Abstract
Recent progress in using stem cells for tissue repair and functional restoration has aroused much attention due to its potential to provide a cue for many diseases such as myocardial infarction. Stem cell therapy for cardiovascular disease has been studied extensively at both experimental and clinical levels. Pluripotent stem cells and mesenchymal stem cells were proven to be effective for myocardial regeneration, angiogenesis, and cardiac functional restoration. In this review, we will concisely discuss advantages and disadvantages of currently-used stem cells for cardiovascular repair and regeneration. The limitations and uniqueness of some types of stem cells will also be discussed. Although substantial progress has been made over the last decade about stem cells in cardiovascular regeneration, many challenges lie ahead before the therapeutic potentials of stem cells can be fully recognized.
Keywords: Adult stem cells, Cardiovascular disease, Embryonic stem cells, Endothelial progenitor cells, Hematopoietic stem cells, Mesenchymal stem cells, Myocardial repair, Pluripotent potent stem cells
Introduction
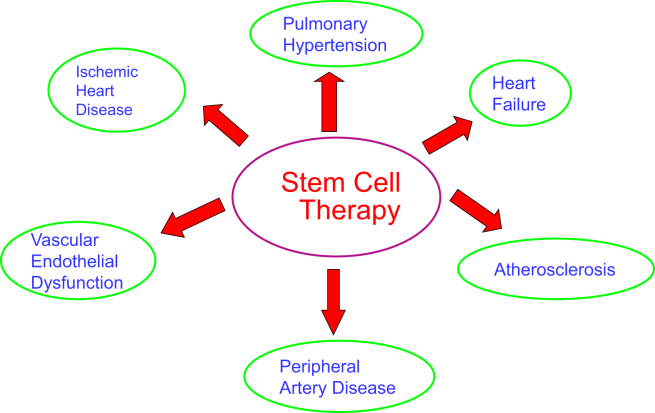
Although numerous pharmacological drugs are available, cardiovascular disease remains the leading cause of death worldwide.1, 2 There are over 5 million patients suffering from chronic heart failure post acute myocardial infarction due to no effective treatment.1 With the advent of an aging society in developed countries, an increased risk for cardiovascular disease and huge burden on human resources and health budgets are promised in the near future.3 Therefore, there is an urgent need to develop more effective therapeutic approaches for cardiovascular disease. The emerging of cardiovascular regenerative medicine may provide an encouraging direction for future therapeutics, which focuses on replacing or regenerating damaged myocardium and blood vessels to restore or establish normal cardiac function.4 Great advantages exist in this new treatment compared to traditional therapy, which was considered only palliative strategy. Traditional cardiac therapy are effective in resolving the acute processes of the disease and extending the patient's lifespan, however, it does not provide the patients a cure, but rather leaving them with chronic diseases as sequelae.5 In contrast, cardiovascular regenerative medicine makes it possible to replace damaged myocardial cells with the patient-specific pluripotent stem cell-derived cardiac myocytes, avoiding the aforementioned medical problems, and prevents or reverses the disease development.4 Moreover, most of the pluripotent stem cells, except for embryonic stem cells, are derived from the patients and possess the same genotype and phenotype, and thus are significantly valuable tools for studying the molecular mechanisms of the disease and for developing patient-specific therapy.6 Stem cell therapy has demonstrated beneficial effects on several cardiovascular diseases including ischemic heart disease,7, 8 heart failure,9 endothelial dysfunction,10, 11 peripheral artery disease,12, 13 atherosclerosis,14 and pulmonary hypertension15, 16 (Fig. 1).
Fig. 1.
Potential stem cell therapy for major cardiovascular disorders.
Stem cells have potential to differentiate into several specific types of cells. Based on the source of origin, stem cells can be classified into embryonic stem cells (ESCs) and adult stem cells (ASCs), where the former comes from embryos and the latter originates from mature adults.17 Furthermore, the ASCs can be divided into tissue-specific stem cells and bone marrow-derived stem cells (BMCs). Bone marrow contains at least two types of stem cells: hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs), and endothelial progenitor cells (EPCs) can also be found in the bone marrow.18 In this review, we will summarize the properties of different types of stem cells and their regenerative capabilities to restore or repair cardiac structure and function. We will also discuss selection and acquisition of stem cells and evaluation of effectiveness of stem cell therapy for cardiovascular disease.
Pluripotent stem cells
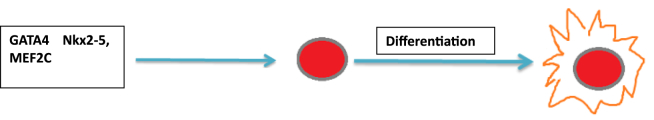
The goal of cardiac stem cell therapy is to restore or regenerate myocardium. The challenge was to identify a suitable source for generating sufficient and phenotypically confirmed cardiomyocytes. Over the past decade, rapid progress has been made in identification, derivation, and characterization of stem cells or progenitor cells. Among these, the embryonic stem cells (ESCs) have attracted attention due to their unique properties.6 ESCs are pluripotent stem cells which are derived from the inner cell mass of the blastocyst-stage embryo.19 Specifically, these cells remain in an undifferentiated state in culture for a long period but retain the potential to differentiate into all cell types in the human body, including cardiomyocytes. Human ESCs (hESCs) were isolated by Thomson et al – more than a decade after the first isolation of mouse ESCs (mESCs) in 1981.20, 21 Since then, the potential for using these unending multipotent cells to treat congenital and degenerative diseases has aroused great interest. Theoretically, the hESCs are capable to differentiate into all three germ layers-endoderm, ectoderm, and mesoderm, so it is important to investigate the signaling pathways and transcription factors that direct its specific differentiation process.19 From a large number of studies, researchers have identified several signaling molecules that are involved in early cardiac differentiation. Over-expression of transcriptional factors such as GATA4, Nkx2-5, or MEF2C can induce differentiation of hESCs into cardiomyocytes (Fig. 2), while inhibition of these factors halts the formation of cardiomyocytes.22 Other factors like growth factors TGFβ1 and FGF2, cardiotrophin, reactive oxygen species (ROS), and dimethyl sulfoxide (DMSO) might also affect the differentiation process.23, 24
Fig. 2.
Effects of transcription factors on hESC differentiation. Over-expression of key transcriptional factors such as GATA4, Nkx2-5, or MEF2C leads to differentiation of human embryonic stem cells (hESCs) into cardiomyocytes. Inhibition of these transcription factors maintain hESC pluripotency.
Despite the exciting achievements of hESCs-differentiated cardiomyocytes in both murine and human models, several pressing issues limit its clinical application. First of all, hESCs research has raised some serious ethical problems due to the fact that the establishment hESCs required the destruction of early human embryos, which was considered crimes against humanity.21 Additionally, the ESCs are generated from embryos and do not retain the same genome with the patients, thus has potential risk of immune rejection after transplantation.6 Furthermore, grafted ESCs in mice only generated a minor population of cardiomyocytes, which was even less in the human model.25 Since the number of differentiated cardiomyocytes significantly compromises the ultimate success of future cell-grafting procedures, strategies to enhance the generation of myocytes by ESCs is of crucial importance. Finally, in vivo implantation of hESCs gave rise to formation of teratocarcinomas (Table 1), although the malignant tumorigenic potential of ESCs is not well defined yet, this finding raises concerns about the safety of its clinical use.26
Table 1.
Comparison of advantages and limitations of different types of stem cells.
| Stem cell type | Limitations | Advantages |
|---|---|---|
| Embryonic stem cells |
|
Can differentiate into cells of all three germ layers |
| Pluripotent stem cells |
|
Avoids ethical concerns |
| Adult stem cells |
|
|
| Mesenchymal stem cells | More research needed |
|
| Hematopoietic stem cells |
|
|
| Endothelial progenitor cells | Extremely low numbers in peripheral blood and bone marrow makes ex vivo expansion difficult | Increase its numbers in response to ischemia/cytokine stimuli and can migrate to injury site and differentiate into new myocytes |
In contrast, the use of human inducible pluripotent stem cells (hiPSCs) overcomes the limitations and ethical concerns of hESCs (Table 1).27 The hiPSCs are derived by reprogramming somatic cells to a pluripotent state.28, 29 Several research teams have compared hESCs and hiPSCs and their differentiated progeny. Laurent et al reported that both hESCs and hiPSC demonstrate similar degree of genomic instability,30, 31 suggesting that much investigation is needed before evaluating their potential for cardiovascular repair and regeneration. Other groups reported that the ability of hESCs and hiPSCs to differentiate into cardiomyocytes is different.32, 33 Parthenogenetic genetic stem cells, another class of pluripotent stem cells, may also avoid disadvantages of hESCs with possibility of reduced immune rejection.27 Didie et al showed that mouse parthenogenetic stem cell-derived cardiomyocytes integrated electrically with host myocardium and improved cardiac function in a mouse model of myocardial infarction.34 Table 1 summarizes the limitations and advantages of using different types of stem cells for cardiovascular therapy.
Adult stem cells
As discussed above, there are political, ethnical and technical concerns in clinical application of hESCs. Recent advances in adult stem cell research might offer a promising advanced alternative for cell-based therapies.4 The use of adult stem cells avoids the ethical and religious fights triggered by hESCs; they possess the same genotype of the patient since they are isolated from adults, thus minimizing the risk of immune rejection (Table 1).35 For all these reasons, the adult stem cells seem more applicable to be used in cell-based cardiovascular repair and regeneration. The heart was previously considered a terminally differentiated organ without the capacity for self-renewal. However, recent evidence suggests the existence of endogenous cardiac stem cells (CSCs) that are able to generate new myocytes and blood vessels.36 Typically, only a small number of CSCs cells (one for every 1 × 103 myocytes) are distributed in the atria, ventricles, and epicardium. In physiological conditions, CSCs are inactive and exiting cell cycle, with only 2–3% actively differentiated for normal myocardial turnover. However, most of the CSCs can be activated and differentiated into new myocytes or vascular cells in response to pathological or physiological stimuli such as ischemia or cellular damage.37 Although some experiments demonstrated that injection of CSCs can generate new myocardium to replace the cells lost in MI,36 the natural regeneration capacity of CSCs are too limited to be used in clinical therapy, and therefore required ex vivo expansion (Table 1).38 There is great advantage to use this organ origin stem cell for myocyte replacement and repair as long as it has been effectively stimulated. However, major difficulties exist in the acquisition and isolation of CSCs from myocardial samples, reducing available CSCs to be used for implantation.35 Furthermore, the molecular mechanism that regulates the CSCs proliferation and differentiation into myocardium has not been elucidated. Despite numerous publications, no consensus has been reached on the identity and actual regenerative or renewal effects of CSCs. Thus, the application of CSCs in cardiovascular disease will remain difficult until all these limitations are appropriately addressed. In addition, attention and effort should be paid to restoration of the fibroblasts function which provides a favorable environment for repair and regeneration of cardiomyocytes.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) were reported by Friedenstein et al who identified a sub-population of bone marrow cells that adhered to plastic and demonstrated fibroblast-like properties.39 MSCs have potential to differentiate into a variety of mesoderma lineage cells (e.g., osteoblasts, adipocytes, and cadiomyocytes).40, 41 Therefore, MSCs, also termed bone marrow stromal cells, are pluripotent progenitor cells of bone marrow origin.42 Human MSCs have distinct surface markers from hematopoietic stem cells: CD105 (SH2), SH3, Stro-1, and CD13.43 MSCs are considered immunologically privileged stem cells due to their lack of surface markers (antigens) required for activation of T lymphocytes.40 In an MHC-mismatched rat heart transplantation model, MSCs can induce tolerance and long-term graft acceptance.44 It was reported that the immunosuppressive effect of MSCs may be mediated by inhibiting the maturation of dendritic cells and suppressing the function of T, B, and natural killer cells.41, 45 Interestingly, transplanted MSCs also secrete paracrine factors to regulate the immune system and modulate inflammatory responses.40 These unique features make MSCs attractive for future regenerative medicine such as tissue repair and gene delivery, allowing allogenic grafting without the use of immunosuppressive agents (Table 1).
MSCs are an ideal source of replacement cells because of their potential for self-renewal, proliferation and differentiation.46, 47, 48 It was shown that human MSCs injected into the left ventricle of an adult mouse heart effectively engrafted in the myocardium and differentiated into cardiomyocytes that were morphologically indistinguishable from the native cardiomyocytes.49 Notably, MSCs also promote the growth and proliferation of adjacent cells via their paracrine function.41 Although MSCs are known to secrete a variety of regulatory and trophic factors including growth factors, cytokines, and chemokines, the nature of the secretome remains to be determined.50 MSCs can enter the circulation and follow chemotactic gradients to home to sites of injury or inflammation participating in wound healing and tissue repair via its regenerative and paracrine function.51, 52, 53, 54 In addition, MSCs also have other characteristics that facilitate their clinical application, such as their expansion potential, ease of collection, and decreased susceptibility to genetic mutations during in vitro passages.55 As a guide for future directions, MSCs engineered with desired therapeutic genes may expand and enhance their therapeutic potentials.
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) are the foundation of adult hematopoiesis and give rise to all types of blood cells throughout the lifespan.56 HSCs are of clinical significance in bone marrow transplantation for the treatment of blood–related genetic deficiency and leukemia.57, 58 HSCs are defined as multipotent stem cells, which have the capacity to differentiate into a number of cells, including cardiomyocytes and endothelial cells.38 HSCs can be isolated from the bone marrow as well as the peripheral blood, but its circulating forms are much lower than in the bone marrow.17 In the normal condition, the number of quiescent HSCs is limited in the bone marrow (one for every 1 × 104 bone marrow cells).17 In response to physiological or pathological stimuli, these stem cells can quickly proliferate and mobilize from their resident bone marrow to peripheral circulation, and then migrate to the site of injury.18 Murine progenitor cells do not have specific surface markers, whereas human HSCs express surface markers: CD34 and AC133, which can be used for positive selection and isolation.59 By characterization of CD34+-CD38- phenotype stem cells, researchers found that blood from the human umbilical cord is a relatively abundant source of HSCs.60 Moreover, highly purified CD34+-CD38- hematopoietic progenitors were also isolated from human fetal livers.61 It was reported that HSCs could result in cardiomyocyte generation via myeloid intermediates by fusion-dependent mechanism.62 The employment of myeloid derivatives as donor cells may provide more effective cell-based therapy for cardiac repair.62 The advantage of using HSCs in cardiovascular repair underlies that they potentially fulfill the goals required for stem cell transplantation—myogenesis and angiogenesis (Table 1). However, their low frequencies, difficult maintenance in cell culture, as well as the unknown signaling pathways that regulate the HSCs proliferation and differentiation, must be addressed.17 In addition, specific induction of HSCs into cardiomyocytes must be guaranteed to avoid tumorigenesis.
Endothelial progenitor cells
Endothelial progenitor cells (EPCs) and their derivatives have been reported to have therapeutic potential for cardiovascular disease.11 EPCs were reported in 1997 by Asahara et al who showed that purified CD34+ hematopoietic progenitor cells from adults can differentiate into endothelial cells and participate in postnatal angiogenesis.63 Although originated in the bone marrow, EPCs can also be found in the peripheral blood and human umbilical cord blood.18 EPCs share two surface antigens with HSCs, CD34 and the VEGF receptor Flk-1, and double labeling of these two markers can be used for positive selection of highly homogenous EPCs populations.38 Under normal condition, the number of EPCs is extremely low in the peripheral blood and bone marrow, which makes it difficult for the ex vivo expansion of sufficient EPCs required for clinical applications.17 EPCs are angiogenic cells that mobilized in response to ischemia or exogenous cytokines which further guide EPCs homing into sites of ischemic tissue, and thus participate in the repair and maintenance of vascular homeostasis (Table 1).38 It was reported that EPCs have proliferative potential and play a vital role in vascular regeneration by replacing or restoring damaged endothelial cells.64 In the field of cardiac research, EPCs are found to increase its circulating numbers in response to myocardial ischemia or cytokine stimuli, homing to the site of myocardium injury and possibly differentiating into new myocytes.65, 66 EPCs have the capacity to circulate, proliferate and differentiate into mature endothelial cells but have neither acquired mature endothelial markers nor formed a lumen.67 It was reported that transplanted autologous EPCs improved mean pulmonary artery pressure, cardiac output and pulmonary vascular resistance in a dog model of pulmonary hypertension.68
Technical challenges of using stem cells for cardiovascular repair
To select appropriate stem cells for cardiovascular tissue regeneration and functional repair, the following issues might be considered: 1) acquisition of adequate stem cells, 2) ex vivo expansion efficiency, 3) the optimal approach of administration, 4) In vivo differentiation efficiency and functional integration.
Acquisition of adequate stem cells
It is challenging for acquisition of adequate ESCs due to limitations as discussed above. In contrast, isolation of BMCs such as hematopoietic, mesenchymal, or endothelial progenitor stem cells are relatively easier because they can be collected either from the peripheral blood or bone barrow. In addition, the organ-originated stem cells such as CSCs may also be difficult to collect because of their unique location and limited number of stem cells.
Ex vivo expansion efficiency
The ex vivo expansion and purification of specific subpopulations are required to facilitate their utilization because the number of stem cells from direct in vivo isolation is limited. Moreover, for ESCs, in vitro pre-differentiation can help prevent the risk of teratoma formation.69 The stimulation methods for optimal expansion and specific selection vary with cell types. For instance, administration of exogenous cytokines is often used to facilitate the differentiation of ESCs to specific cells.55 Flowcytometry is used for positive selection of new cardiomyocytes. The common cardiac markers for fluorescent labeling include GATA4, Nkx2.5, α-MHC, β-MHC, and ANP.19 Another popular method to track the generation of cardiomyocytes is encoding target gene with enhanced green fluorescent protein (GFP). The subsequent sorting of the GFP-positive cells yields a high level of purified myocyte subpopulation.55 The selected cells must be screened to ensure functional properties of cardiomyocytes before transplantation. The myocytes should have automaticity and exhibit action potentials. The early myocytes may show immature AP properties, but the functional maturity is expected with prolonged duration in culture.
Optimal administration approach
Three approaches have been tested for delivery of stem cells to the cardiovascular system (intravenous vs. intracoronary vs. intramyocardial). Stem cell transplantation into heart can be carried out via the intracoronary route or coronary sinus, or directly administered to the site within myocardium using endocardial mapping devices.38 Moreover, bone marrow-derived cells can be administered intravenously, and homing of these cells to sites of injured myocardium can be confirmed using specific markers.55 To determine the best administration approach for candidate stem cells, it is necessary to test and compare all three delivery methods in an animal model of MI. Thus, the maximal homing rate of stem cells to the site of injured heart in conjunction with the most efficient approach for delivery (e.g. intravenous) can be selected as the optimal approach for future clinical transplantation. The delivery of stem cells into the pulmonary circulation via jugular veins is selected as an effective approach for the treatment of pulmonary arterial hypertension.
In vivo differentiation efficiency and functional integration
Several clinical trials indicated that the implanted stem cells may not be able to function as competent myocytes. The improvement of cardiac function may be due to other possibilities, including activation of resident myocyte function or amplification of the endogenous repair process via the paracrine effect mediated by transplanted cells.19, 35 The structural and functional characteristics of stem cell-derived cardiomyocytes, and their ability to integrate into the host tissue, will need to be evaluated. First, the structural proteins in mature myocytes such as sarcomeric organization, e.g., α-actin, cardiac troponins, myosin heavy chain, myosin light chain, desmin, and tropomyosin, need to be confirmed. Second, the electrophysiological studies should be performed to study the ion channels and propagation of action potential in stem cell-derived cardiomyocyte. Furthermore, other parameters (e.g., the intracellular calcium) that depict normal myocyte function should also be examined.70 In addition, whether gap junctions are present between host and donor cardiomyocytes, and whether the electromechanical and structural integration have been formed between host and donor cardiomyocytes need to be examined. Finally, to assess whether stem cell transplantation can restore, maintain, or improve impaired heart function, it is necessary to assess clinical parameters such as cardiac pump function, coronary blood flow, cardiac remodeling, and cardiac oxygenation. Vascular endothelial function can be assessed by in vivo blood pressure responses to endothelial-derived vasodilators. The long-term therapeutic effect also need to be monitored to determine whether this treatment improves lifespan and decreases mortality. Lastly, the potential side effects of stem cell therapy must be fully evaluated to guide future clinical application.
Conflicts of interest
The authors have no conflict of interest.
Acknowledgment
This work was supported by the NIH R01 HL105302, HL102074, HL118558, HL116863, and DK 093403.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauer M.S. Advancing cardiovascular research. Chest. 2012;141(2):500–505. doi: 10.1378/chest.11-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart S., MacIntyre K., Capewell S., McMurray J.J. Heart failure and the aging population: an increasing burden in the 21st century? Heart (Br Card Soc. 2003;89(1):49–53. doi: 10.1136/heart.89.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacic J.C., Harvey R.P., Dimmeler S. Cardiovascular regenerative medicine: digging in for the long haul. Cell Stem Cell. 2007;1(6):628–633. doi: 10.1016/j.stem.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Nadal-Ginard B., Torella D., Ellison G. [Cardiovascular regenerative medicine at the crossroads. Clinical trials of cellular therapy must now be based on reliable experimental data from animals with characteristics similar to human's] Rev Espanola Cardiol. 2006;59(11):1175–1189. [PubMed] [Google Scholar]
- 6.Murata M., Tohyama S., Fukuda K. Impacts of recent advances in cardiovascular regenerative medicine on clinical therapies and drug discovery. Pharmacol Ther. 2010;126(2):109–118. doi: 10.1016/j.pharmthera.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y., Jiang S., Hu R., Lv L. Potential mechanism for endothelial progenitor cell therapy in acute myocardial infarction: activation of VEGF- PI3K/Akte-NOS pathway. Ann Clin Lab Sci. 2013;43(4):395–401. [PubMed] [Google Scholar]
- 8.Traverse J.H., Henry T.D., Pepine C.J., Willerson J.T., Ellis S.G. One-year follow-up of intracoronary stem cell delivery on left ventricular function following ST-elevation myocardial infarction. JAMA. 2014;311(3):301–302. doi: 10.1001/jama.2013.282674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez L.A., Guerrero-Beltran C.E., Cordero-Reyes A.M., Garcia-Rivas G., Torre-Amione G. Use of stem cells in heart failure treatment: where we stand and where we are going. Methodist Debakey Cardiovasc J. 2013;9(4):195–200. doi: 10.14797/mdcj-9-4-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong M., Yu B., Wang Y.G., Xu M. Bone marrow rejuvenation. An excellent potential therapy for age-related endothelial dysfunction. Circ J. 2013;77(12):2886–2888. doi: 10.1253/circj.cj-13-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jian-Yong X., Yee-Ki L., Wang Y., Hung-Fat T. Therapeutic application of endothelial progenitor cells for treatment of cardiovascular diseases. Curr Stem Cell Res Ther. 2014;9(5):401–414. doi: 10.2174/1574888x09666140619121318. [DOI] [PubMed] [Google Scholar]
- 12.Gupta N.K., Armstrong E.J., Parikh S.A. The current state of stem cell therapy for peripheral artery disease. Curr Cardiol Rep. 2014;16(2):447. doi: 10.1007/s11886-013-0447-2. [DOI] [PubMed] [Google Scholar]
- 13.Botham C.M., Bennett W.L., Cooke J.P. Clinical trials of adult stem cell therapy for peripheral artery disease. Methodist Debakey Cardiovasc J. 2013;9(4):201–205. doi: 10.14797/mdcj-9-4-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Zhang Y., Peng H., Liu P. [Progress in mesenchymal stem cells for treatment of atherosclerosis] Sheng Wu Gong Cheng Xue Bao. 2013;29(11):1538–1547. [PubMed] [Google Scholar]
- 15.Kanki-Horimoto S., Horimoto H., Mieno S. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114(1 Suppl):I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 16.Takemiya K., Kai H., Yasukawa H., Tahara N., Kato S., Imaizumi T. Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic Res Cardiol. 2010;105(3):409–417. doi: 10.1007/s00395-009-0065-8. [DOI] [PubMed] [Google Scholar]
- 17.Asahara T., Kalka C., Isner J.M. Stem cell therapy and gene transfer for regeneration. Gene Ther. 2000;7(6):451–457. doi: 10.1038/sj.gt.3301142. [DOI] [PubMed] [Google Scholar]
- 18.Borlongan C.V., Glover L.E., Tajiri N., Kaneko Y., Freeman T.B. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lev S., Kehat I., Gepstein L. Differentiation pathways in human embryonic stem cell-derived cardiomyocytes. Ann N. Y Acad Sci. 2005;1047:50–65. doi: 10.1196/annals.1341.005. [DOI] [PubMed] [Google Scholar]
- 20.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 21.Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 22.Skerjanc I.S., Petropoulos H., Ridgeway A.G., Wilton S. Myocyte enhancer factor 2C and Nkx2-5 up-regulate each other's expression and initiate cardiomyogenesis in P19 cells. J Biol Chem. 1998;273(52):34904–34910. doi: 10.1074/jbc.273.52.34904. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T., Takahashi T., Esaki M. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circulation journal. Off J Jpn Circ Soc. 2004;68(7):691–702. doi: 10.1253/circj.68.691. [DOI] [PubMed] [Google Scholar]
- 24.Sauer H., Neukirchen W., Rahimi G., Grunheck F., Hescheler J., Wartenberg M. Involvement of reactive oxygen species in cardiotrophin-1-induced proliferation of cardiomyocytes differentiated from murine embryonic stem cells. Exp Cell Res. 2004;294(2):313–324. doi: 10.1016/j.yexcr.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Amit M., Carpenter M.K., Inokuma M.S. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227(2):271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 26.Chambers I., Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23(43):7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 27.Lundy S.D., Gantz J.A., Pagan C.M., Filice D., Laflamme M.A. Pluripotent stem cell derived cardiomyocytes for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16(7):319. doi: 10.1007/s11936-014-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K., Tanabe K., Ohnuki M. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Laurent L.C., Ulitsky I., Slavin I. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8(1):106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller F.J., Schuldt B.M., Williams R. A bioinformatic assay for pluripotency in human cells. Nat Methods. 2011;8(4):315–317. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toivonen S., Ojala M., Hyysalo A. Comparative analysis of targeted differentiation of human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells reveals variability associated with incomplete transgene silencing in retrovirally derived hiPSC lines. Stem Cells Transl Med. 2013;2(2):83–93. doi: 10.5966/sctm.2012-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sepac A., Si-Tayeb K., Sedlic F. Comparison of cardiomyogenic potential among human ESC and iPSC lines. Cell Transpl. 2012;21(11):2523–2530. doi: 10.3727/096368912X653165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Didie M., Christalla P., Rubart M. Parthenogenetic stem cells for tissue-engineered heart repair. J Clin Invest. 2013;123(3):1285–1298. doi: 10.1172/JCI66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leri A., Kajstura J., Anversa P., Frishman W.H. Myocardial regeneration and stem cell repair. Curr Problems Cardiol. 2008;33(3):91–153. doi: 10.1016/j.cpcardiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Beltrami A.P., Barlucchi L., Torella D. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 37.Urbanek K., Quaini F., Tasca G. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U.S.A. 2003;100(18):10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbott J., Giordano F. Stem cells and cardiovascular disease. J Nucl Cardiol. 2003;10(4):403–412. doi: 10.1016/s1071-3581(03)00580-4. [DOI] [PubMed] [Google Scholar]
- 39.Friedenstein A.J., Piatetzky S., II, Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–390. [PubMed] [Google Scholar]
- 40.Krause K., Schneider C., Kuck K.H., Jaquet K. Stem cell therapy in cardiovascular disorders. Cardiovasc Ther. 2010;28(5):e101–110. doi: 10.1111/j.1755-5922.2010.00208.x. [DOI] [PubMed] [Google Scholar]
- 41.Thakker R., Yang P. Mesenchymal stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16(7):323. doi: 10.1007/s11936-014-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittenger M.F., Mackay A.M., Beck S.C. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 43.Stem Cell Repair and Regeneration: Imperial College Press; 2007.
- 44.Popp F.C., Eggenhofer E., Renner P. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20(1–2):55–60. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Bassi E.J., Aita C.A., Camara N.O. Immune regulatory properties of multipotent mesenchymal stromal cells: where do we stand? World J Stem Cells. 2011;3(1):1–8. doi: 10.4252/wjsc.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caplan A.I. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 47.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caplan A.I., Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 49.Toma C., Pittenger M.F., Cahill K.S., Byrne B.J., Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 50.Ranganath S.H., Levy O., Inamdar M.S., Karp J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10(3):244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weil B.R., Manukyan M.C., Herrmann J.L. The immunomodulatory properties of mesenchymal stem cells: implications for surgical disease. J Surg Res. 2011;167(1):78–86. doi: 10.1016/j.jss.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapel A., Bertho J.M., Bensidhoum M. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5(12):1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 53.Chavakis E., Dimmeler S. Homing of progenitor cells to ischemic tissues. Antioxid Redox Signal. 2011;15(4):967–980. doi: 10.1089/ars.2010.3582. [DOI] [PubMed] [Google Scholar]
- 54.Chavakis E., Urbich C., Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45(4):514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Stem Cell Biology and Regenerative Medicine; 2012.
- 56.Balmer G.M., Riley P.R. Harnessing the potential of adult cardiac stem cells: lessons from haematopoiesis, the embryo and the niche. J Cardiovasc Transl Res. 2012;5(5):631–640. doi: 10.1007/s12265-012-9386-3. [DOI] [PubMed] [Google Scholar]
- 57.Johnson F.L., Look A.T., Gockerman J., Ruggiero M.R., Dalla-Pozza L., Billings F.T., 3rd Bone-marrow transplantation in a patient with sickle-cell anemia. N. Engl J Med. 1984;311(12):780–783. doi: 10.1056/NEJM198409203111207. [DOI] [PubMed] [Google Scholar]
- 58.Johnson F.L., Sanders J.E., Ruggiero M., Chard R.L., Jr., Thomas E.D. Bone marrow transplantation for the treatment of acute nonlymphoblastic leukemia in children aged less than 2 years. Blood. 1988;71(5):1277–1280. [PubMed] [Google Scholar]
- 59.Peichev M., Naiyer A.J., Pereira D. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 60.Hao Q.L., Shah A.J., Thiemann F.T., Smogorzewska E.M., Crooks G.M. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood. 1995;86(10):3745–3753. [PubMed] [Google Scholar]
- 61.Humeau L., Chabannon C., Firpo M.T. Successful reconstitution of human hematopoiesis in the SCID-hu mouse by genetically modified, highly enriched progenitors isolated from fetal liver. Blood. 1997;90(9):3496–3506. [PubMed] [Google Scholar]
- 62.Fukata M., Ishikawa F., Najima Y. Contribution of bone marrow-derived hematopoietic stem/progenitor cells to the generation of donor-marker(+) cardiomyocytes in vivo. PLoS One. 2013;8(5):e62506. doi: 10.1371/journal.pone.0062506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urbich C., Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 64.Barst R. Is it possible to reverse the endothelial dysfunction in pulmonary arterial hypertension? J Am Coll Cardiol. 2007;49(14):1572–1574. doi: 10.1016/j.jacc.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 65.Shintani S., Murohara T., Ikeda H. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 66.Badorff C., Brandes R.P., Popp R. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107(7):1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 67.Wang X.X., Zhang F.R., Shang Y.P. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49(14):1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi M., Nakamura T., Toba T., Kajiwara N., Kato H., Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004;10:771–779. doi: 10.1089/1076327041348563. [DOI] [PubMed] [Google Scholar]
- 69.Dressel R., Schindehütte J., Kuhlmann T. The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients' immune response. PLoS One. 2008;3(7):e2622. doi: 10.1371/journal.pone.0002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mauritz C., Schwanke K., Reppel M. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118(5):507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]