of penetrating charge carriers at the surface of the negatively charged lipid leaflet and their depletion at the surface of the positively charged one.
Figure 3.
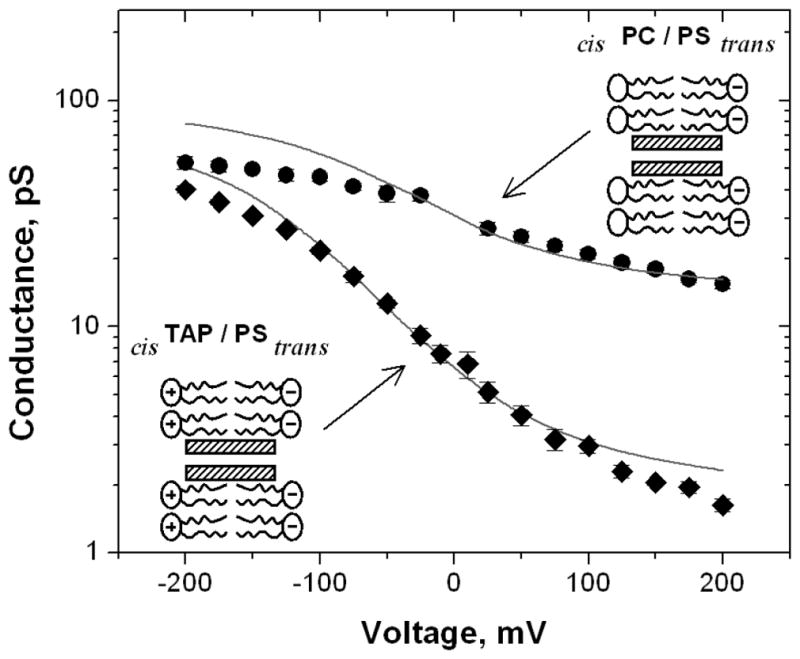
The dependence of the channel conductance on voltage in asymmetrically charged bilayers can be attributed to the different density of cations at the two surfaces of the membrane. The solid lines through the data are for both positive/negative and neutral/negative membranes are drawn according to Eqs. (2) and (4) with two adjustable parameters. They are the effective distance z from the nearest lipid charge to the channel entrance, Eq. (2), and the product of the channel cross section by diffusion coefficient, Eq. (4). The deviations at high voltage are expected due to the saturation effects mentioned in the text.
Addition of trivalent cations to the negatively charged membranes (PS/PS) decreases the channel conductance at both polarities of the applied voltage, but much more profoundly at voltages that are positive at the side of trivalent cation addition. Figure 4 gives raw data in the form of channel current records (represented as conductance for the convenience of comparison) at plus and minus 100 mV at increasing La+3 concentrations. While in symmetrically charged membranes in the absence of La+3 the amplitudes of the currents at negative and positive voltages are indeed equal, the one-sided La+3 addition introduces asymmetry: the conductance at positive voltages is larger than its counterpart at negative ones. The effect is hardly discernible at 1 μM of lanthanum chloride, but is quite obvious for the higher concentrations.
Figure 4.

The effect of trivalent cation addition is seen as a reduction of the currents through the single channels initially reconstituted into PS/PS (negative/negative) membrane. Increased lanthanum chloride concentrations, added from cis side only, progressively decrease the cationic current through the channel for both polarities; however, the effect is larger at potentials positive from the side of trivalent cation addition.
The trivalent cation accumulation at the membrane surface can be analyzed and quantified with the help of Eqs. (2) and (4) with the membrane surface charge used as an adjustable parameter. As we are interested only in the trivalent cation concentration that inverses the charge of the membrane surface, the straightforward way is to compare the channel conductance in the presence of trivalent cations with that of the channel in an asymmetric charged/neutral membrane. Figure 5 shows such a comparison for the channel conductances interpolated to zero voltage for PS/PS membranes in the presence of trivalent cations in the cis side of the cell and the channels in PS/PC membranes. It is seen that at their increasing concentrations, all three cations first decrease the effective surface charge of the cis leaflet of the membrane and then inverse its sign when the channel conductance becomes decreases below its value in PS/PC membranes. Specifically, charge inversion takes place at the concentrations exceeding (0.048±0.004) mM for lanthanum, (0.25±0.03) mM for hexaamminecobalt, and (4.3±0.8) mM for spermidine. The charge inverting concentration for lanthanum is close to that reported in recent experiments with PS liposomes 64.
Figure 5.
Addition of trivalent cations to the membrane-bathing solution not only compensates the negative charge of the cis leaflet of the initially negative/negative membrane, but, at a certain concentration, overcompensates it. Trivalent cations were added to the cis side of the reconstitution cell. The data-points are interpolations of single-channel conductance, which was measured in a range of applied voltages similarly to the data in Figs. 1 and 3, to zero voltage. The inversion of the effective surface charge happens when the channel conductance, as a function of trivalent cation concentration, crosses the level corresponding to its value in the neutral/negative membrane.
To verify our finding with the cation-selective channel conductance, we also performed more traditional measurements of liposome ζ-potential, which were repeatedly used to study the charge inversion phenomenon (e.g., 11, 12 and references therein). The data obtained by the two methods are compared in Table 1 that shows reasonable agreement.
Table 1.
| Trivalent cation | Cation concentration producing the effective surface charge inversion of membrane surface, mM
|
|
|---|---|---|
| GrA channel conductance | ζ-potential of liposomes | |
| Lanthanum | 0.048±0.004 | 0.058±0.014 |
| Hexaamminecobalt | 0.25±0.03 | 0.42±0.14 |
| Spermidine | 4.3±0.8 | 3.4±0.9 |
Though there is still no consensus on the adequate theoretical description of the charge inversion phenomenon, our data suggest that the charge reversing concentrations correlate with the cation size. The smaller the cation size, the smaller is the concentration. This observation may support one of the available approaches that explains the phenomenon by the gain of entropy upon release of counter-ions 15 (see also ref. 18). The impact of the ion size on short-range correlations and on the charge inversion in electrolyte mixtures was recently investigated using Monte Carlo simulations 65, 66.
4. CONCLUSIONS
We use the effects of the surface charge on the conductance of an ion channel embedded inside the object under study – a lipid bilayer. We show that analysis of the current-voltage dependences of the cation-selective channel, gramicidin A, is a reliable method for studies of multivalent ion adsorption to the membrane surface. Control experiments with the asymmetrically charged membranes serve as a consistent calibration of the membrane surface potential and its modification by multivalent ions and permit characterization of the surface charge state. In particular, we find that:
In asymmetric membranes formed by opposing monolayers of pure PS and TAP at the salt concentrations close to physiological, the current-voltage relationship of the channel at small voltages displays rectification characterized by the parameters that are typical for semiconductor n/p diodes.
Accumulation of multivalent ions at the membrane surface and the corresponding changes of the effective surface charge can be seen through the changes in the channel conductance. For the negatively charged PS membrane in 0.1 M CsCl its surface charge can be compensated and overcompensated by trivalent cations with the surface charge inversion observed at the concentrations in a series lanthanum < hexaamminecobalt ≪ spermidine, which correlates with the cation size.
Acknowledgments
Funding Sources:
This study was supported by the Intramural Research Program of the NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
We are grateful to Vicente Aguilella, Brian Todd, and Adrian Parsegian for fruitful discussions.
References
- 1.McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–36. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- 2.Kekicheff P, Marcelja S, Senden TJ, Shubin VE. Charge reversal seen in electrical double-layer interaction of surfaces immersed in 2–1 calcium electrolyte. J Chem Phys. 1993;99(8):6098–6113. [Google Scholar]
- 3.Besteman K, Zevenbergen MAG, Heering HA, Lemay SG. Direct observation of charge inversion by multivalent ions as a universal electrostatic phenomenon. Physical Review Letters. 2004;93(17):170802. doi: 10.1103/PhysRevLett.93.170802. [DOI] [PubMed] [Google Scholar]
- 4.Besteman K, Zevenbergen MAG, Lemay SG. Charge inversion by multivalent ions: dependence on dielectric constant and surface-charge density. Physical Review E. 2005;72(6):061501. doi: 10.1103/PhysRevE.72.061501. [DOI] [PubMed] [Google Scholar]
- 5.Pittler J, Bu W, Vaknin D, Travesset A, McGillivray DJ, Loesche M. Charge inversion at minute electrolyte concentrations. Physical Review Letters. 2006;97(4):046102. doi: 10.1103/PhysRevLett.97.046102. [DOI] [PubMed] [Google Scholar]
- 6.van der Heyden FHJ, Stein D, Besteman K, Lemay SG, Dekker C. Charge inversion at high ionic strength studied by streaming currents. Physical Review Letters. 2006;96(22):224502. doi: 10.1103/PhysRevLett.96.224502. [DOI] [PubMed] [Google Scholar]
- 7.Besteman K, Van Eijk K, Lemay SG. Charge inversion accompanies DNA condensation by multivalent ions. Nat Phys. 2007;3(9):641–644. [Google Scholar]
- 8.Alcaraz A, Nestorovich EM, Lopez ML, Garcia-Gimenez E, Bezrukov SM, Aguilella VM. Diffusion, exclusion, and specific binding in a large channel: a study of OmpF selectivity inversion. Biophys J. 2009;96(1):56–66. doi: 10.1016/j.bpj.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Gillespie D, Boda D, Vlassiouk I, Eisenberg RS, Siwy ZS. Tuning transport properties of nanofluidic devices with local charge inversion. J Am Chem Soc. 2009;131(14):5194–5202. doi: 10.1021/ja808717u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wernersson E, Kjellander R, Lyklema J. Charge inversion and ion-ion correlation effects at the mercury/aqueous MgSO4 Interface: toward the solution of a long-standing issue. J Phys Chem C. 2010;114(4):1849–1866. [Google Scholar]
- 11.Jimenez ML, Delgado AV, Lyklema J. Hydrolysis versus ion correlation models in electrokinetic charge inversion: establishing application ranges. Langmuir. 2012;28(17):6786–6793. doi: 10.1021/la3010773. [DOI] [PubMed] [Google Scholar]
- 12.Kubickova A, Krizek T, Coufal P, Vazdar M, Wernersson E, Heyda J, Jungwirth P. Overcharging in biological systems: reversal of electrophoretic mobility of aqueous polyaspartate by multivalent cations. Phys Rev Lett. 2012;108(18):186101. doi: 10.1103/PhysRevLett.108.186101. [DOI] [PubMed] [Google Scholar]
- 13.Greberg H, Kjellander R. Charge inversion in electric double layers and effects of different sizes for counterions and coions. J Chem Phys. 1998;108(7):2940–2953. [Google Scholar]
- 14.Borukhov I, Andelman D, Orland H. Scaling laws of polyelectrolyte adsorption. Macromolecules. 1998;31(5):1665–1671. [Google Scholar]
- 15.Park SY, Bruinsma RF, Gelbart WM. Spontaneous overcharging of macro-ion complexes. Europhys Lett. 1999;46(4):454–460. [Google Scholar]
- 16.Shklovskii BI. Screening of a macroion by multivalent ions: correlation-induced inversion of charge. Physical Review E. 1999;60(5):5802–5811. doi: 10.1103/physreve.60.5802. [DOI] [PubMed] [Google Scholar]
- 17.May S, Harries D, Ben-Shaul A. Lipid demixing and protein-protein interactions in the adsorption of charged proteins on mixed membranes. Biophys J. 2000;79(4):1747–1760. doi: 10.1016/S0006-3495(00)76427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosberg AY, Nguyen TT, Shklovskii BI. Colloquium: the physics of charge inversion in chemical and biological systems. Rev Mod Phys. 2002;74(2):329–345. [Google Scholar]
- 19.Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrob Agents Ch. 1999;43(5):1003–1012. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felgner PL, Rhodes G. Gene Therapeutics. Nature. 1991;349(6307):351–352. doi: 10.1038/349351a0. [DOI] [PubMed] [Google Scholar]
- 21.van der Schoot P, Bruinsma R. Electrostatics and the assembly of an RNA virus. Physical Review E. 2005;71(6):061928. doi: 10.1103/PhysRevE.71.061928. [DOI] [PubMed] [Google Scholar]
- 22.Belyi VA, Muthukumar M. Electrostatic origin of the genome packing in viruses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17174–17178. doi: 10.1073/pnas.0608311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wernersson E, Heyda J, Kubickova A, Krizek T, Coufal P, Jungwirth P. Counterion condensation in short cationic peptides: Limiting mobilities beyond the Onsager-Fuoss theory. Electrophoresis. 2012;33(6):981–989. doi: 10.1002/elps.201100602. [DOI] [PubMed] [Google Scholar]
- 24.Yang ST, Zaitseva E, Chernomordik LV, Melikov K. Cell-Penetrating Peptide Induces Leaky Fusion of Liposomes Containing Late Endosome-Specific Anionic Lipid. Biophys J. 2010;99(8):2525–2533. doi: 10.1016/j.bpj.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montal M, Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proceedings of the National Academy of Sciences of the United States of America. 1972;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen OS, Feldberg S, Nakadomari H, Levy S, Mclaughlin S. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys J. 1978;21(1):35–70. doi: 10.1016/S0006-3495(78)85507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin SG, Szabo G, Eisenman G, Ciani SM. Surface charge and the conductance of phospholipid membranes. Proc Natl Acad Sci U S A. 1970;67(3):1268–75. doi: 10.1073/pnas.67.3.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung L, Kaloyanides G, McDaniel R, McLaughlin A, McLaughlin S. Interaction of gentamicin and spermine with bilayer membranes containing negatively charged phospholipids. Biochemistry. 1985;24(2):442–52. doi: 10.1021/bi00323a030. [DOI] [PubMed] [Google Scholar]
- 29.Frohlich O. Asymmetry of the gramicidin channel in bilayers of asymmetric lipid composition: I. Single channel conductance. J Membr Biol. 1979;48(4):365–83. doi: 10.1007/BF01869447. [DOI] [PubMed] [Google Scholar]
- 30.Apell HJ, Bamberg E, Lauger P. Effects of surface charge on the conductance of the gramicidin channel. Biochimica et Biophysica Acta. 1979;552(3):369–78. doi: 10.1016/0005-2736(79)90181-0. [DOI] [PubMed] [Google Scholar]
- 31.Rostovtseva TK, Osipov VV, Lev AA. Dependence of single gramicidin channel conductance on potential induced by adsorption of 1-anilino-8-naphthalene sulfonate anions on lipid membranes. Biol Membrany. 1987;4(9):955–964. [Google Scholar]
- 32.Osipov VV, Rostovtseva TK, Lev AA. Current-Voltage characteristics of single gramicidin-A channels under symmetrical and asymmetrical adsorption of ANS - anions on the membranes. Biol Membrany. 1988;5(2):162–172. [Google Scholar]
- 33.Rostovtseva TK, Aguilella VM, Vodyanoy I, Bezrukov SM, Parsegian VA. Membrane surface-charge titration probed by gramicidin A channel conductance. Biophys J. 1998;75(4):1783–1792. doi: 10.1016/S0006-3495(98)77620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguilella VM, Bezrukov SM. Alamethicin channel conductance modified by lipid charge. Eur Biophys J. 2001;30(4):233–241. doi: 10.1007/s002490100145. [DOI] [PubMed] [Google Scholar]
- 35.Bell JE, Miller C. Effects of phospholipid surface-charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys J. 1984;45(1):279–287. doi: 10.1016/S0006-3495(84)84154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moczydlowski E, Alvarez O, Vergara C, Latorre R. Effect of phospholipid surface-charge on the conductance and gating of a Ca2+ - activated K+ channel in planar lipid bilayers. Journal of Membrane Biology. 1985;83(3):273–282. doi: 10.1007/BF01868701. [DOI] [PubMed] [Google Scholar]
- 37.Coronado R, Affolter H. Insulation of the conduction pathway of muscle transverse tubule - calcium channels from the surface-charge of bilayer phospholipid. Journal of General Physiology. 1986;87(6):933–953. doi: 10.1085/jgp.87.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurnev PA, Queralt-Martin M, Aguilella VM, Rostovtseva TK, Bezrukov SM. Probing tubulin-blocked state of VDAC by varying membrane surface charge. Biophys J. 2012;102(9):2070–2076. doi: 10.1016/j.bpj.2012.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majd S, Yusko EC, Billeh YN, Macrae MX, Yang J, Mayer M. Applications of biological pores in nanomedicine, sensing, and nanoelectronics. Curr Opin Biotechnol. 2010;21(4):439–76. doi: 10.1016/j.copbio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koeppe RE, Andersen OS. Engineering the gramicidin channel. Annu Rev Bioph Biom. 1996;25:231–258. doi: 10.1146/annurev.bb.25.060196.001311. [DOI] [PubMed] [Google Scholar]
- 41.Hladky SB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- 42.Jain A, Kapoor A. A new method to determine the diode ideality factor of real solar cell using Lambert W-function. Sol Energ Mat Sol C. 2005;85(3):391–396. [Google Scholar]
- 43.Kienker PK, Degrado WF, Lear JD. A helical-dipole model describes the single-channel current rectification of an uncharged peptide ion-channel. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(11):4859–4863. doi: 10.1073/pnas.91.11.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malev VV, Schagina LV, Gurnev PA, Takemoto JY, Nestorovich EM, Bezrukov SM. Syringomycin E channel: A lipidic pore stabilized by lipopeptide? Biophys J. 2002;82(4):1985–1994. doi: 10.1016/S0006-3495(02)75547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiedt B, Healy K, Morrison AP, Neumann R, Siwy Z. Transport of ions and biomolecules through single asymmetric nanopores in polymer films. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2005;236:109–116. [Google Scholar]
- 46.Constantin D, Siwy ZS. Poisson-Nernst -Planck model of ion current rectification through a nanofluidic diode. Physical Review E. 2007;76(4):041202. doi: 10.1103/PhysRevE.76.041202. [DOI] [PubMed] [Google Scholar]
- 47.Vlassiouk I, Siwy ZS. Nanofluidic diode. Nano Letters. 2007;7(3):552–556. doi: 10.1021/nl062924b. [DOI] [PubMed] [Google Scholar]
- 48.Capone R, Blake S, Restrepo MR, Yang J, Mayer M. Designing nanosensors based on charged derivatives of gramicidin A. J Am Chem Soc. 2007;129(31):9737–9745. doi: 10.1021/ja0711819. [DOI] [PubMed] [Google Scholar]
- 49.Verdia-Baguena C, Queralt-Martin M, Aguilella VM, Alcaraz A. Protein ion channels as molecular ratchets. switchable current modulation in outer membrane protein f porin induced by millimolar La3+ ions. J Phys Chem C. 2012;116(11):6537–6542. [Google Scholar]
- 50.Krasilnikov OV, Yuldasheva LN, Merzlyak PG, Capistrano MF, Nogueira RA. The hinge portion of the S. aureus alpha-toxin crosses the lipid bilayer and is part of the trans-mouth of the channel. Biochimica et Biophysica Acta. 1997;1329(1):51–60. doi: 10.1016/s0005-2736(97)00087-4. [DOI] [PubMed] [Google Scholar]
- 51.Alcaraz A, Ramirez P, Garcia-Gimenez E, Lopez ML, Andrio A, Aguilella VM. A pH-tunable nanofluidic diode: electrochemical rectification in a reconstituted single ion channel. J Phys Chem B. 2006;110(42):21205–9. doi: 10.1021/jp063204w. [DOI] [PubMed] [Google Scholar]
- 52.Ninham BW, Parsegian VA. Electrostatic potential between surfaces bearing ionizable groups in ionic equilibrium with physiologic saline solution. J Theor Biol. 1971;31(3):405. doi: 10.1016/0022-5193(71)90019-1. [DOI] [PubMed] [Google Scholar]
- 53.Bu W, Vaknin D, Travesset A. How accurate is Poisson-Boltzmann theory for monovalent ions near highly charged interfaces? Langmuir. 2006;22(13):5673–5681. doi: 10.1021/la053400e. [DOI] [PubMed] [Google Scholar]
- 54.Eisenberg M, Gresalfi T, Riccio T, McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979;18(23):5213–23. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- 55.Green WN, Andersen OS. Surface charges and ion channel function. Annual review of physiology. 1991;53:341–59. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- 56.Neumcke B. Ion flux across lipid bilayer membranes with charged surfaces. Biophysik. 1970;6(3):231–40. doi: 10.1007/BF01189084. [DOI] [PubMed] [Google Scholar]
- 57.Markin VS, Chizmadzhev Yu A. Induced Ionic Transport (Indutsirovanniy Ionniy Transport) Nauka Publishers; Moscow: 1974. [Google Scholar]
- 58.Ramirez P, Mafe S, Aguilella VM, Alcaraz A. Synthetic nanopores with fixed charges: an electrodiffusion model for ionic transport. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68(1 Pt 1):011910. doi: 10.1103/PhysRevE.68.011910. [DOI] [PubMed] [Google Scholar]
- 59.Elliott JR, Needham D, Dilger JP, Haydon DA. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim Biophys Acta. 1983;735(1):95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- 60.Loosley-Millman ME, Rand RP, Parsegian VA. Effects of monovalent ion binding and screening on measured electrostatic forces between charged phospholipid bilayers. Biophys J. 1982;40(3):221–32. doi: 10.1016/S0006-3495(82)84477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tristram-Nagle S, Kim DJ, Akhunzada N, Kucerka N, Mathai JC, Katsaras J, Zeidel M, Nagle JF. Structure and water permeability of fully hydrated diphytanoylPC. Chem Phys Lipids. 2010;163(6):630–7. doi: 10.1016/j.chemphyslip.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrache HI, Tristram-Nagle S, Gawrisch K, Harries D, Parsegian VA, Nagle JF. Structure and fluctuations of charged phosphatidylserine bilayers in the absence of salt. Biophys J. 2004;86(3):1574–86. doi: 10.1016/S0006-3495(04)74225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andersen OS. Ion movement through gramicidin-A channels - single-channel measurements at very high potentials. Biophys J. 1983;41(2):119–133. doi: 10.1016/S0006-3495(83)84414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin-Molina A, Rodriguez-Beas C, Faraudo J. Charge reversal in anionic liposomes: experimental demonstration and molecular origin. Physical Review Letters. 2010;104(16):168103. doi: 10.1103/PhysRevLett.104.168103. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z-Y, Ma Y-Q. Insights from Monte Carlo simulations on charge inversion of planar electric double layers in mixtures of asymmetric electrolytes. J Chem Phys. 2010;133:064704. doi: 10.1063/1.3469795. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z-Y, Ma Y-Q. A molecular simulation study on the role of ion sizes and dielectric images in near-surface ion distribution far from the strong coupling limit. J Chem Phys. 2012;136:234701. doi: 10.1063/1.4729311. [DOI] [PubMed] [Google Scholar]




