Abstract
The combination of efavirenz with HIV-1 protease inhibitors (PI) results in complex interactions secondary to mixed induction and inhibition of oxidative metabolism. ACTG A5043 was a prospective, open-label, controlled, two-period, multiple-dose study with 55 healthy volunteers. The objective of the present study was to evaluate the potential pharmacokinetic interaction between efavirenz and dual PIs. The subjects received a daily dose of 600 mg efavirenz for 10 days with amprenavir 600 mg twice daily added at day 11 and were randomized to receive nelfinavir, indinavir, ritonavir, saquinavir, or no second PI on days 15–21. Intensive pharmacokinetic studies were conducted on day 14 and 21. Efavirenz plasma concentrations were fit to candidate models using weighted non-linear regression. The disposition of efavirenz was described by a linear two-compartment model with first order absorption following a fitted lag time. Apparent clearance (CLt/F), volume of distribution at steady state (Vss/F), inter-compartmental clearance, and the central and peripheral volume of distribution were estimated. The mean CLt/F and Vss/F of efavirenz were 0.126 l/h/kg and 4.412 l/kg, respectively. Both AUC and CLt/F of efavirenz remained unchanged after 7 days of dual PI dosing. The mean Vss/F of efavirenz increased an average of 89% across arms, ranging from 52% (nelfinavir) to 115% (indinavir) relative to efavirenz with amprenavir alone. Increases were also observed in Vp/F after the addition of nelfinavir, indinavir, ritonavir and saquinavir by 85%, 170%, 162% and 111%, respectively. In conclusion, concomitant administration of dual PIs is unlikely to have any clinically significant effect on the pharmacokinetics of CYP2B6 substrates in general or oral efavirenz specifically.
Keywords: pharmacokinetic modeling, drug interactions, non-nucleoside reverse transcriptase inhibitor, efavirenz, HIV-1 protease inhibitors
Introduction
The combination of efavirenz with HIV-1 protease inhibitors (PI) results in complex pharmacokinetic interactions secondary to mixed induction and inhibition of oxidative metabolism [1]. Efavirenz is converted to inactive metabolites by the cytochrome P450 enzymes, primarily CYP3A4 and CYP2B6 [2,3]. Since all currently available PIs are CYP3A4 inhibitors with varying inhibitory and induction activities, significant drug interactions have been reported when efavirenz and PIs are used in combination [4–7]. It has been reported that ritonavir produced a 21% increase in efavirenz exposure, whereas the hepatic clearance of efavirenz was unaltered by weaker inhibitors, such as indinavir, nelfinavir or saquinavir [8]. However, few data are available on the administration of efavirenz with dual PIs and the pharmacokinetic interactions that result at steady-state.
Adult AIDS Clinical Trial Group (ACTG) protocol A5043 was developed to examine these interactions, in which a second PI was introduced after a prior period of efavirenz and amprenavir in HIV-seronegative subjects [1]. Previous ACTG studies utilizing similar NNRTI-PI combinations have reported clinical efficacy and pharmacokinetic data for these combinations when taken together [9]. The primary goal of A5043 was to extend these studies and obtain additional data on indinavir, nelfinavir and saquinavir containing regimens and their dosage requirements when combined with amprenavir and efavirenz in healthy volunteers. At the time A5043 was developed, the routine use of low-dose ritonavir was not considered to be the standard of care, and the optimal approach to combining two PIs with efavirenz was under investigation. One of the objectives of A5043 was to obtain additional data to examine influence of dual PIs on efavirenz pharmacokinetics. Some results from A5043 are already published [1]. This report presents a compartmental model analysis for efavirenz and pharmacokinetic evaluation of efavirenz with dual PI administration.
Materials and methods
Study population
Healthy males or females were included in the study according to the following criteria: age between 18 and 65 years; standard medical examination, including electrocardiogram and laboratory tests, without signs of acute or chronic illness; seronegative for HIV. Individuals with a history of alcohol abuse, suspected allergic or other serious reactions to drugs, psychiatric disease or diseases that could interfere with drug metabolism were excluded. All participants gave written informed consent and the protocol was approved by the Institutional Review Board of all participating institutions.
Study design
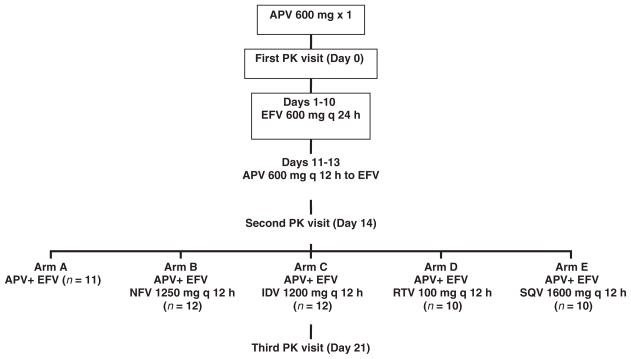
This was a prospective, open-label, controlled, three-period multiple dose study with 55 healthy volunteers. All participants were instructed to initiate efavirenz dosing (600 mg, once daily) in the evening and to switch to morning administration on day 8. On day 11, participants began 600 mg amprenavir twice daily in addition to the efavirenz followed by a 12 h pharmacokinetic evaluation on day 14 after the morning dose of efavirenz and amprenavir. After the pharmacokinetic evaluation, participants were randomized to receive the combination of efavirenz and amprenavir (arm A), or these two agents plus nelfinavir 1250 mg twice daily (arm B), indinavir 1200 mg twice daily (arm C), ritonavir 100 mg twice daily (arm D), or saquinavir 1600 mg twice daily (arm E) for at least 7 days followed by a 12 h pharmacokinetic evaluation (Figure 1).
Figure 1.
Schematic representation of the study design and samples timing for A5043. APV: amprenavir; EFV: efavirenz; IDV: indinavir; NFV: nelfinavir; RTV: ritonavir; SQV: saquinavir. Pharmacokinetic evaluation of amprenavir was performed during the first PK visit on day 0
Blood sampling
Participants reported to the research facility early in the morning on day 14 and day 21 (Figure 1) after fasting overnight, and abstained from drinking alcohol or from performing high-impact exercise. They were allowed to drink water throughout the study. The first blood sample (0 h) was taken from a venous catheter, and a standardized breakfast was served. After the assigned medications were taken, blood samples were then collected in 7 ml sodium heparin tubes at 1, 2, 3, 4, 6, 8, 10 and 12 h post-dose. Samples were centrifuged within 30 min after collection for 10 min at 800 × g to separate the plasma, which was then frozen at −70°C until analysis.
Safety assessment and adverse events
All participants underwent clinical and laboratory evaluations at all pharmacokinetic study visits and at the final safety visit. Adverse events with a severity of grade 1 or above, as defined by the National Institute of Allergy and Infectious Diseases, Division of AIDS Toxicity Tables, were monitored by the study team. For each regimen within each treatment arm, the number of rashes and the number of other severe reactions were counted and were previously reported [1].
Determination of efavirenz concentrations
Plasma concentrations of efavirenz were determined by a validated liquid chromatography-tandem mass spectrometry (LC/MS/MS) method performed at the University at Buffalo AACTG Pharmacology Specialty Laboratory, which was also a participant in the quality assurance and proficiency testing program of the ACTG [10,11]. Briefly, efavirenz was isolated from plasma samples by liquid–liquid extraction with a mixture of hexane and ethyl acetate (1:1). The organic phase was transferred into clean glass tubes and evaporated to dryness at 50°C for 30 min. The residue was reconstituted with 125 μl of mobile phase and centrifuged at 6000 × g for 5 min to remove particulates. Two injections of each sample were made into the LC/MS/MS system. The first injection was for the analysis of protease inhibitor species, and the second injection was for the analysis of efavirenz. Efavirenz was eluted from a reversed phase Waters Symmetry™ C18 column (Milford, MA) using an isocratic mobile phase and was introduced into the MS (Applied Biosystems PE/Sciex API 3000, Applied Biosystems, Foster City, CA) via electrospray ionization operated in the negative mode. Data were collected and analysed utilizing the Analyst™ software program (Foster City, CA). The calibration range was 0.128 to 20.0 μg/ml. In each run, quality control samples of 0.48, 2.40 and 12.0 μg/ml were included. Efavirenz inter-assay variation expressed as the percentage coefficient of variance (%CV), was 7%, 7% and 6%, at 480, 1200 and 2400 ng/ml, respectively. The limit of determination was 205 ng/ml.
Pharmacokinetic analysis
The pharmacokinetics of efavirenz was evaluated based on concentration-time data obtained on days 14 and 21, using compartmental methods (ADAPT II, Los Angeles, CA). Candidate structural pharmacokinetic models were fit to the data using maximum likelihood followed by MAP-Bayesian analysis. Efavirenz concentration data were weighted by the inverse of the estimated measurement variance; it was assumed that the measurement standard deviation was linearly related to the fitted efavirenz concentration. Model discrimination was based upon Akaike’s Information Criteria. Pharmacokinetic parameters estimated by the model included: volume of distribution at steady-state, of central and peripheral compartments (Vss/F, Vc/F, Vp/F); total and distributional clearance (CLt/F, CLd/F); and terminal half life (T1/2). The area under the concentration-time curve (AUC) was determined by using the following equation: .
Statistical analysis
Sample size calculations were based on a two-sided paired t-test, with the type I error rate at 0.05, assuming a within-subject coefficient of variation (CV) in AUC of 20% for amprenavir. A sample size of 12 eligible subjects per arm provided 80% power to detect a 25% difference in AUC (e.g. percent change in amprenavir AUC without versus with co-administration of a second PI). Only subjects who were able to provide pharmacokinetic data on all study days were included in the statistical analysis of pharmacokinetic parameters. Within-subject differences between parameters on days 21 and 14 were evaluated using non-parametric Wilcoxon signed rank test, and a p value of 0.05 or less was considered statistically significant. The statistical analyses were performed using SYSTAT (SPSS Inc., Chicago, IL, USA).
Results
Study population
Fifty-nine participants completed all pharmaco-kinetic assessments; however, due to protocol errors, 55 were included in pharmacokinetic analyses. Data on the study population were summarized as medians and ranges: age 28 years (19–51), weight 78.6 kg (61.8–104.1) and height 1.80 m (1.64–1.95). Distributions by age, sex and race were similar for the subjects in each study arm who were included in the pharmacokinetic analyses. Only one of the 55 participants was female. Detailed demographic information has been previously reported [1].
Pharmacokinetics
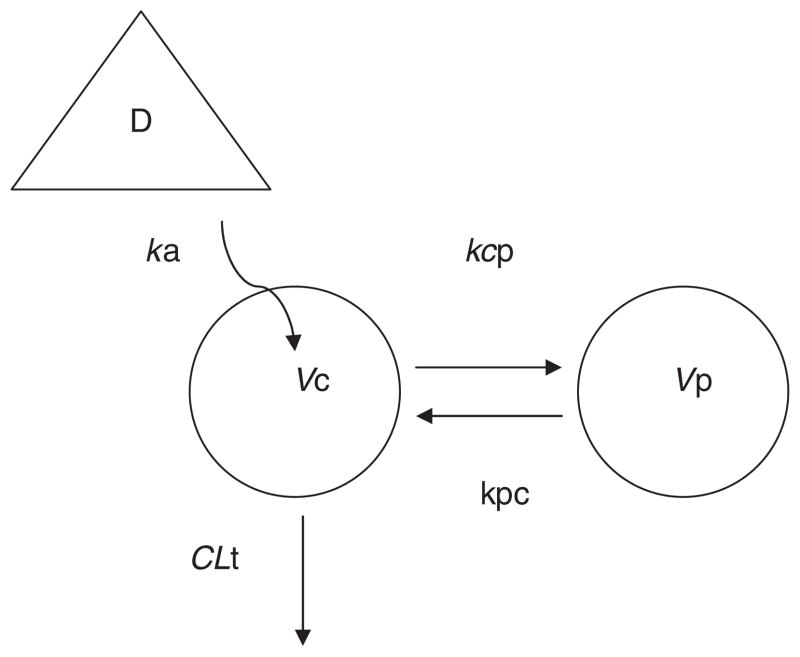
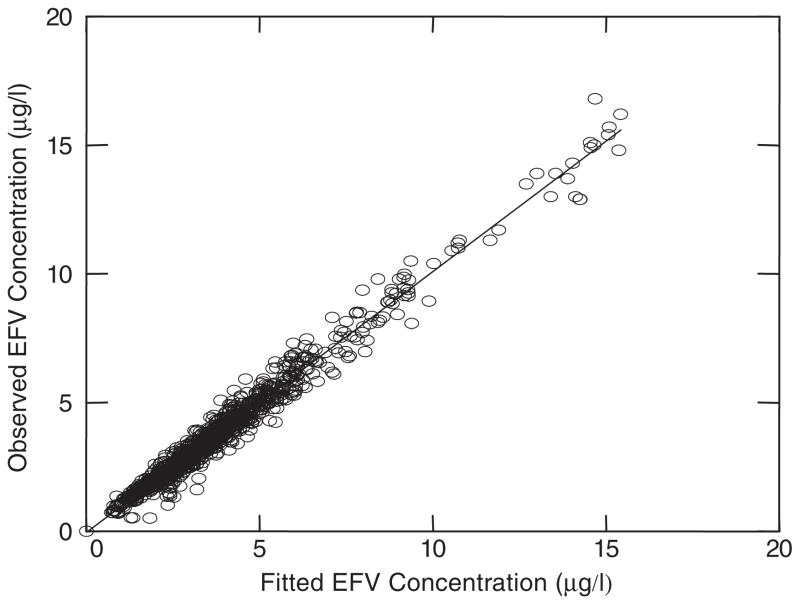
A linear two-compartment model with first order absorption was used to describe efavirenz pharmacokinetics. Figure 2 illustrates the schematic of this model. The goodness of fit of the final model to the plasma efavirenz data is presented in Figure 3. The fitted functions had no regions of bias and had excellent precision with an overall r2 value of 0.972.
Figure 2.
Schematic representation of the basic pharmacokinetic model of orally administered efavirenz. A linear model with two compartments (plus an absorption site, D=Dose); fitted TLag followed by first order absorption (ka) into the central compartment (Vc); distributional clearance (CLd), between Vc and a peripheral compartment (Vp); inter-compartmental rate constants: kcp=CLd/Vc and kpc=CLd/Vp; total volume of distribution (Vss) is the sum of Vc and Vp; total clearance (CLt) out of the central compartment (T1/2, terminal half life)
Figure 3.
Precision of fitted functions. Model predicted concentrations highly correlated with observed plasma concentrations of efavirenz (r2=0.972). Data represented 1137 observations from 55 subjects who completed two pharmacokinetic studies (EFV: efavirenz)
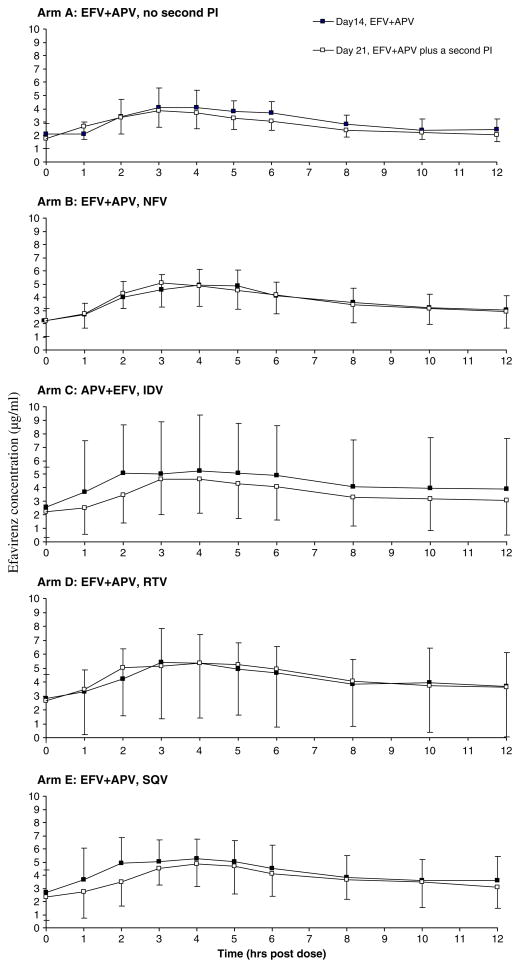
The pharmacokinetic parameters of efavirenz, as estimated by the final model, are summarized in Table 1. Figure 4 provides the mean plasma efavirenz concentration-time profiles in each arm. Co-administration of amprenavir and a second PI from day 14 to 21 affected efavirenz disposition only moderately. Both AUC and CLt/F of efavirenz remained unchanged after 7 days of dual-PI dosing. There were statistically significant changes but median percent differences were within 80–125%. Except for the RTV arm (D), the same is true for CLd/F. The mean Vss/F of efavirenz increased an average of 89% across arms, ranging from 52% (arm B nelfinavir, range −3–111%, p = 0.003) to 115% (arm C indinavir, range 68–152%, p = 0.002) relative to amprenavir alone (no second PI, day 14). Significant increases were also observed in Vp/F that addition of nelfinavir, indinavir, ritonavir and saquinavir into efavirenz and amprenavir regimen raised Vp/F by 85% (range 8–168%, p = 0.002), 170% (range 94–320%, p = 0.002), 162% (range 22–630%, p = 0.005) and 111% (range 56218%, p = 0.005), respectively. The mean Vc/F, however, considerably decreased for most of the arms. Compared with efavirenz t1/2 on day 14, it was prolonged by 66% (range 6–152%, p = 0.002), 116% (range 47–260%, p = 0.002), 167% (range 6–690%, p = 0.005) and 78% (range 13–123%, p = 0.005) for nelfinavir, indinavir, ritonavir and saquinavir, respectively. Relative to efavirenz ka and tlag after 14 days of amprenavir co-administration, the adding a second PI was associated with moderate changes in these parameters on day 21.
Table 1.
Pharmacokinetic data for efavirenz (EFV, given as a single 600-mg daily dose) with protease inhibitors (PI)
| Variable | Arm | Second PI | Day 14 (EFV+APV, mean ± SD) | Day 21 (EFV+APV+Second PI, mean ± SD)) | Day 21 percentage of day 14 (median, range) | P (Wilcoxon signed rank test) |
|---|---|---|---|---|---|---|
| AUC (μg*h/mL) | A | None | 37.07 ± 9.586 | 33.78 ± 8.168 | 93 (61–123) | 0.465 |
| B | NFV | 44.95 ± 10.37 | 44.87 ± 13.65 | 99 (76–127) | 0.850 | |
| C | IDV | 52.78 ± 43.21 | 42.82 ± 27.06 | 89 (67–138) | 0.052 | |
| D | RTV | 50.79 ± 22.88 | 52.05 ± 41.39 | 97 (79–162) | 0.193 | |
| E | SQV | 50.48 ± 20.06 | 45.00 ± 19.76 | 89 (70–123) | 0.037 | |
| CLt/F (L/h/kg) | A | None | 0.126 ± 0.041 | 0.123 ± 0.020 | 104 (72–161) | 0.790 |
| B | NFV | 0.111 ± 0.025 | 0.117 ± 0.034 | 105 (80–134) | 0.480 | |
| C | IDV | 0.123 ± 0.058 | 0.136 ± 0.058 | 117 (68–148) | 0.041 | |
| D | RTV | 0.110 ± 0.040 | 0.125 ± 0.047 | 112 (61–134) | 0.047 | |
| E | SQV | 0.105 ± 0.036 | 0.122 ± 0.047 | 115 (81–151) | 0.037 | |
| CLd/F (L/h/kg) | A | None | 0.438 ± 0.145 | 0.466 ± 0.191 | 111 (45–195) | 0.657 |
| B | NFV | 0.367 ± 0.062 | 0.303 ± 0.049 | 83 (62–98) | 0.002 | |
| C | IDV | 0.388 ± 0.076 | 0.347 ± 0.101 | 90 (59–143) | 0.099 | |
| D | RTV | 0.396 ± 0.066 | 0.268 ± 0.137 | 69 (21–116) | 0.028 | |
| E | SQV | 0.366 ± 0.065 | 0.295 ± 0.099 | 83 (37–120) | 0.093 | |
| Vss/F (L/kg) | A | None | 4.412 ± 1.750 | 4.550 ± 1.258 | 122 (42–201) | 0.929 |
| B | NFV | 3.055 ± 0.972 | 4.412 ± 0.913 | 152 (97–211) | 0.003 | |
| C | IDV | 3.039 ± 0.678 | 6.344 ± 0.659 | 215 (168–292) | 0.002 | |
| D | RTV | 3.147 ± 1.169 | 5.960 ± 0.365 | 214 (119–441) | 0.005 | |
| E | SQV | 3.012 ± 0.510 | 5.109 ± 0.424 | 174 (133–217) | 0.005 | |
| Vc/F (L/kg) | A | None | 0.760 ± 0.113 | 0.683 ± 0.106 | 91 (61–108) | 0.131 |
| B | NFV | 0.706 ± 0.092 | 0.427 ± 0.217 | 59 (33–130) | 0.005 | |
| C | IDV | 0.718 ± 0.090 | 0.459 ± 0.118 | 64 (43–98) | 0.002 | |
| D | RTV | 0.700 ± 0.135 | 0.888 ± 0.452 | 127 (63–238) | 0.285 | |
| E | SQV | 0.737 ± 0.081 | 0.556 ± 0.141 | 77 (48–114) | 0.022 | |
| Vp/F (L/kg) | A | None | 3.653 ± 1.735 | 3.867 ± 1.209 | 134 (35–251) | 1.000 |
| B | NFV | 2.349 ± 0.922 | 3.985 ± 0.886 | 185 (108–268) | 0.002 | |
| C | IDV | 2.321 ± 0.694 | 5.885 ± 0.636 | 270 (194–420) | 0.002 | |
| D | RTV | 2.447 ± 1.137 | 5.072 ± 0.400 | 262 (122–730) | 0.005 | |
| E | SQV | 2.266 ± 0.513 | 4.553 ± 0.427 | 211 (156–318) | 0.005 | |
| T1/2 (h) | A | None | 30.75 ± 13.07 | 32.26 ± 10.75 | 126 (48–230) | 1.000 |
| B | NFV | 23.08 ± 6.670 | 37.29 ± 12.34 | 166 (106–252) | 0.002 | |
| C | IDV | 25.83 ± 12.77 | 51.11 ± 19.71 | 216 (147–360) | 0.002 | |
| D | RTV | 24.77 ± 8.853 | 58.12 ± 36.04 | 267 (106–790) | 0.005 | |
| E | SQV | 25.87 ± 9.826 | 45.78 ± 18.82 | 178 (113–223) | 0.005 | |
| Ka (1/h) | A | None | 0.410 ± 0.132 | 0.335 ± 0.145 | 98 (16–218) | 0.374 |
| B | NFV | 0.394 ± 0.190 | 0.245 ± 0.097 | 71 (24–123) | 0.012 | |
| C | IDV | 0.367 ± 0.196 | 0.229 ± 0.108 | 80 (27–194) | 0.050 | |
| D | RTV | 0.403 ± 0.327 | 0.590 ± 0.516 | 213 (31–718) | 0.285 | |
| E | SQV | 0.388 ± 0.227 | 0.255 ± 0.106 | 78 (25–138) | 0.017 |
Figure 4.
Arm-specific efavirenz concentrations b sample time when efavirenz was co-administered with amprenavir and with amprenavir plus a second PI. For each arm separately, mean efavirenz concentrations were plotted against scheduled sample times when subjects had taken efavirenz plus amprenavir only (day 14) and when subjects had taken efavirenz plus amprenavir and (on all but arm A) a second PI (day 21). Error bars indicate the standard deviation of efavirenz concentrations (APV: amprenavir; EFV: efavirenz; IDV: indinavir; NFV: nelfinavir; RTV: ritonavir; SQV: saquinavir)
Adverse events
No serious clinical adverse events were reported. In general, these combinations were very well tolerated and adverse events were only mild to moderate (grade 1 or 2). The most frequently reported adverse events during the study were central nervous system (CNS) toxicities. Among the subjects who completed the study on day 14, 75% and 6% experienced grade 1 and grade 2 CNS toxicities, respectively. Rash occurred in nine subjects who reported a grade 1 rash. The rashes were most often maculopapular and lasted 3–4 days, with no other symptoms. There was no significant difference among the arms with regard to CNS symptoms and rashes.
Discussion
This work reports the lack of pharmacokinetic interaction between efavirenz and co-administered dual PIs in AACTG A5043 study. The use of compartmental pharmacokinetic analysis for examining efavirenz disposition in ACTG 5043 yielded estimates and comparisons of pharmacokinetic microconstants that would not have been identified with standard non-compartmental analysis. The rationale for our modeling approach relates to known characteristics of efavirenz that include, ~50% bioavailability [12,13] and oxidative metabolism via CYP3A and CYP2B6 to inactive hydroxylated metabolites including 8- and 7-hydroxyefavirenz, with subsequent urinary and biliary excretion after glucuronidation in the liver [3,14,15]. In a previous investigation, nevirapine, an inducer of CYP2B6 and CYP3A4, significantly decreased the plasma concentrations of efavirenz, indicating that either CYP2B6 or CYP3A4 or both contribute to the biotransformation of efavirenz [16]. In genetic association studies, the CYP2B6 516 TT genotype was associated with a decreased clearance and increased plasma concentrations of efavirenz in HIV-infected patients [17–19], supporting a significant role for CYP2B6 in its biotransformation.
Previous studies have examined efavirenz with PIs and found that while PIs interact with a number of CYP450 isoforms and P-glycoprotein (P-gp), there is little influence on efavirenz disposition [20]. All currently available PIs are inhibitors of CYP3A with inhibitory activities ranging from weak (saquinavir) to potent (ritonavir) [4–7]. Both indinavir and ritonavir also inhibit CYP2C and −2D6 but to a much lesser extent than CYP3A [7]. PIs, however, have minimal effects on CYP2B6. Recent data indicate that most PIs are substrates for the P-gp drug efflux pump, and ritonavir has even been reported to be a potent P-gp inhibitor [21–23]. Thus, a multiple dose study on efavirenz and ritonavir in combination in healthy volunteers found ritonavir increased the AUC of efavirenz by 20% [2]. The magnitude of this effect is unlikely to be of clinical significance, and it indicates CYP3A (and possibly P-gp) plays a minor role in efavirenz metabolism.
The other drug that was an important component of ACTG 5043 was amprenavir, a moderately potent inhibitor of CYP3A4 [4,24] without notable effects on CYP1A2, −2C9, −2C19, −2D6, −2E1, or uridine glucuronosyltransferase activities [Product Information of Amprenavir (Agenerase), GSK, May, 2005]. Although amprenavir exhibits both CYP3A4 inhibition and induction, it has minimal effect on the pharmacokinetics of efavirenz [25] suggesting efavirenz is metabolized primarily by CYP2B6. Thus, the pharmacokinetic parameters of efavirenz obtained in ACTG 5043 on day 14 in the presence of amprenavir were comparable to published values for efavirenz monotherapy. The estimated average clearance of efavirenz (0.105–0.126 l/h/kg) in the present study was smaller than that previously reported in patients (0.186 l/h/kg) [26], although a recent report based on population analysis presented a value in patients given efavirenz at 600 mg/day almost identical to that found in this study [27]. For volume of distribution at steady state (Vss), estimates ranging from 3.012 to 4.412 l/kg were consistent with literature [8,27,28]. These data along with previous observations indicate that amprenavir does not substantially alter efavirenz pharmacokinetics.
There were only moderate changes in the efavirenz pharmacokinetic parameters after addition of the second PI. The finding that the second PIs had no significant effect on the exposure [1] or clearance of efavirenz, whereas they increased its Vss/F by 52–115% and prolonged its half-life by 66–167%, suggests that the interaction might occur mainly during the distribution phases, probably through protein binding and/or interactions with membrane transporters that contribute to the efavirenz metabolism [3,29]. However, it is unlikely interactions between PIs and P-gp play a role in this phenomenon. Recent studies showed that efavirenz was not a P-gp substrate on P-gp-expressing LS180 V cells [30], nor did it modulate intestinal P-gp expression in healthy volunteers [31]. There are two possibilities: (1) since efavirenz and most PIs are highly protein bound, the addition of a second PI may displace efavirenz from protein binding sites resulting in increased free fraction, CLt/F and Vss/F; (2) an improved bioavailability of efavirenz by a second PI may also result in larger Vss/F and CLt/F. Both mechanisms, however, are not likely to be true because no CLt/F change was observed for efavirenz in this study. Nevertheless, the magnitude of Vss/F increase and t1/2 prolongation seems unlikely to be of clinical significance, and dosage adjustments may not be necessary. Neither Vss/F increase nor t1/2 prolongation was observed using non-compartmental analysis (data not shown), indicating the advantage of efavirenz modeling identifying pharmacokinetic changes during dual PI administration.
The lack of pharmacokinetic interactions between efavirenz and dual PIs may be mainly due to the fact that efavirenz is primarily metabolized by CYP2B6 whereas CYP3A4 only plays a minor role. According to a pilot clinical study [31], there was no correlation between efavirenz systemic exposure and CYP3A4 activity in healthy volunteers. An intensive in vitro study indicated the efavirenz is metabolized by CYP2B6 with some involvement of CYP3A [3]. These data suggest that CYP2B6 not CYP3A is responsible for the metabolism of efavirenz. Since PIs are primary CYP3A4 inhibitors, they should have little effect on efavirenz metabolism.
In ACTG 5043, the pharmacokinetics of efavirenz was adequately described with the developed pharmacokinetic model. This two-compartment model with first-order absorption and lag-time was similar to the model with transition compartments [28], but slightly different from the one-compartment models used for population analyses of efavirenz [8,27]. However, pharmacokinetic estimates of efavirenz obtained from the present and previous studies using different models were in good agreement. It sufficiently described the absorption of efavirenz and its slow disposition. The developed model in the present study can be used to further optimize efavirenz-containing therapy in HIV-infected patients, such as to predict the toxicity of efavirenz based on pharmacokinetic profiles. A relationship between blood concentrations of efavirenz and development of side effects in CNS has been recently suggested in 17 patients receiving long-term therapy [32].
Several limitations of our present study should be outlined. First of all, the pharmacokinetic interactions investigated, namely efavirenz together with amprenavir and four other PIs seem to be somewhat irrelevant for current therapy. This is mainly due to the fact that the routine use of low-dose ritonavir was not considered to be the standard of care at the time when A5043 was developed, and the optimal approach to combining two PIs with efavirenz was under investigation in A5043 protocol. Nevertheless, the results from our present study that PIs have negligible effects on efavirenz pharmacokinetics suggest no dose adjustment needed for efavirenz with concurrent use of dual PIs in clinical practice. Secondly, there were no measures of efavirenz exposure prior to addition of amprenavir. Previous studies have explored the effects of amprenavir on efavirenz pharmacokinetics showing minimal interactions between these two agents [25]. Furthermore, the pharmacokinetic parameters of efavirenz obtained in our present study in the presence of amprenavir (on day 14) were comparable with published values for efavirenz monotherapy. This observation reinforces the indication that amprenavir does not substantially alter efavirenz pharmacokinetics, which makes the problem less important.
Since fosamprenavir, a prodrug of amprenavir, has become the only amprenavir produced available for adult dosing, the clinical relevance of the present findings could be extrapolated for concurrent use of fosamprenavir/ritonavir and efavirenz. In a recent study, fosamprenavir with and without ritonavir was added to the anti-retroviral regimens of healthy volunteers receiving efavirenz to evaluate this drug interaction. Despite significant reductions in plasma amprenavir exposure were observed following coadministration of 1395/200 mg of fosamprenavir/ritonavir once daily and 600 mg of efavirenz, no dose adjustment of efavirenz was required [33] suggesting negligible effects of dual PIs on efavirenz pharmacokinetics. However, this notion should be interpreted with caution as darunavir, a new PI, with ritonavir has been reported to increase efavirenz exposure by 21% [34]. Therefore, interaction studies between boosted novel PIs and efavirenz are warranted.
In conclusion, efavirenz pharmacokinetics with dual-PIs can be evaluated using a compartmental analysis and co-administration of dual PIs had only a limited effect on the efavirenz pharmacokinetics suggesting no dose adjustment needed for efavirenz with concurrent use of dual PIs.
Acknowledgments
The participation of the study volunteers is appreciated to help answer the clinical research questions in A5043. The research staff at the following Adult ACTG units and General Clinical Research Centers that participated in A5043: AIDS Clinical Trials Units: Indiana University, Johns Hopkins University, Ohio State University, Stanford University, University of Colorado, University of Rochester, University of Washington, Vanderbilt University, Washington University, Adult ACTG Pharmacology Support Laboratory- University at Buffalo School of Pharmacy and Pharmaceutical Sciences faculty and laboratory staff, ACTG Operation Center: Barbara Brizz, Statistical Data Analysis Center: Yoninah Cramer, Data Management Center: Ann Walawander, Courtney Ashton, DAIDS: Elaine Ferguson, Pharmaceutical Company Representatives: Pascal J. de Caprariis (Roche), Alfred J. Saah (Merck), Mark Becker (Agouron), Mary Wire and Mark Shelton (GlaxoSmithKline).
Support was provided by the following grants: NIAID U01-AI-25859, 25903, 25924, 27658, 27664, 27666, 27668, 32770, 38855 and 38858; GCRC support from the NIH National Center of Research Resources M01-RR-00034, 00036, 00037, 00044, 00051, 00052, 00070 and 00750; and NIDA R01-DA-015024-02 (QM, AF, GDM).
References
- 1.Morse GD, Rosenkranz S, Para MF, et al. Amprenavir and efavirenz pharmacokinetics before and after the addition of nelfinavir, indinavir, ritonavir, or saquinavir in seronegative individuals. Antimicrob Agents Chemother. 2005;49:3373–3381. doi: 10.1128/AAC.49.8.3373-3381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Q, Okusanya OO, Smith PF, et al. Pharmacokinetic drug interactions with non-nucleoside reverse transcriptase inhibitors. Expert Opin Drug Metab Toxicol. 2005;1:473–485. doi: 10.1517/17425255.1.3.473. [DOI] [PubMed] [Google Scholar]
- 3.Ward BA, Gorski JC, Jones DR, et al. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 4.Decker CJ, Laitinen LM, Bridson GW, et al. Metabolism of amprenavir in liver microsomes: role of CYP3A4 inhibition for drug interactions. J Pharm Sci. 1998;87:803–807. doi: 10.1021/js980029p. [DOI] [PubMed] [Google Scholar]
- 5.Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44:190–194. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lillibridge JH, Liang BH, Kerr BM, et al. Characterization of the selectivity and mechanism of human cytochrome P450 inhibition by the human immunodeficiency virus-protease inhibitor nelfinavir mesylate. Drug Metab Dispos. 1998;26:609–616. [PubMed] [Google Scholar]
- 7.von Moltke LL, Greenblatt DJ, Grassi JM, et al. Protease inhibitors as inhibitors of human cytochromes P450: high risk associated with ritonavir. J Clin Pharmacol. 1998;38:106–111. doi: 10.1002/j.1552-4604.1998.tb04398.x. [DOI] [PubMed] [Google Scholar]
- 8.Pfister M, Labbe L, Hammer SM, et al. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398. Antimicrob Agents Chemother. 2003;47:130–137. doi: 10.1128/AAC.47.1.130-137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammer SM, Vaida F, Bennett KK, et al. Dual vs single protease inhibitor therapy following antiretroviral treatment failure: a randomized trial. JAMA. 2002;288:169–180. doi: 10.1001/jama.288.2.169. [DOI] [PubMed] [Google Scholar]
- 10.Frerichs VA, DiFrancesco R, Morse GD. Determination of protease inhibitors using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:393–403. doi: 10.1016/s1570-0232(02)01002-4. [DOI] [PubMed] [Google Scholar]
- 11.Holland DT, DiFrancesco R, Stone J, et al. Quality assurance program for clinical measurement of antiretrovirals: AIDS clinical trials group proficiency testing program for pediatric and adult pharmacology laboratories. Antimicrob Agents Chemother. 2004;48:824–831. doi: 10.1128/AAC.48.3.824-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289–304. doi: 10.2165/00003088-199936040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Martinez E, Arnaiz JA, Podzamczer D, et al. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med. 2003;349:1036–1046. doi: 10.1056/NEJMoa021589. [DOI] [PubMed] [Google Scholar]
- 14.Adkins JC, Noble S. Efavirenz. Drugs. 1998;56:1055–1064. doi: 10.2165/00003495-199856060-00014. discussion 1065–1056. [DOI] [PubMed] [Google Scholar]
- 15.Mutlib AE, Chen H, Nemeth GA, et al. Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos. 1999;27:1319–1333. [PubMed] [Google Scholar]
- 16.Veldkamp AI, Harris M, Montaner JS, et al. The steady-state pharmacokinetics of efavirenz and nevirapine when used in combination in human immunodeficiency virus type 1-infected persons. J Infect Dis. 2001;184:37–42. doi: 10.1086/320998. [DOI] [PubMed] [Google Scholar]
- 17.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. Aids. 2004;18:2391–2400. [PubMed] [Google Scholar]
- 18.Rotger M, Colombo S, Furrer H, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya K, Gatanaga H, Tachikawa N, et al. Homozygous CYP2B6 *6 (Q172 H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319:1322–1326. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 20.DiCenzo R, Forrest A, Squires KE, et al. Indinavir, efavirenz, and abacavir pharmacokinetics in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2003;47:1929–1935. doi: 10.1128/AAC.47.6.1929-1935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsenz J, Steffen H, Alex R. Active apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayers. Pharm Res. 1998;15:423–428. doi: 10.1023/a:1011924314899. [DOI] [PubMed] [Google Scholar]
- 22.Lee CG, Gottesman MM. HIV-1 protease inhibitors and the MDR1 multidrug transporter. J Clin Invest. 1998;101:287–288. doi: 10.1172/JCI2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Profit L, Eagling VA, Back DJ. Modulation of P-glycoprotein function in human lymphocytes and Caco-2 cell monolayers by HIV-1 protease inhibitors. AIDS. 1999;13:1623–1627. doi: 10.1097/00002030-199909100-00004. [DOI] [PubMed] [Google Scholar]
- 24.Adkins JC, Faulds D. Amprenavir. Drugs. 1998;55:837–842. doi: 10.2165/00003495-199855060-00015. discussion 843–834. [DOI] [PubMed] [Google Scholar]
- 25.Falloon J, Piscitelli S, Vogel S, et al. Combination therapy with amprenavir, abacavir, and efavirenz in human immunodeficiency virus (HIV)-infected patients failing a protease-inhibitor regimen: pharmacokinetic drug interactions and antiviral activity. Clin Infect Dis. 2000;30:313–318. doi: 10.1086/313667. [DOI] [PubMed] [Google Scholar]
- 26.Villani P, Regazzi MB, Castelli F, et al. Pharmacokinetics of efavirenz (EFV) alone and in combination therapy with nelfinavir (NFV) in HIV-1 infected patients. Br J Clin Pharmacol. 1999;48:712–715. doi: 10.1046/j.1365-2125.1999.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Csajka C, Marzolini C, Fattinger K, et al. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 28.Kappelhoff BS, Huitema AD, Yalvac Z, et al. Population pharmacokinetics of efavirenz in an unselected cohort of HIV-1-infected individuals. Clin Pharmacokinet. 2005;44:849–861. doi: 10.2165/00003088-200544080-00006. [DOI] [PubMed] [Google Scholar]
- 29.Fellay J, Marzolini C, Meaden ER, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–36. doi: 10.1016/S0140-6736(02)07276-8. [DOI] [PubMed] [Google Scholar]
- 30.Stormer E, von Moltke LL, Perloff MD, Greenblatt DJ. Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm Res. 2002;19:1038–1045. doi: 10.1023/a:1016430825740. [DOI] [PubMed] [Google Scholar]
- 31.Mouly S, Lown KS, Kornhauser D, et al. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72:1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez F, Navarro A, Padilla S, et al. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis. 2005;41:1648–1653. doi: 10.1086/497835. [DOI] [PubMed] [Google Scholar]
- 33.Wire MB, Ballow C, Preston SL, et al. Pharmacokinetics and safety of GW433908 and ritonavir, with and without efavirenz, in healthy volunteers. AIDS. 2004;18:897–907. doi: 10.1097/00002030-200404090-00007. [DOI] [PubMed] [Google Scholar]
- 34.Sekar VJ, De Pauw M, Marien K, et al. Pharmacokinetic interaction between TMC114/r and efavirenz in healthy volunteers. Antiviral therapy. 2007;12:509–514. [PubMed] [Google Scholar]