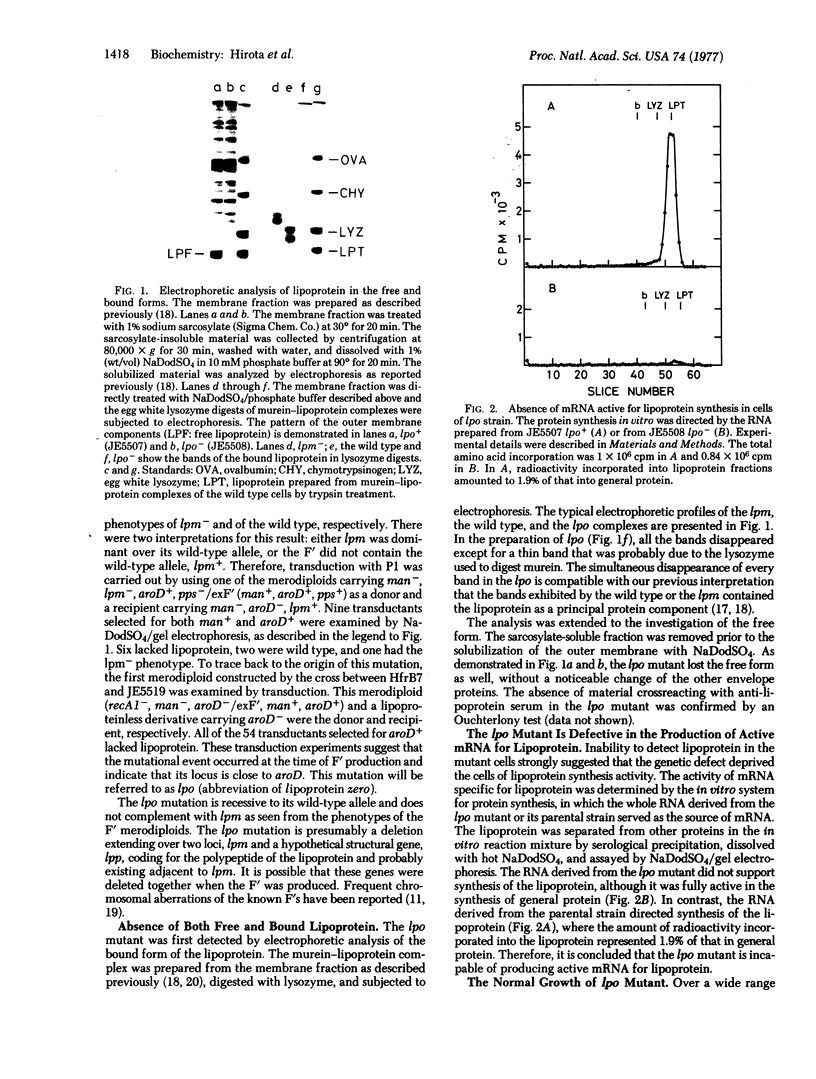
Abstract
A mutant of E. coli lacking a specific outer membrane lipoprotein was found. Both the free and the bound form have been lost in this mutant. No material that cross-reacted with antiserum against lipoprotein was detected by the Ouchterlony test. The mutant was defective in producing mRNA active for lipoprotein synthesis. The mutation leading to the loss of lipoprotein synthesis, referred to as lpo, seems to have arisen during production of an F'. The map position of lpo was at 36.5 min on the E. coli K12 map, in the order man, uidA, lpo, aroD, pps. The lpo mutant grew and divided normally and remained susceptible to bacteriophages lambda, phi80, P1, P2, the T series, and f1, f2, and MS2 in its male derivatives. The mutant was hypersensitive to EDTA and cationic dves and somewhat sensitive to detergents. There was considerable leakage of periplasmic enzymes but passive transport of beta-galactoside was unchanged. These physiological characteristics of the mutant suggest that lipoprotein is involved in maintaining the integrity of the outer envelope structure, by bridging the outer membrane and murein, but not in the vital processes of growth and division.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch V., Braun V. Distribution of murein-lipoprotein between the cytoplasmic and outer membrane of Escherichia coli. FEBS Lett. 1973 Aug 15;34(2):307–310. doi: 10.1016/0014-5793(73)80818-x. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Hantke K. Biochemistry of bacterial cell envelopes. Annu Rev Biochem. 1974;43(0):89–121. doi: 10.1146/annurev.bi.43.070174.000513. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K., Wolff H. Supramolecular structure of the rigid layer of the cell wall of Salmonella, Serratia, Proteus, and Pseudomonas fluorescens. Number of lipoprotein molecules in a membrane layer. Biochemistry. 1970 Dec 22;9(26):5041–5049. doi: 10.1021/bi00828a001. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Wang S., Inouye M. Cell-free synthesis of a specific lipoprotein of the Escherichia coli outer membrane directed by purified messenger RNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4149–4153. doi: 10.1073/pnas.71.10.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. A three-dimensional molecular assembly model of a lipoprotein from the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2396–2400. doi: 10.1073/pnas.71.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Hellerqvist C. G. Bacteriophage attachment sites, serological specificity, and chemical composition of the lipopolysaccharides of semirough and rough mutants of Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):57–64. doi: 10.1128/jb.105.1.57-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel G., Novel M. Mutants d'Escherichia coli K 12 affectés pour leur croissance sur méthyl-beta-D-glucuronide: localisation of gène de structure de la beta-D-glucuronidase (uid A. Mol Gen Genet. 1973;120(4):319–335. [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Iino T. In vitro synthesis of phase-specific flagellin of Salmonella. J Mol Biol. 1973 Nov 25;81(1):57–70. doi: 10.1016/0022-2836(73)90247-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Iketani H., Campisi J., Hirashima A. Novel mutation that causes a structural change in a lipoprotein in the outer membrane of Escherichia coli. J Bacteriol. 1976 Sep;127(3):1494–1501. doi: 10.1128/jb.127.3.1494-1501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti S. V., Park J. T. Lipoprotein of gram-negative bacteria is essential for growth and division. Nature. 1976 Sep 23;263(5575):323–326. doi: 10.1038/263323a0. [DOI] [PubMed] [Google Scholar]
- Voll M. J., Leive L. Release of lipopolysaccharide in Escherichia coli resistant to the permeability increase induced by ethylenediaminetetraacetate. J Biol Chem. 1970 Mar 10;245(5):1108–1114. [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Weigand R. A., Vinci K. D., Rothfield L. I. Morphogenesis of the bacterial division septum: a new class of septation-defective mutants. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1882–1886. doi: 10.1073/pnas.73.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Lin J. J. Escherichia coli mutants altered in murein lipoprotein. J Bacteriol. 1976 Apr;126(1):147–156. doi: 10.1128/jb.126.1.147-156.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]