Abstract
GnRH plays an essential role in neuroendocrine control of reproductive function. In mammals, the pattern of gonadotropin secretion includes both pulse and surge phases, which are regulated independently. The pulsatile release of GnRH and LH plays an important role in the development of sexual function and in the normal regulation of the menstrual cycle. The importance of GnRH pulsatility was established in a series of classic studies. Fertility is impaired when GnRH pulsatility is inhibited by chronic malnutrition, excessive caloric expenditure, or aging. A number of reproductive disorders in women with including hypogonadotropic hypogonadism, hypothlamic amenorrhea, hyperprolactinemia and polycystic ovary syndrome (PCOS) are also associated with disruption of the normal pulsatile GnRH secretion. Despite these findings, the molecular mechanisms of this pulsatile GnRH regulation are not well understood. Here, we review recent studies about GnRH pulsatility, signaling and transcriptional response, and its implications for disease.
Introduction
The decapeptide gonadotropin-releasing hormone (GnRH) is the master hormone regulating reproduction. GnRH is released from some 1000 neurons within the hypothalamus in a pulsatile manner. Hypothalamic GnRH gene expression occurs intermittently and is dependent on a 300-bp promoter region termed the neuron specific enhancer (NSE) 1. The primary target of hypothalamic GnRH is the anterior pituitary gonadotrope, which responds to stimulation by increasing the synthesis and secretion of the gonadotropins luteinzing hormone (LH) and follicle stimulating hormone (FSH), which in turn regulate gonadal development and function2, 3. The gonadotropins are members of the glycoprotein hormone family, and stimulate spermatogenesis, folliculogenesis, and ovulation. In addition to the hypothalamic and pituitary peptides, steroidal hormones from the gonad, including androgen, estrogen and progesterone, are also important for regulation for the reproductive function. Generally, the gonadal steroids act on the hypothalamus and pituitary in a classical negative feedback loop inhibiting GnRH and gonadotropin expression.
In the female, the pattern of GnRH and gonadotropin release varies during different reproductive stages and among different species. In mammals, the pattern of gonadotropin secretion includes both pulse and surge phases, which are regulated independently. Several central and peripheral signals modulate GnRH neuronal activity. Some of these signals are stimulatory for GnRH release (e.g. norepinephrine, kisspeptin and neuropeptide Y), others are inhibitory (e.g. beta-endorphin, progesterone and interleukin-1), others still can be either stimulatory or inhibitory (e.g. estradiol). The neuronal structures and chemical interactions that result in normal pulsatile GnRH release remain unclear, but the GnRH pulse-generator seems to involve both norepinephrine and kisspeptin as well as components of the circadian clock. Many of the stimulatory and inhibitory signals may influence the pulse-generator by acting on secondary neurons. In contrast, the surge GnRH release is triggered either by increasing levels of circulating estradiol during the preovulatory period in spontaneous-ovulating species, or by coitus in species exhibiting coitus-induced ovulation. The pulsatile and surge release may involve different subsets of neurons with those in the arcuate nucleus mediating the normal pulsatile release whereas others in the AVPV mediating the surge 4.
The pulsatile release of GnRH and LH plays an important role in the development of sex function and in the normal regulation of the menstrual cycle. In 1970, Dierschke et al. first observed LH pulses in the ovariectomized monkey5. Later studies also showed this phenomenon in the human and rat. This LH pulse is produced by a corresponding GnRH pulse from the hypothalamus 6. Both the frequency and amplitude of the GnRH pulse are critical for normal gonadotropin release 7. One reason for the GnRH secretion in a pulsatile manner is to avoid the down-regulation of the GnRH receptor in the pituitary. In rhesus monkeys with hypothalamic lesions that abolish pituitary gonadotropin release, the constant infusion of exogenous GnRH fails to restore sustained gonadotropin secration but intermittent administration of GnRH once per hour reestablishes gonadotropin secretion 8. Westel et al. showed that intrinsic pulsatile secretory activity was seen in immortalized GnRH neurons indicating that the pulse generator is cell autonomous 9. Furthermore, it is known that the activity of each GnRH neuron is synchronized in vivo and immortalized GnRH neurons will spontaneously synchronize in perfusion culture10.
Even though pulsatility of GnRH is recognized as a major determinant for differential gonadotropin subunit gene expression and gonadotropin secretion, very little is yet known about the signaling circuits governing GnRH action at the pituitary level. In this article, we review the current knowledge of the role of pulsatile GnRH on pituitary function and reproduction.
GnRH pulses, gonadotropins and fertility
GnRH pulses stimulate the synthesis and secretion of LH and FSH from the anterior pituitary. Although produced in same gonadotrope cell, concentrations of LH and FSH vary throughout the menstrual cycle. In the early follicular and luteal phases FSH is predominant over LH, whereas LH is dominant over FSH in the late follicular phase. It is well known that both LH and FSH synthesis is regulated by the frequency of GnRH pulses, with LH favored by fast pulse frequencies (> 1 pulse per h) and FSH favored by slow pulse frequencies (< 1 pulse per 2-3 h). During the follicular phase of the ovulatory cycle, increasing estrogen production activates Kiss-1 neurons in the AVPV overriding the normal tonic repression by ovarian steroids and increasing GnRH pulse frequency and amplitude. This in turn favors LH synthesis leading to the LH surge and triggering ovulation. Following ovulation, luteinization of the granulosa cells increases progesterone production which slows GnRH pulse frequency to decrease LH production and increase FSH production to stimulate the next round of ovulation. This phenomenon has been demonstrated in many animals and cell culture systems but the molecular basis for this differential regulation in not understood well.
Both GnRH and the gonadotropins are necessary for this reproductive cycle in mice. The well-characterized hypogonadal (hpg) mouse does not produce GnRH and is infertile. Plasma LH levels are undetectable but FSH is only reduced 50% as FSH expression is primarily driven by activin in vivo. Female hpg mice do not cycle but show normal numbers of primordial and primary follicles with reduced numbers of secondary and antral follicles consistent with a block in follicle development at the pre-antral stage. Mice lacking a functional LHβ gene are viable but demonstrate postnatal defects in gonadal growth and function resulting in infertility similar to the hpg mice. Mutant males show decreased testes size, a block in Leydig cell differentiation, and a reduction in serum and intra-testicular testosterone levels, whereas females are hypogonadal and demonstrate decreased levels of serum estradiol and progesterone 11. Deletion of the FSHβ gene, on the other hand, does not cause infertility in male mice but does reduce testicular tubule size, sperm number and motility. In contrast, the females ware infertile with a block in folliculogenesis at the pre-antral stage similar to the hpg mouse.
Negative feedback by gonadal steroids
It is well established that negative feedback regulates the hypothalamic pituitary axis12. Testosterone administration to GnRH-deficient men suppresses both LH and FSH13. Conversely, chemical castration of normal men with ketoconazole elevates serum LH and FSH14 and surgical castration of male rats causes an increase in LH pulse frequency and amplitude15. Similarly, estradiol suppresses LH and FSH in GnRH-deficient men and aromatase inhibition increases LH and FSH indicating a role for estrogens in negative feedback in men. These effects are blocked by a GnRH agonist indicating the changes in hypothalamic GnRH secretion are responsible.
The regulation in women is a more complex as progesterone has inhibitory effects but estradiol can have both stimulatory and inhibitory effects depending upon the stage of the menstrual cycle. Ovariectomy of female rats in diestrus or early proestrus causes an increase in LH pulse frequency and amplitude, but ovariectomy during late proestrus reduces LH as estrogen is important in driving the GnRH/LH surge 16, 17. Replacement of estrogen reduces pulse frequency but not amplitude, replacement of both estradiol and progesterone reduces both pulse frequency and amplitude. Replacement of progesterone alone has little effect without estrogen replacement to induce the progesterone receptor. Androgens also appear to prevent the normal negative feedback suppression of LH in PCOS women as anti-androgen therapy with flutamide restores the sensitivity of LH secretion to inhibition by estradiol and progesterone 18 .
At the pituitary level, gonadal steroids have been shown to alter gonadotropin subunit expression. Ovariectomy causes a 2-3-fold increase in LHβ transcription and a 6-9 fold increased in FSHβ transcription. These effects are primarily due to effects of steroids on hypothalamic GnRH secretion but there is also evidence for direct effects on pituitary gonadotropes. Low levels of testosterone enhance the GnRH stimulation of LHβ in primary pituitary cultures and sensitize cells to lower pulses of GnRH, partly due to effects on the GnRH-R. Estradiol also augments GnRH-R expression and sensitivity to GnRH 19.
Signaling response to pulsatile GnRH
If GnRH pulse frequency and amplitude is important for differential expression and secretion of gonadotropins, how does the gonadotrope decode pulse frequency and amplitude? GnRH binds to a high affinity G-protein coupled receptor on the plasma membrane of the gonadotrope. The GnRH receptor can couple to the Gq/11, Gs, Gi and possibly G12/13 subfamilies of G-proteins depending on the cell type 20-23. In αT3-1 cells, the receptor couples exclusively to Gq/11. In primary pituitary cells, LβT2 and GT1-7 cells, the GnRH receptor couples to Gs in addition to Gq/1124, and in ovarian carcinomas and uterine leiomyosarcomas, the receptor also couples to Gi 25. The Gq/11 proteins activate phospholipase C to generate inositol triphosphate (IP3) and diacylglycerol (DAG) causing release of intracellular calcium and activation of Protein Kinase C (PKC) signaling 26. The GnRH-induced calcium increase has been studied in primary pituitary cells, LβT2 and αT3-1 cells and is due to both IP3-mediated intracellular release and influx through L-type calcium channels. The Gs and Gi proteins act on adenylate cyclase to modulate cAMP levels in GT1-7 cells and LβT2 cells 27,22.
Most of these studies, however, have used tonic GnRH treatment, either acute or chronic, to activate signaling. While acute stimulation is useful for studying the kinetics of activation of signaling, it does not shed any light on the dynamics of the process in vivo where GnRH is secreted in pulses. Chronic GnRH studies have defined the mechanisms of down-regulation of signaling, which is useful pharmacologically, but is not physiological. Investigating the cellular response to pulsatile GnRH is important to understand the physiological response of the pituitary in the whole animal to regulate reproduction.
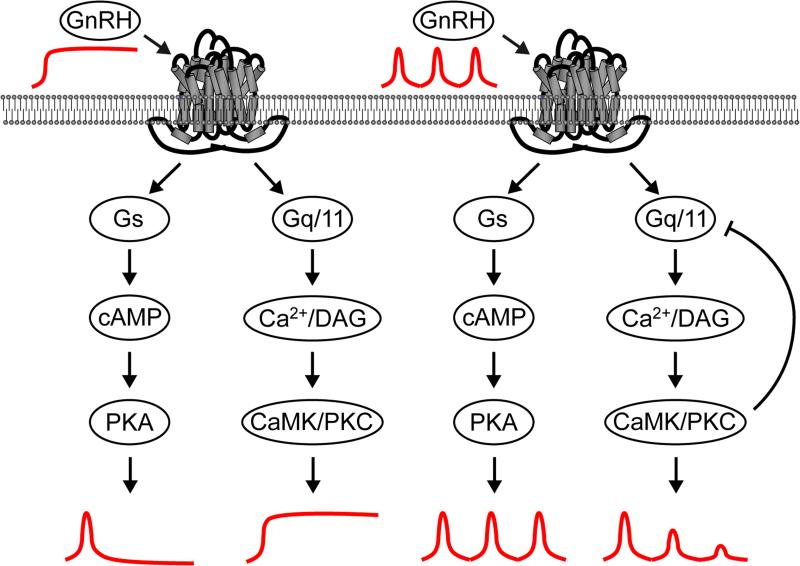
As a first step to understand how the Gs and Gq pathways are differentially activated by GnRH pulse frequency, we monitored the dynamics of the response in live cells following GnRH pulse treatment using fluorescence resonance energy transfer (FRET) reporters, as these genetic reporters allow signal to be recorded over > 4 h. We monitored the Gs pathway using cAMP and PKA-dependent reporters in the immortalized LβT2 gonadotrope cell line. A cAMP-dependent reporter based on the Epac protein allowed us to analyze the dynamics of cAMP changes. The response of this reporter showed a strong FRET signal even at low concentrations of GnRH and did not show a dose-dependence. The FRET signal was rapidly deactivated even in the presence of tonic GnRH. A strong FRET signal was observed with each pulse at both high and low pulse frequencies and did not diminish with subsequent pulses. Similar effects were seen with a PKA-dependent reporter (AKAR3). Manipulation of the deactivation rate of the reporter allowed us to change a sharp pulsatile response into a saw-tooth response. These saw-tooth responses were cumulative and approximated to an artificial dose-dependent tonic treatment at high pulse frequencies. These data suggest that the Gs pathway is adapted to mediate signaling in response to pulses of GnRH due to the rapid kinetics of cAMP effects, cumulative effect of multiple pulses and lack of desensitization (Figure 1). We monitored activation of the Gq/11 pathway using the diacylglycerol-specific reporter (DAGR) and Ca2+ indicator (GCaMP2). These two reporters showed an increase in FRET signal with increasing doses of tonic GnRH which continued for the duration of the stimulation. With multiple pulses of GnRH, a strong FRET signal was observed with the initial pulse of GnRH but the response to subsequent pulses diminished rapidly independently of the pulse frequency suggesting desensitization (Figure 1).
Figure 1. Model for regulation of signaling by the GnRH-R in response to tonic or pulsatile GnRH.
Tonic GnRH stimulation (left) causes a transient increase in Gs/cAMP signaling that rapidly returns to baseline in spite of the continued presence of GnRH. In contrast, Gq/11/DAG/Ca2+ signaling remains elevated during the entire period of GnRH stimulation. Pulsatile GnRH (right) causes matching pulses of Gs/cAMP signaling of constant amplitude over time. Gq/11/DAG/Ca2+ signaling shows an initial pulse matching the GnRH pulse but each subsequent pulse has lower amplitude until no further pulses are seen after 2 h. This may reflect a PKC or CaMK induced negative feedback loop to desensitize Gq/11 signaling.
We propose a model where the GnRH receptor activates Gs to increase cAMP. This signal is transient because cAMP is degraded by phosphodiesterases and the Gs-bound GTP is rapidly hydrolyzed to GDP with a kcat for GTP hydrolysis of 4 min−1. The GDP-bound Gs can re-associate with Gβγ subunits but does not functionally re-engage the GnRH receptor unless the GnRH is removed. In contrast, the GnRH-R activates Gq/11 causing the PLC-mediated production of DAG and IP3. The GTPase activity of Gq/11 (0.8 min−1) is less than Gs so the signal is not extinguished as rapidly, but the GDP-bound Gq can re-associate with Gβγ and re-engage the GnRH receptor to allow continual signaling in the presence of GnRH. Downstream signaling, possibly by PKC or CaMKII then induces a negative feedback loop that desensitizes Gq signaling. Thus, the differential activation of Gs and Gq/11 may provide the basis for frequency and amplitude-dependent signaling by the GnRH-R.
Transcriptional response to pulsatile GnRH
How do these differences in signaling alter gonadotropin expression? The tractability of the αT3-1 and LβT2 immortalized gonadotrope cells has made them a popular model for the study of the molecular mechanism for gonadotropin synthesis 28, 29. LH and FSH consist of a common α-subunit and distinct β-subunit which determine the specificity of the gonadotropins. αT3-1 cells express only the α-subunit gene (Cga) whereas LβT2 cells express all subunits of LHβ (Lhb) and FSHβ (Fshb). Both cells express GnRH receptors and GnRH induces a robust transcriptional response. These cells also express receptors for pituitary adenylate cyclase-activating polypeptide (PACAP), dopamine, and activin. Many groups have focused on the transcription factors that regulate the LHβ and FSHβ gene promoters to understand molecular basis of GnRH pulse sensing at the genomic level. A number of transcriptional factors are known to be important for gonadotrope-specific expression of LHβ and FSHβ gene promoter-reporter constructs but few have been verified directly on the endogenous genes. Such studies have suggested that the effects of GnRH on LHβ and FSHβ are distinct.
The induction of the LHβ promoter by GnRH is mediated by two distinct regions of the promoter. An obligate proximal promoter region contains two tandem binding sites for steroidogenic factor 1 (SF-1) and early growth response 1 (Egr-1), and a single central binding site for Pitx-1/Otx-1 that may also mediate Smad binding. An upstream enhancer region is required for full GnRH induction of the promoter and contains a CArG box and binding sites for Sp1 and NFY. Most of these sites are conserved across mammalian species 30. Regulation of the FSHβ promoter is more complicated as both GnRH and activin independently and synergistrically activate transcription. Many groups have identified binding sites required for tissue-specific or hormonally-regulated expression including sites for SF-1, Ptx1, Lhx, Pbx/Prep, AP-1, NFY, and Smads. The GnRH response appears to require the AP-1 binding sites 31. Most of these studies, however, have used tonic treatment of cells in monolayer culture to identify these factors and sites, which raises the question whether the same factors are involved in regulation by pulses of GnRH.
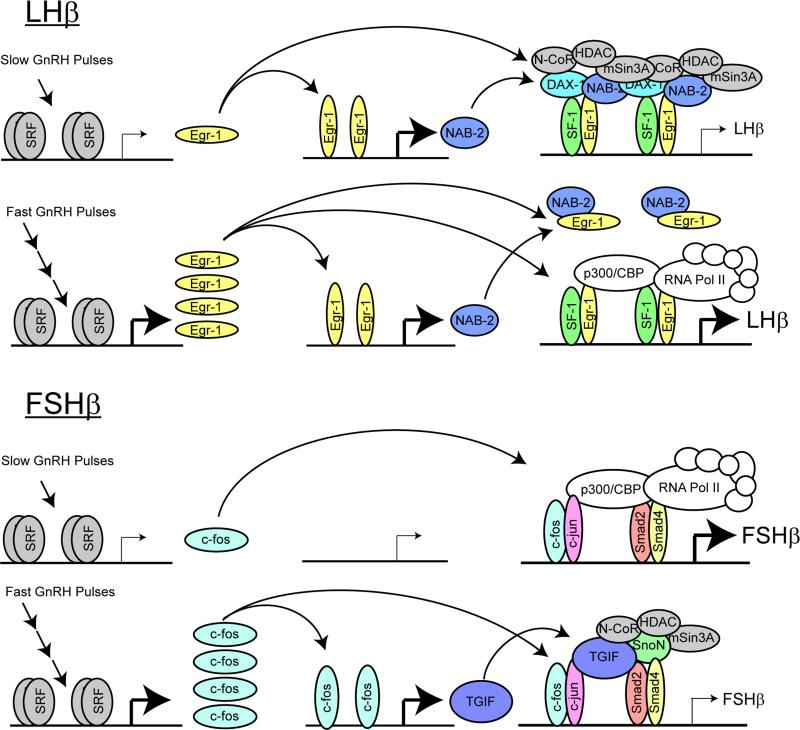
Previously, our group examined the genome-wide impact of pulsatile GnRH to provide a clear assessment of the role of GnRH pulsatility in gonadotrope gene expression32. Using a perifusion system to administer pulsatile GnRH without mechanical, thermal, or atmospheric disturbance, we have examined the frequency-dependent changes in LßT2 gonadotrope cell gene expression. For the LHβ promoter, this analysis revealed that stable activation of Egr mRNAs and proteins requires fast GnRH pulse frequencies. In contrast, the Ngfi-A-binding protein (Nab) family of Egr corepressors is readily activated by a single pulse or low-frequency stimulation. Additionally, the SF-1 corepressor, Dax1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita-critical region on the X-chromosome gene 1), is also stimulated by low-frequency GnRH pulses but is reduced at high frequency. In vitro studies confirmed that overexpression or knock-down of these co-repressors reduced or increased the GnRH induction of the LHβ promoter. Furthermore, forced expression of Nab2 eliminated the differential pulse sensitivity in perfusion culture. The different sensitivity of Egr and Nab to GnRH pulses suggests a model of frequency-dependent transcriptional modulation in which gonadotropes exhibit resistance to low frequency or random signaling input due to tonic stimulation of Nab co-repressors but respond to high-frequency input by inducing higher Egr expression to titrate away the co-repressor thus increasing LH ß-subunit mRNA synthesis (Figure 2).
Figure 2. Model for pulse regulation of the LHβ and FSHβ promoters.
For the LHβ promoter (top), under a low pulse frequency, transient stimulation of Egr1 expression leads to a secondary increase in Nab2 protein. The transiently increased Egr1 level is insufficient for sustained activation of the LHβ promoter due to the repression by Nab2 and Dax1. Under high pulse frequency, high Egr1 expression is sustained, but does not lead to further increases in Nab expression. The increased Egr1 level quenches Nab2 activity, allowing increased activity of the LHβ promoter through association with transcription-activating factors. For the FSHβ promoter (bottom), under a low pulse frequency, transient stimulation of fos/jun family member expression leads to a secondary increase in FSHβ transcription but does not trigger co-repressor expression. Under high pulse frequency, TGIF/SnoN co-repressor expression is also stimulated, leading to decreased FSHβ transcription through association of co-repressors with transcription-activating factors.
We have also developed a model for pulse regulation of FSHβ. From our microarray dataset we observed that positive regulators of FSHβ expression, such as AP-1 family members c-fos, c-jun, Jun B and Jun D, were upregulated at slower pulse frequencies than a number of potential negative regulators such as SnoN, TGIF and Crem. Again, we confirmed that the co-repressors SnoN, TGIF and Crem repress FSHβ promoter activity driven by GnRH and activin or by individual transcription factors c-fos, c-jun, Jun B, Jun D, or Smads 2, 3 & 4 in the transfected mouse pituitary LßT2 gonadotropes. Our model for the differential pulse sensitivity of the FSHβ promoter is therefore based on the induction of the co-repressors at the high GnRH pulse frequencies to dampen FSHβ transcription driven by promoter-bound AP-1 and Smads (Figure 2).
The role of pulsatile GnRH for disease
It is known that dysregulation of the GnRH pulse is associated with various disorders. Reproduction can be inhibited by chronic malnutition or excessive caloric expenditure. Rather than being independent determinants of fertility, it is balance between intake and expenditure that matters. Thus, a prolonged state of negative energy balance provoked by either fasting or exercise causes functional hypothalamic amenorrhea (FHA). This has been demonstrated in women athletes where exercise-induced amenorrhea can be reversed by halting the exercise regimen with no change in body weight or food intake. Similarly, small rodents kept at low temperatures become infertile as energy is diverted for thermogenesis, but if they have access to an unlimited food supply they continue to reproduce33. This phenomenon can be partly explained by the inhibition of the GnRH pulse-generator. Decreases in metabolic fuel availability appear to be detected by cells in the caudal hindbrain and hypothalamus. Hindbrain and hypothalamic neurons producing neuropeptide Y and catecholamines project to forebrain where they contact GnRH neurons both directly and also indirectly via corticotropin-releasing hormone (CRH) neurons to inhibit GnRH secretion34.
Aging can also induce decremental changes in hypothalamic, pituitary and gonadal function35. GnRH and LH pulse amplitude decrease causing decreased testosterone, and hence increased GnRH pulse frequency due to less negative feedback to the hypothalamus36. In humans, the interplay between GnRH dose and frequency is very delicate and determines pituitary LH gonadotropin responsiveness. Hughes et al. administered 2 log orders of GnRH doses to men with GnRH deficiency and idiopathic hypogonadotropic hypogonadism (IHH) at different frequencies from 0.5 to 8 hourly37. As GnRH stimulation slowed, pulse amplitudes of LH increased whereas mean nadirs decreased. It should be noted that curvilinear does-response curves were found for each frequency and showed an increasing slope as the frequency of GnRH stimulation slowed. In women, the changes in the expression of neurotransmitters and peptides regulating the activity of the GnRH pulse generator are altered during aging. LH and FSH decrease progressively after menopause, as does GnRH pulse frequency. The level of GnRH increases with aging, however, suggesting that aging alters pituitary responsiveness to GnRH 38.
Disruption of the normal pulsatile GnRH secretion in women is associated with a number of reproductive disorders including hypogonadotropic hypogonadism, hypothalamic amenorrhea, hyperprolactinemia and polycystic ovary syndrome (PCOS). PCOS was first described in 1935 as the association of amenorrhea, obesity and polycystic ovaries in reproductive age women. It is a common clinical disorder characterized by ovulatory dysfunction and hyperandrogenemia. A neuroendocrine hallmark of PCOS is persistently rapid GnRH pulsatility, which favors pituitary synthesis of LH over FSH and contributes to the increased LH concentrations and LH:FSH ratios typical of this disorder39. PCOS is also associated with insulin resistance and hyperinsulinaemia 40, 41. In women with PCOS, treatment with GnRH antagonists results in an acute, dose-dependent reduction in both LH and testosterone levels 42-45 suggesting that LH is the physiological stimulus for androgen synthesis by ovarian theca cells and plays an important role in maintaining hyperandrogenemia. The origin of elevated androgens in PCOS patients is probably multifunctional resulting from abnormal ovarian steroidogenesis, hyperinsulinemia and increased LH drive. Indeed, hyperandrogenemia before and during puberty may predispose women to PCOS and hyperandrogenemic girls often do not establish regular menstrual periods. However, neuroendocrine abnormalities, wherever primary or secondary, make an important contribution to the pathogenesis and development of PCOS.
Conclusion
Understanding how the pituitary gonadotrope decodes the GnRH pulse frequency is crucial if we are to understand the pathophysiology of various reproductive disorders. The combination of genetic models in mice, genomic profiling, and in vivo imaging methods have the potential to uncover the molecular and cellular mechanisms underlying this regulation. Future work should be dedicated to testing these models for pulse signaling and transcriptional regulation, and deriving mathematical models to explain this phenomenon.
References
- 1.Clark ME, Mellon PL. The POU homeodomain transcription factor Oct-1 is essential for activity of the gonadotropin-releasing hormone neuron-specific enhancer. Mol Cell Biol. 1995;15:6169–6177. doi: 10.1128/mcb.15.11.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conn PM, Crowley WF., Jr. Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser UB, et al. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18:46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- 4.Smith JT, et al. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 5.Dierschke DJ, et al. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87:850–853. doi: 10.1210/endo-87-5-850. [DOI] [PubMed] [Google Scholar]
- 6.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 7.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- 8.Belchetz PE, et al. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 9.Wetsel WC, et al. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc Natl Acad Sci U S A. 1992;89:4149–4153. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Advis JP, et al. Regulation of gonadotropin releasing hormone release by neuropeptide Y at the median eminence during the preovulatory period in ewes. Neuroendocrinology. 2003;77:246–257. doi: 10.1159/000070280. [DOI] [PubMed] [Google Scholar]
- 11.Kumar TR. Functional analysis of LHbeta knockout mice. Mol Cell Endocrinol. 2007;269:81–84. doi: 10.1016/j.mce.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 12.McCartney CR, et al. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20:317–326. doi: 10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein JS, et al. Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin- releasing hormone-deficient men. J Clin Endocrinol Metab. 1991;73:609–620. doi: 10.1210/jcem-73-3-609. [DOI] [PubMed] [Google Scholar]
- 14.Hayes FJ, et al. Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J Clin Endocrinol Metab. 2001;86:53–58. doi: 10.1210/jcem.86.1.7101. [DOI] [PubMed] [Google Scholar]
- 15.Steiner RA, et al. Regulation of luteinizing hormone pulse frequency and amplitude by testosterone in the adult male rat. Endocrinology. 1982;111:2055–2061. doi: 10.1210/endo-111-6-2055. [DOI] [PubMed] [Google Scholar]
- 16.Rispoli LA, Nett TM. Pituitary gonadotropin-releasing hormone (GnRH) receptor: structure, distribution and regulation of expression. Anim Reprod Sci. 2005;88:57–74. doi: 10.1016/j.anireprosci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, et al. Estrogen receptor alpha-induced cholecystokinin type A receptor expression in the female mouse pituitary. J Endocrinol. 2007;195:393–405. doi: 10.1677/JOE-07-0358. [DOI] [PubMed] [Google Scholar]
- 18.Eagleson CA, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 19.Weiss JM, et al. Regulation of GnRH I receptor gene expression by the GnRH agonist triptorelin, estradiol, and progesterone in the gonadotroph-derived cell line alphaT3-1. Endocrine. 2006;30:139–144. doi: 10.1385/ENDO:30:1:139. [DOI] [PubMed] [Google Scholar]
- 20.Naor Z, et al. Signal transduction of the gonadotropin releasing hormone (GnRH) receptor: cross-talk of calcium, protein kinase C (PKC), and arachidonic acid. Cell Mol Neurobiol. 1995;15:527–544. doi: 10.1007/BF02071315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger LL, et al. Regulation of Intracellular Signaling Cascades by GNRH Pulse Frequency in the Rat Pituitary: Roles for CaMK II, ERK, and JNK Activation. Biol Reprod. 2008 doi: 10.1095/biolreprod.108.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, et al. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002;277:32099–32108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, et al. GnRH activates ERK1/2 leading to the induction of c-fos and LHbeta protein expression in LbetaT2 cells. Mol Endocrinol. 2002;16:419–434. doi: 10.1210/mend.16.3.0791. [DOI] [PubMed] [Google Scholar]
- 24.Kuphal D, et al. Stable transfection of GH3 cells with rat gonadotropin- releasing hormone receptor complementary deoxyribonucleic acid results in expression of a receptor coupled to cyclic adenosine 3′,5′-monophosphate-dependent prolactin release via a G-protein. Endocrinology. 1994;135:315–320. doi: 10.1210/endo.135.1.8013367. [DOI] [PubMed] [Google Scholar]
- 25.Imai A, et al. Coupling of gonadotropin-releasing hormone receptor to Gi protein in human reproductive tract tumors. J Clin Endocrinol Metab. 1996;81:3249–3253. doi: 10.1210/jcem.81.9.8784077. [DOI] [PubMed] [Google Scholar]
- 26.Stojilkovic SS, Catt KJ. Novel aspects of GnRH-induced intracellular signaling and secretion in pituitary gonadotrophs. J Neuroendocrinol. 1995;7:739–757. doi: 10.1111/j.1365-2826.1995.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 27.Krsmanovic LZ, et al. Regulation of Ca2+-sensitive adenylyl cyclase in gonadotropin-releasing hormone neurons. Mol Endocrinol. 2001;15:429–440. doi: 10.1210/mend.15.3.0610. [DOI] [PubMed] [Google Scholar]
- 28.Windle JJ, et al. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4:597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]
- 29.Alarid ET, et al. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- 30.Fortin J, et al. Conservation of mechanisms mediating gonadotrophin- releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol Hum Reprod. 2009;15:77–87. doi: 10.1093/molehr/gan079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coss D, et al. p38 mitogen-activated protein kinase is critical for synergistic induction of the FSH(beta) gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol. 2007;21:3071–3086. doi: 10.1210/me.2007-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawson MA, et al. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21:1175–1191. doi: 10.1210/me.2006-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider JE, Wade GN. Decreased availability of metabolic fuels induces anestrus in golden hamsters. Am J Physiol. 1990;258:R750–755. doi: 10.1152/ajpregu.1990.258.3.R750. [DOI] [PubMed] [Google Scholar]
- 34.Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1277–1296. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- 35.Veldhuis JD, et al. The aging male hypothalamic-pituitary-gonadal axis: Pulsatility and feedback. Mol Cell Endocrinol. 2008 doi: 10.1016/j.mce.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu PY, et al. Age-specific changes in the regulation of LH-dependent testosterone secretion: assessing responsiveness to varying endogenous gonadotropin output in normal men. Am J Physiol Regul Integr Comp Physiol. 2005;289:R721–728. doi: 10.1152/ajpregu.00138.2005. [DOI] [PubMed] [Google Scholar]
- 37.Hughes VA, et al. Interplay between dose and frequency of GnRH administration in determining pituitary gonadotropin responsiveness. Neuroendocrinology. 2008;87:142–150. doi: 10.1159/000112421. [DOI] [PubMed] [Google Scholar]
- 38.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344–351. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- 39.Taylor AE, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 40.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 41.Dunaif A. Insulin action in the polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:341–359. doi: 10.1016/s0889-8529(05)70073-6. [DOI] [PubMed] [Google Scholar]
- 42.Coccia ME, et al. GnRH antagonists. Eur J Obstet Gynecol Reprod Biol 115 Suppl. 2004;1:S44–56. doi: 10.1016/j.ejogrb.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Hohmann FP, et al. LH suppression following different low doses of the GnRH antagonist ganirelix in polycystic ovary syndrome. J Endocrinol Invest. 2005;28:990–997. doi: 10.1007/BF03345337. [DOI] [PubMed] [Google Scholar]
- 44.Sagnella F, et al. Suppression and recovery of gonadotropin and steroid secretion by a gonadotropin-releasing hormone receptor antagonist in healthy women with normal ovulation versus women with polycystic ovary syndrome in the early follicular phase. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.02.120. [DOI] [PubMed] [Google Scholar]
- 45.Ertunc D, et al. Gonadotropin-releasing hormone antagonist use in controlled ovarian stimulation and intrauterine insemination cycles in women with polycystic ovary syndrome. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2008.11.030. [DOI] [PubMed] [Google Scholar]