Abstract
Purpose of review
This review describes the current treatment of human epidermal growth factor receptor-2 (HER2) positive breast cancer with a focus on recently reported clinical trials. Treatment of resistant disease and central nervous system metastases will be reviewed as will new agents that are being developed to target HER2 amplified breast cancers.
Recent Findings
Recent studies evaluating trastuzumab-resistant breast cancer have shown a benefit of continuing trastuzumab with chemotherapy or with another HER2-targeted agent. Targeting the vascular endothelial growth factor (VEGF), mammalian target of rapamycin (mTOR), and PI3 kinase pathways in addition to HER2 may enhance efficacy compared with individual agents. Several novel anti-HER2 compounds are being evaluated with promising early data.
Summary
HER2-positive breast cancer has traditionally been associated with poor prognosis. However, treatment with HER2-targeted therapies has changed the natural history of this disease. Greater success depends on elucidating mechanisms of resistance and exploring new methods of blocking signal transduction via HER2 and related pathways.
Keywords: Breast cancer, HER2, trastuzumab, lapatinib, brain metastases
INTRODUCTION
Breast cancer is the most common female malignancy in the USA and the second leading cause of cancer death in women [1]. It has become evident, through gene expression profiling, that breast cancer is a heterogeneous disease, comprised of at least five subtypes [2]. Approximately 25% of breast cancers are classified as HER2-positive, which denotes an aggressive phenotype [3, 4]. However, with the advent of HER2 targeted therapy, most notably, trastuzumab, the natural history of HER2-positive breast cancer has been dramatically improved [5, 6].
BIOLOGY OF HER2+ BREAST CANCER
The ErbB family of receptor tyrosine kinases is comprised of four cell-surface receptors, HER1 (epidermal growth factor receptor [EGFR]), HER2, HER3, and HER4. Upon receptor dimerization an intracellular signaling cascade is activated, resulting in cell proliferation, survival, invasion, and angiogenesis (Figure 1). In contrast to the other ErbB family members, no ligand has been identified for HER2 [7]. However, HER2 is the preferred dimerization partner within the ErbB family, a feature which contributes to its importance [8].
Figure 1. The HER2 Signaling Pathway.
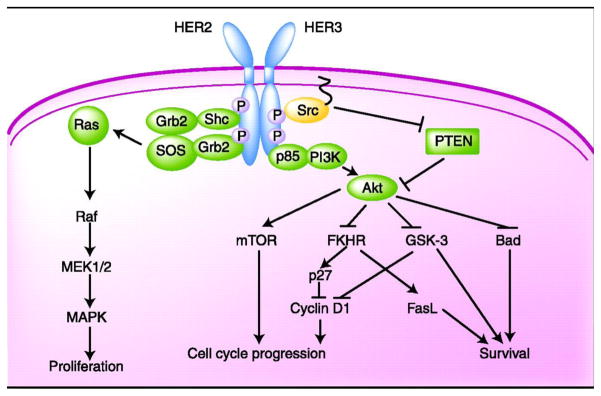
Ligand binding induces dimerization, leading to activation of the intracellular tyrosine kinase. On auto- and cross-phosporylation of the receptor complex, key downstream effectors are recruited. This figure illustrates a HER2-HER3 heterodimer, but HER2 can also form homodimers or heterodimerize with other members of the HER2 family. FKHR, forkhead in rhabdomyosarcoma; Grb2, growth factor receptor-bound protein 2; GSK-3, glycogen kinase synthase-3; MAPK, mitogen-activated protein kinase; mTOR, molecular target of rapamycin; PI3K, phospatidyl-inositol 3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome 10; SOS, son-of-sevenless guanine nucleotide exchange factor.
The diagnosis of HER2-positive breast cancer is made via immunohistochemistry (IHC), which identifies overexpression of the HER2 gene product and fluorescence in situ hybridization (FISH) analysis, which identifies amplification of the HER2 gene. According to the College of American Pathology (CAP) guidelines, tumors that have indeterminate results by IHC (2+) should have reflex testing by FISH [9]. Some institutions use FISH routinely as it is less subjective than IHC interpretation and is associated with a greater predictive value of response to HER2-directed therapy [10].
Trastuzumab is a humanized murine IgG monoclonal antibody that binds to the HER2 extracellular domain. Its mechanism of action has not been fully ascertained, however, it has been shown to reduce signaling through the PI3K/Akt and Ras/Raf/MEK/MAPK pathways, leading to cell cycle arrest, inhibition of DNA repair after chemotherapy [11] and induction of apoptosis [12]. Two small clinical studies have suggested that trastuzumab may promote antibody dependent cellular cytotoxicity (ADCC), in which the antibody Fc portion is bound to Fc receptors expressed by immune effector cells, such as NK cells [13, 14]. However, a recent analysis of genomic DNA samples from trastuzumab treated breast cancer patients found no significant correlation between FcR genotype and disease free survival (DFS) or progression-free survival (PFS), and, therefore, did not support ADCC as a major mechanism of action of trastuzumab [15].
CURRENT TREATMENT OPTIONS
The following section discusses the current treatment options available.
Metastatic Breast Cancer
The use of trastuzumab with or without chemotherapy is the backbone of systemic treatment of HER2-positive breast cancer. The incorporation of trastuzumab into the treatment of HER2-positive breast cancer was based on a groundbreaking Phase III trial in which 469 women with HER2-positive metastatic breast cancer (MBC) were randomized to receive standard chemotherapy with or without trastuzumab. The addition of trastuzumab extended time to progression (TTP) from 4.6 months to 7.4 months (p<0.001) and reduced the relative risk of death by 20% (hazard ratio (HR) 0.80, p<0.046) [16].
Single agent trastuzumab has also been evaluated, but yields lower overall response rate (ORR; 15% in treatment-refractory MBC [17]; 30% in the first-line setting [18, 19]) than when combined with chemotherapy. In the past decade, numerous studies have demonstrated safety and efficacy of various chemotherapeutic agents combined with trastuzumab [20–25]. Cardiotoxicity is unacceptably high with the concurrent use of anthracyclines and trastuzumab and is not recommended [26]. About half of HER2-positive tumors are also estrogen receptor (ER) positive. Patients with HER2-positive, ER-positive breast cancer may derive less benefit from endocrine therapy [27], possibly due to HER2 and ER crosstalk [28]. These findings support strategies that combine endocrine therapy with trastuzumab. In a randomized Phase III trial (the TAnDEM study) the combination of anastrozole and trastuzumab was shown to significantly improve progression free survival (PFS; 4.8 vs 2.4 months) and ORR (20.3% vs 6.8%) over anastrozole alone [29].
Progression on trastuzumab
Despite its remarkable impact on HER2-positive breast cancer, resistance to trastuzumab remains a therapeutic challenge. Trastuzumab resistance from the outset of treatment, known as de novo resistance is seen in approximately 50% of patients with MBC and acquired resistance after initial response to trastuzumab develops in most patients. Putative mechanisms of trastuzumab resistance include upregulation of alternative cell signaling pathways [30], expression of a truncated HER2 protein which lacks the trastuzumab binding site [31], and autophagy, in which cancer cells recycle and repackage proteins needed for their survival [32]. Expression of membrane-associated glycoprotein mucin-4 (MUC4), may confer resistance to trastuzumab by masking HER2 [33].
Trastuzumab with chemotherapy after progression
Evidence is emerging that supports continued use of trastuzumab with second- and third-line chemotherapeutic agents after disease progression on trastuzumab [34–36]. The German Breast Group 26/Breast International Group 03–05 trial randomly assigned 156 patients who had progressed on trastuzumab to capecitabine alone versus capecitabine with trastuzumab. Median TTP (8.2 mos vs. 5.6 mos, p=0.0338) and ORR (48.1% vs 27.0%, p=0.0115) were significantly better in the combination arm compared to capecitabine alone [35].
Lapatinib
Lapatinib is an oral, small molecule tyrosine kinase inhibitor that targets both HER2 and EGFR. Since the action of lapatinib is intracellular, it may avoid resistance mechanisms involving the HER2 extracellular domain. Lapatinib is primarily used to treat trastuzumab-resistant tumors based on a Phase III study in which patients pretreated with an anthracycline, taxane, and trastuzumab were randomized to receive capecitabine and lapatinib versus capecitabine alone. A significant improvement in ORR and TTP was seen with the combination regimen. However, there was no difference in overall survival (OS) [37]. Lapatinib monotherapy for patients who progress on trastuzumab is well tolerated, but only of modest benefit. In a Phase II study of 78 women with heavily pretreated HER2-positive breast cancer, ORR was 5.1% and clinical benefit rate was 9% for single agent lapatinib [38].
Brain metastases
Brain metastases have become more common as the initial site of recurrence as patients live longer. One study suggests that nearly 50% of patients treated with trastuzumab develop brain metastases [39]. Their development may relate to biologic characteristics specific to HER2-amplified malignancies [40–42]. Local therapies including surgery, whole brain radiation, and stereotactic radiosurgery have been the primary approach to treating central nervous system (CNS) metastases. However, results of systemic treatment of brain metastases in HER2-positive breast cancer have been disappointing, likely due to the inability of systemic therapies, including trastuzumab, to cross the blood brain barrier (BBB) [40]. In spite of this, there is evidence that continuing trastuzumab beyond CNS progression improves outcomes [43] and patients with HER2-positive CNS metastases do appear to live longer than those that are HER2-negative [44].
Lapatinib is a small molecule with the ability to cross the BBB making it an attractive candidate for the treatment of CNS metastases. In the original Phase III study of lapatinib and capecitabine, an exploratory analysis demonstrated a decrease in CNS relapse in lapatinib treated patients [45]. While a Phase II study of lapatinib in 39 HER2-positive patients with brain metastases did not meet its primary endpoint of objective tumor response, volumetric decrease in CNS lesions suggested that lapatinib did have activity [46]. This finding prompted another Phase II study of 242 patients with CNS metastases treated with lapatinib. A modest 6% of patients responded. However, of 50 patients who entered an extension phase of the study in which capecitabine was added to lapatinib, 20% responded [47]. Studies combining lapatinib with cytotoxic chemotherapy and other biologically targeted agents are ongoing.
Early Stage Breast Cancer
After its approval in the metastatic setting, trastuzumab was evaluated in the adjuvant (post-operative) setting in several large prospective randomized trials that enrolled over 13,000 patients in total. The addition of trastuzumab to chemotherapy led to a significant improvement in DFS (36–58%) and OS (24–59%) [48–52]. As expected, the benefit of trastuzumab is limited to HER2-positive disease [53, 54]. The first regimen evaluated with trastuzumab was doxorubicin, cyclophosphamide followed by paclitaxel, trastuzumab (AC→TH). While effective, this comes at the cost of a 4.1% risk of grade 3/4 heart failure which is attributable to the use of an anthracycline with trastuzumab [55]. A non-anthracycline-based regimen was also evaluated in a trial that enrolled 3,222 women with HER2-amplified early breast cancer and randomly assigned them to receive chemotherapy alone (AC→T;doxorubicin, cyclophosphamide, docetaxel), anthracycline-based chemotherapy plus trastuzumab (AC→TH), or a non-anthracycline based chemotherapy plus trastuzumab (TCH; docetaxel, carboplatin, and trastuzumab). Efficacy analysis at 65 months demonstrated that women receiving either trastuzumab-containing regimen had significantly improved DFS and OS compared to women receiving AC→T. Additionally, there was no statistically significant difference in DFS between the TCH and AC→TH arms. However, there were fivefold greater congestive heart failure events with AC→TH than with TCH [51]. These data support the use of trastuzumab with a non-anthracycline based chemotherapy regimen in the adjuvant treatment of early breast cancer.
Recent evidence suggests that the biology of a tumor may be more important than its size at diagnosis. Two recent studies indicate that sub-centimeter HER2-positive tumors carry a higher risk of recurrence than their HER2-negative counterparts [56, 57]. Moreover, two small, hypothesis-generating retrospective analyses suggest that adjuvant trastuzumab-based therapy may improve outcomes for T1 tumors that are HER2-positive. [58, 59]. Larger, prospective studies to evaluate the use of trastuzumab-based therapy for subcentimeter HER2+ tumors are ongoing.
Locally Advanced Breast Cancer: Neoadjuvant therapy
In locally advanced breast cancer, neoadjuvant (preoperative) chemotherapy has increased rates of breast conserving surgery [60, 61]. Furthermore, neoadjuvant therapy provides an in vivo assessment of response to therapy based on pathologic response. For these reasons, neoadjuvant therapy is the standard of care in locally advanced and inflammatory breast cancer, and may be considered in patients with early breast cancer.
Neoadjuvant treatment with trastuzumab and chemotherapy has been evaluated in recently reported Phase III trials. Concurrent administration of trastuzumab and paclitaxel followed by anthracycline-based chemotherapy produced a higher pathologic complete response (pCR) rate than the same chemotherapy alone (65.2% vs 26.3%, p=.016) [62]. Updated safety and efficacy analyses conducted with an additional 22 patients who all received trastuzumab and chemotherapy, demonstrated an improvement in 3-year DFS with the addition of trastuzumab (100% vs 85.3%) [63]. The Neoadjuvant Herceptin (NOAH) trial compared treatment with trastuzumab (for 1 year, starting preoperatively; n=117) with no trastuzumab (n=118), in women with HER2-positive locally advanced or inflammatory breast cancer treated with a neoadjuvant chemotherapy regimen containing doxorubicin, cyclophosphamide, paclitaxel, methotrexate, and fluorouracil. At 3 years, event free survival was significantly improved with the addition of trastuzumab (HR 0.59 [95% CI 0.38–0.90]; p=0.013) [64]. In the German Breast Group/Gynecologic Oncologic Study Group (GeparQuattro) trial, the combination of trastuzumab and anthracycline based chemotherapy (four cycles of epirubicin/cyclophosphamide followed by four cycles of docetaxel with or without capecitabine) in a group of patients with HER2-positive breast cancer produced a greater pCR rate than chemotherapy alone in a reference group of patients with HER2-negative breast cancer (pCR 31.7% vs 15.7% respectively) [65]. TCH has also been investigated in the neoadjuvant setting and has produced pCR rates of 31–43% [66, 67]. Ongoing Phase III clinical trials evaluating neoadjuvant chemotherapy with trastuzumab and other biologic agents, such as lapatinib will further define the role of preoperative administration of HER2 targeted agents [68, 69].
FUTURE DIRECTIONS
Treatment strategies that involve biologic agents with different mechanisms of action are ongoing. These compounds, outlined in Table 1, target several intracellular pathways that contribute to the propagation of HER2-positive breast cancer.
Table 1.
Targeted Therapies for HER2-positive breast cancer
| Name | Type | Mechanism of action |
|---|---|---|
| Trastuzumab | Receptor antibody | HER2 binding and inhibition |
| Pertuzumab | Receptor antibody | HER2 dimerization inhibition |
| Trastuzumab-MCC-DM1 | Receptor antibody-toxin conjugate | HER2 binding, maytansine toxin delivery |
| Lapatinib | Small molecule TKI | Reversible EGFR(HER1) and HER2 inhibition |
| Neratinib | Small molecule TKI | Irreversible pan-ErbB inhibition |
| Everolimus | STI | Inhibition of mTOR |
| Bevacizumab | Antibody | VEGFR ligand inhibition |
| Pazopanib | Small molecule TKI | VEGFr, c-kit, PDGFr inhibition |
| Metformin | Biguanide | AMPK activation and mTOR inhibition |
AMPK, adenosine monophosphate-activated protein kinase; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; mTOR, mammalian target of rapamycin; PDGFr, platelet-derived growth factor receptor; STI, signal transduction inhibitor; TKI, tyrosine kinase inhibitor; VEGFr, vascular endothelial growth factor receptor.
Combination HER2 directed therapy
The combination of lapatinib and trastuzumab has demonstrated synergy in preclinical models [70] and has recently been shown to improve PFS in patients with MBC that had progressed on trastuzumab [71]. In a phase III trial, 296 HER2-positive patients whose disease had progressed on trastuzumab-containing regimens were randomized to receive lapatinib alone or lapatinib with trastuzumab. The median PFS was 12.0 weeks in the lapatinib plus trastuzumab arm and 8.1 weeks in the lapatinib arm (HR 0.73; 95% CI, 0.57 to 0.93; P = .008). The combination of trastuzumab and lapatinib was well tolerated, with fewer serious adverse events than would be expected with a chemotherapy containing regimen [71]. In an updated analysis, the median OS was 14 months for the lapatinib plus trastuzumab arm, compared with 9.5 months for the lapatinib arm (HR=0.74, P=.026) [72].
mTOR inhibition
Agents that decrease signaling through the PI3K/Akt/mTOR potentially overcome both trastuzumab and lapatinib resistance. In preclinical models, the mTOR inhibitor, rapamycin, demonstrated synergy with trastuzumab [73]. These compounds, when added to trastuzumab showed clinical activity in Phase I trials of pretreated patients [74]. PI3K inhibitors also impair signaling through this pathway and have shown promise in preclinical studies [75]. The antidiabetic drug, metformin, has been shown to inhibit mTOR and is being evaluated in breast cancer for this reason [76]. Ongoing Phase III studies of mTOR inhibitors in combination with anti-HER2 and chemotherapy will define their role in the treatment of HER2-positive breast cancer.
Angiogenesis Inhibition
Preclinical data demonstrated an interaction between the HER2 and VEGF pathway [77], providing a rationale for combining therapies targeting each. A phase II study of trastuzumab and bevacizumab as first-line therapy for HER2-overexpressing MBC showed that this combination produced an ORR of 48% with acceptable toxicity [78]. Dual tyrosine kinase inhibition with the anti-angiogenesis agent, pazopanib, combined with lapatinib was evaluated in a phase II study of advanced HER2-positive breast cancer. Response rates were improved with combination therapy over lapatinib alone (44% vs 30%, respectively) and a decrease in progressive disease at 12 weeks was observed [79]. Combination VEGF/HER2 blockade is under study in the adjuvant [80] and metastatic settings (with chemotherapy) [81] in two large Phase III trials.
Novel HER2 targeted agents
T-DM1 is an antibody-drug conjugate (ADC) which combines trastuzumab with a potent antimicrotubule agent, DM1, using a stable linker (MCC). This molecule is targeted to HER2-positive cancer cells and is internalized, which may render it less toxic and more effective [82]. A phase II study of T-DM1 in 110 patients with heavily pretreated, HER2-positive MBC demonstrated an ORR of 33% and PFS of 7.3 months. The most common toxicities were nausea, fatigue and thrombocytopenia. No dose limiting cardiotoxicity was observed [83]. Trials comparing first-line treatment with T-DM1 to combination chemotherapy and anti-HER2 agents are ongoing.
Pertuzumab is a monoclonal antibody that targets a different region of the HER2 extracellular domain than trastuzumab. A recent phase II study that evaluated the combination of pertuzumab and trastuzumab demonstrated an ORR of 24.2%, and CBR of 50% of 66 patients whose disease progressed on prior trastuzumab therapy [84].
Neratinib is an oral, pan-ErbB tyrosine kinase inhibitor in development that has shown promise in a Phase II clinical trial [85].
CONCLUSIONS
In the past, HER2 amplification connoted aggressive disease associated with an increased risk of recurrence and death. However, a greater understanding of the molecular mechanisms underlying the pathogenesis of HER2-positive breast cancer has generated treatment options to combat this poor prognosis for many women. By further defining mechanisms of resistance to current treatments and developing new agents to subvert them, the prognosis of HER2-positive breast cancer will continue to be improved.
References and Recommended Reading
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000 Aug 17;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Slamon D, Clark G, Wong S, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 5.Ferretti G, Fabi A, Felici A, et al. Improved prognosis by trastuzumab of women with HER2-positive breast cancer compared with those with HER2-negative disease. J Clin Oncol. 2010 Jul 10;28(20):e337. doi: 10.1200/JCO.2010.28.2525. author reply e8–9. [DOI] [PubMed] [Google Scholar]
- 6.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010 Jan 1;28(1):92–8. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klapper LN, Glathe S, Vaisman N, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A. 1999 Apr 27;96(9):4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996 Oct;16(10):5276–87. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Journal of Clinical Oncology. 2007 Jan 1;25(1):118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 10.Sauter G, Lee J, Bartlett JMS, et al. Guidelines for Human Epidermal Growth Factor Receptor 2 Testing: Biologic and Methodologic Considerations. J Clin Oncol. 2009 Mar 10;27(8):1323–33. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 11.Le XF, Pruefer F, Bast RC., Jr HER2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27Kip1 via multiple signaling pathways. Cell Cycle. 2005 Jan;4(1):87–95. doi: 10.4161/cc.4.1.1360. [DOI] [PubMed] [Google Scholar]
- 12.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009 Dec 1;27(34):5838–47. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 13.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G Fragment C Receptor Polymorphisms and Clinical Efficacy of Trastuzumab-Based Therapy in Patients With HER-2/neu-Positive Metastatic Breast Cancer. J Clin Oncol. 2008;26(11):1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 14.Tamura K, Shimizu C, Koizumi F. Correlation of FcgammaR IIa-H131R and IIIa-V158F polymorphisms and clinical outcome of trastuzumab in both neoadjuvant and metastatic setting in patients with HER-2 positive breast cancer. J Clin Oncol. 2009;27:15s, abstract 110. [Google Scholar]
- 15.Hurvitz S, Betting D, Stern H. Analysis of Fcgamma receptor IIA & IIIA polymorphisms: correlation with outcome in trastuzumab-treated HER2-Neu amplified early and metastatic breast cancer patients. Oral presentation at the 2009 San Antonio Breast Cancer Symposium; December 12, 2009; p. Abstract 64. [Google Scholar]
- 16.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. New England Journal of Medicine. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 17.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational Study of the Efficacy and Safety of Humanized Anti-HER2 Monoclonal Antibody in Women Who Have HER2-Overexpressing Metastatic Breast Cancer That Has Progressed After Chemotherapy for Metastatic Disease. Journal of Clinical Oncology. 1999 Sep 1;17(9):2639. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 18.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002 Feb 1;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 19.Baselga J, Carbonell X, Castaneda-Soto NJ, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005 Apr 1;23(10):2162–71. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Michalaki V, Fotiou S, Gennatas S, et al. Trastuzumab plus Capecitabine and Docetaxel as First-line Therapy for HER2-positive Metastatic Breast Cancer: Phase II Results. Anticancer Res. 2010 Jul;30(7):3051–4. [PubMed] [Google Scholar]
- 21.Moulder S, Li H, Wang M, et al. A phase II trial of trastuzumab plus weekly ixabepilone and carboplatin in patients with HER2-positive metastatic breast cancer: an Eastern Cooperative Oncology Group Trial. Breast Cancer Res Treat. 2010 Feb;119(3):663–71. doi: 10.1007/s10549-009-0658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Untch M, Muscholl M, Tjulandin S, et al. First-line trastuzumab plus epirubicin and cyclophosphamide therapy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: cardiac safety and efficacy data from the Herceptin, Cyclophosphamide, and Epirubicin (HERCULES) trial. J Clin Oncol. 2010 Mar 20;28(9):1473–80. doi: 10.1200/JCO.2009.21.9709. [DOI] [PubMed] [Google Scholar]
- 23.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005 Jul 1;23(19):4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 24.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006 Jun 20;24(18):2786–92. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 25.Wardley AM, Pivot X, Morales-Vasquez F, et al. Randomized phase II trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. J Clin Oncol. 2010 Feb 20;28(6):976–83. doi: 10.1200/JCO.2008.21.6531. [DOI] [PubMed] [Google Scholar]
- 26.Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast. 2004 Jun;13(3):173–83. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008 Mar 1;26(7):1059–65. doi: 10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 28.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004 Jun 16;96(12):926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009 Nov 20;27(33):5529–37. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 30.Nahta R, Yu D, Hung MC, et al. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006 May;3(5):269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 31.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007 Apr 18;99(8):628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Autophagy facilitates the development of breast cancer resistance to the anti-HER2 monoclonal antibody trastuzumab. PLoS One. 2009;4(7):e6251. doi: 10.1371/journal.pone.0006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagy P, Friedlander E, Tanner M, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005 Jan 15;65(2):473–82. [PubMed] [Google Scholar]
- 34.Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of Treatment of Metastatic Breast Cancer With Trastuzumab Beyond Disease Progression. J Clin Oncol. 2004 Mar 15;22(6):1063–70. doi: 10.1200/JCO.2004.06.557. [DOI] [PubMed] [Google Scholar]
- ••35.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009 Apr 20;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. The first prospective, adequately powered trial to demonstrate that continuing trastuzumab with chemotherapy beyond disease progression on trastuzumab+/−first line chemotherapy, improves ORR and TTP. [DOI] [PubMed] [Google Scholar]
- 36.Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer. 2004 Apr;5(1):52–8. doi: 10.3816/cbc.2004.n.010. discussion 9–62. [DOI] [PubMed] [Google Scholar]
- 37.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006 Dec 28;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 38.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009 Jun;20(6):1026–31. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 39.Lai R, Dang CT, Malkin MG, et al. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004 Aug 15;101(4):810–6. doi: 10.1002/cncr.20418. [DOI] [PubMed] [Google Scholar]
- 40.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007 Mar 15;13(6):1648–55. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 41.Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006 Dec 20;24(36):5658–63. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 42.Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007 May 1;67(9):4190–8. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 43.Park IH, Ro J, Lee KS, et al. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009 Jan;20(1):56–62. doi: 10.1093/annonc/mdn539. [DOI] [PubMed] [Google Scholar]
- 44.Kirsch DG, Ledezma CJ, Mathews CS, et al. Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol. 2005 Mar 20;23(9):2114–6. doi: 10.1200/JCO.2005.05.249. author reply 6–7. [DOI] [PubMed] [Google Scholar]
- •45.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008 Dec;112(3):533–43. doi: 10.1007/s10549-007-9885-0. Updated efficacy and biomarker analyses of the original trial demonstrating that the addition of lapatinib to capecitabine improves TTP vs capecitabine alone in disease that progressed on anthracycline-, taxane-, and trastuzumab-containing regimens. [DOI] [PubMed] [Google Scholar]
- 46.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008 Apr 20;26(12):1993–9. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009 Feb 15;15(4):1452–9. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 48.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. New England Journal of Medicine. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 49.Ian S, Marion P, Richard DG, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. LANCET. 2007;369(9555):29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 50.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. New England Journal of Medicine. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- ••51.Slamon D, Eiermann W, Robert N, et al. Phase III Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Docetaxel (ACT) with Doxorubicin and Cyclophosphamide Followed by Docetaxel and Trastuzumab (ACTH) with Docetaxel, Carboplatin and Trastuzumab (TCH) in Her2neu Positive Early Breast Cancer Patients: BCIRG 006 Study. Cancer Research. 2009;69(24Suppl):Abstract 62. This study demonstrated that in the adjuvant treatment of HER2-positive patients, trastuzumab provided a similar and significant improvement in DFS and OS when used with anthracycline-based (ACTH) or non-anthracycline (TCH) chemotherapy. The non-anthracycline chemotherapy combined with trastuzumab (TCH) had an improved toxicity profile vs the anthracycline-based regimen (ACTH) [Google Scholar]
- 52.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006 Feb 23;354(8):809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 53.Pritchard KI, Shepherd LE, O’Malley FP, et al. HER2 and Responsiveness of Breast Cancer to Adjuvant Chemotherapy. New England Journal of Medicine. 2006;354(20):2103–11. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 54.Seidman AD, Berry D, Cirrincione C, et al. Randomized Phase III Trial of Weekly Compared With Every-3-Weeks Paclitaxel for Metastatic Breast Cancer, With Trastuzumab for all HER-2 Overexpressors and Random Assignment to Trastuzumab or Not in HER-2 Nonoverexpressors: Final Results of Cancer and Leukemia Group B Protocol 9840. J Clin Oncol. 2008 Apr 1;26(10):1642–9. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 55.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005 Nov 1;23(31):7811–9. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009 Dec 1;27(34):5700–6. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009 Dec 1;27(34):5693–9. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues MJ, Kirova Y, Guillot E, et al. Trastuzumab Treatment in T1ab, Node-Negative, Human Epidermal Growth Factor Receptor 2-Overexpressing Breast Carcinomas. J Clin Oncol. 2010 Jul 26; doi: 10.1200/JCO.2010.29.7952. [DOI] [PubMed] [Google Scholar]
- 59.McArthur HLMP, Patil S. Benefits of trastuzumab-based therapy for women with small, node-negative, HER2-positive breast cancer. ASCO Breast Cancer Symposium; San Francisco, CA. October 8–10, 2009. [Google Scholar]
- 60.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008 Feb 10;26(5):778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 61.Esteva FJ, Yu D, Hung MC, et al. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010 Feb;7(2):98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 62.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005 Jun 1;23(16):3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 63.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007 Jan 1;13(1):228–33. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 64.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010 Jan 30;375(9712):377–84. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 65.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010 Apr 20;28(12):2024–31. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 66.Chang HR, Glaspy J, Allison MA, et al. Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer. 2010 Sep 15;116(18):4227–37. doi: 10.1002/cncr.25309. [DOI] [PubMed] [Google Scholar]
- 67.Coudert BP, Largillier R, Arnould L, et al. Multicenter phase II trial of neoadjuvant therapy with trastuzumab, docetaxel, and carboplatin for human epidermal growth factor receptor-2-overexpressing stage II or III breast cancer: results of the GETN(A)-1 trial. J Clin Oncol. 2007 Jul 1;25(19):2678–84. doi: 10.1200/JCO.2006.09.9994. [DOI] [PubMed] [Google Scholar]
- 68.A Randomized Phase III Trial of Neoadjuvant Therapy for Patients with Palpable and Operable HER2-Positive Breast Cancer Comparing the Combination of Trastuzumab Plus Lapatinib to Trastuzumab and to Lapatinib Administered with Weekly Paclitaxel Following AC Accompanied by Correlative Science Studies to Identify Predictors of Pathologic Complete Response. [Accessed on Sept. 3, 2010]; Available at: http://clinicaltrials.gov/ct2/show/NCT00486668.
- 69.A Randomised, Multicenter Open-label Phase III Study of Neoadjuvant Lapatinib, Trastuzumab and Their Combination Plus Paclitaxel in Women With HER2/ErbB2 Positive Primary Breast Cancer. [Accessed on Sept. 3, 2010]; Available at: http://clinicaltrials.gov/ct2/show/NCT00553358.
- 70.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006 Feb 1;66(3):1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- ••71.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010 Mar 1;28(7):1124–30. doi: 10.1200/JCO.2008.21.4437. This randomized, Phase III trial demonstrated that lapatinib in combination with trastuzumab is a chemotherapy-free treatment option that significantly improved PFS versus lapatinib alone in heavily pretreated patients. [DOI] [PubMed] [Google Scholar]
- 72.Blackwell K, Burstein H, Sledge G, et al. Updated Survival Analysis of a Randomized Study of Lapatinib Alone or in Combination with Trastuzumab in Women with HER2-Positive Metastatic Breast Cancer Progressing on Trastuzumab Therapy. Cancer Research. 2009;69(24Suppl):Abstract 61. [Google Scholar]
- 73.Miller TW, Forbes JT, Shah C, et al. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res. 2009 Dec 1;15(23):7266–76. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Regan RAF, Campone M, et al. RAD001 (everolimus) in combination with weekly paclitaxel and trastuzumab in patients with HER-2-overexpressing metastatic breast cancer with prior resistance to trastuzumab: A multicenter phase I clinical trial (abstract 3119) Cancer Res. 2009;69(2 suppl) [Google Scholar]
- 75.Ozbay T, Durden DL, Liu T, et al. In vitro evaluation of pan-PI3-kinase inhibitor SF1126 in trastuzumab-sensitive and trastuzumab-resistant HER2-over-expressing breast cancer cells. Cancer Chemother Pharmacol. 2010 Mar;65(4):697–706. doi: 10.1007/s00280-009-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vazquez-Martin A, Oliveras-Ferraros C, Barco SD, et al. The anti-diabetic drug metformin suppresses self-renewal and proliferation of trastuzumab-resistant tumor-initiating breast cancer stem cells. Breast Cancer Res Treat. 2010 May 11; doi: 10.1007/s10549-010-0924-x. [DOI] [PubMed] [Google Scholar]
- 77.Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004 Mar 1;10(5):1706–16. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- •78.Hurvitz S, Pegram M, Lin L, et al. Final results of a phase II trial evaluating trastuzumab and bevacizumab as first line therapy of HER2-amplified advanced breast cancer (abstract 6094) Cancer Res. 2009;69(24 suppl) This is the first Phase II trial demonstrating that two humanized antibodies given together without chemotherapy has clinical activity and acceptable tolerability in patients with HER-2 positive advanced breast cancer. [Google Scholar]
- 79.Slamon D, Gomez H, Kabbinavar F, et al. Randomized study of pazopanib + lapatinib vs. lapatinib alone in patients with HER2- positive advanced or metastatic breast cancer (abstract 1016) J Clin Oncol. 2008;26(15S):45s. [Google Scholar]
- 80.A Multicenter Phase III Randomized Trial of Adjuvant Therapy for Patients With HER2-Positive Node-Positive or High Risk Node-Negative Breast Cancer Comparing Chemotherapy Plus Trastuzumab With Chemotherapy Plus Trastuzumab Plus Bevacizumab. Available at. [Accessed on Sept. 3, 2010]; http://clinicaltrials.gov/ct2/show/NCT00625898.
- 81.A Randomized, Open-label Study to Compare the Effect of First-line Treatment With Avastin in Combination With Herceptin/Docetaxel and Herceptin/Docetaxel Alone on Progression-free Survival in Patients With HER2 Positive Locally Recurrent or Metastatic Breast Cancer. [Accessed on Sept. 3, 2010]; Available at: http://clinicaltrials.gov/ct2/show/NCT00391092.
- ••82.Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010 Jun 1;28(16):2698–704. doi: 10.1200/JCO.2009.26.2071. The first in-human study of the antibody drug conjugate, T-DM1. This agent was shown to have clinical activity and a favorable toxicity profile in patients with MBC who progressed on trastuzumab. [DOI] [PubMed] [Google Scholar]
- 83.Krop I, LoRusso P, Miller K, et al. A phase II study of trastuzumab-DM1 (T-DM1), a novel HER2 antibody-drug conjugate, in patients with HER2+ metastatic breast cancer who were previously treated with an anthracycline, a taxane, capecitabine, lapatinib, and trastuzumab. Cancer Research. 2009;69(24Suppl):Abstract 710. [Google Scholar]
- 84.Baselga J, Gelmon KA, Verma S, et al. Phase II Trial of Pertuzumab and Trastuzumab in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer That Progressed During Prior Trastuzumab Therapy. J Clin Oncol. 2010 Mar 1;28(7):1138–44. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010 Mar 10;28(8):1301–7. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]


