Abstract
Introduction
Present-day rational drug design approaches are based on exploiting unique features of the target biomolecules, small- or macromolecule drug candidates, and physical forces that govern their interactions. The 2013 Nobel Prize in chemistry awarded “for the development of multiscale models for complex chemical systems” once again demonstrated the importance of the tailored drug discovery that reduces the role of the trial and error approach to a minimum. The “rational drug design” term is rather comprehensive as it includes all contemporary methods of drug discovery where serendipity and screening are substituted by the information-guided search for new and existing compounds. Successful implementation of these innovative drug discovery approaches is inevitably preceded by learning the physics, chemistry, and physiology of functioning of biological structures under normal and pathological conditions.
Areas covered
This article provides an overview of the recent rational drug design approaches to discover inhibitors of anthrax toxin. Some of the examples include small-molecule and peptide-based post-exposure therapeutic agents as well as several polyvalent compounds. The review also directs the reader to the vast literature on the recognized advances and future possibilities in the field.
Expert opinion
Existing options to combat anthrax toxin lethality are limited. With the only anthrax toxin inhibiting therapy (PA-targeting with a monoclonal antibody, raxibacumab) approved to treat inhalational anthrax, in our view, the situation is still insecure. The FDA’s animal rule for drug approval, which clears compounds without validated efficacy studies on humans, creates a high level of uncertainty, especially when a well-characterized animal model does not exist. Besides, unlike PA, which is known to be unstable, LF remains active in cells and in animal tissues for days. Therefore, the effectiveness of the post-exposure treatment of the individuals with anti-PA therapeutics can be time-dependent, requiring coordinated use of membrane permeable small-molecule inhibitors, which block the LF and EF enzymatic activity intracellularly. The desperate search for an ideal anthrax antitoxin allowed researchers to gain important knowledge of the basic principles of small-molecule interactions with their protein targets that could be easily transferred to other systems. At the same time, better identification and validation of anthrax toxin therapeutic targets at the molecular level, which include understanding of the physical forces underlying the target/drug interaction, as well as elucidation of the parameters determining the corresponding therapeutic windows, require further examination.
Keywords: rational drug design, Bacillus anthracis, binary toxins, antitoxins, polyvalent interactions
1. Introduction
Drug discovery strategies continuously evolve. Historically, drugs were discovered by serendipitous empirical approaches, by optimizing biologically active molecules provided by Nature, or by high-throughput screening of diverse chemical libraries (1). In our days, because of the rapidly growing knowledge of the biological origins of many diseases, we are witnessing the emergence of novel rational drug discovery methods, where biologically active compounds are specifically designed and tuned to attack the exact disease targets (2). These methods are based on exploiting unique features of the target biomolecules, small- or macromolecule drug candidates, and physical forces that govern their interactions. Rational drug design approaches often use computer-aided drug discovery methods where the three-dimensional models of druggable targets and druglike molecules are made (3). However, the “rational drug design” term is broader and could include all contemporary medicinal chemistry methods where serendipity and screening are substituted by the innovative and information-guided compound design. Successful implementation of these approaches would inevitably be preceded by learning the physics, chemistry, and physiology of functioning of biological structures under normal and pathological conditions.
The purpose of this article is to review the main recent strategies of drug design using the discovery of inhibitors against anthrax toxin as a prime example. The intentional dissemination of Bacillus anthracis spores in 2001 via the so-called “anthrax letters” and their fatal consequences led to the twelve years of continuing political and scientific efforts to develop medical countermeasures to protect humans from anthrax bioterrorism (4). Those efforts mainly focus on a search for the 1) new immunogenic vaccines, 2) selective antimicrobial agents against Bacillus anthracis, 3) passive immunization with anthrax-toxin targeting therapeutic antibodies, and 4) effective small-molecule antitoxins. Boosted by the demand, the field rapidly moves forward producing hundreds of publications. The current state of anthrax prophylactics and therapeutics as well as ongoing studies aiming to identify new targets for drug design and novel families of anthrax inhibitors were described recently in a series of reviews (4-8). This article is not intended to contribute yet another addition into this series; rather, it is conceived to discuss the rational approaches used for designing small-molecule anthrax toxin inhibitors. Rational design of peptide- and macromolecule-based polyvalent inhibitors is also addressed. The present status of the anthrax vaccine program, passive immunization with antibodies, and discoveries of new antibiotics against B. anthracis are not discussed.
2. Mode of action of anthrax toxin
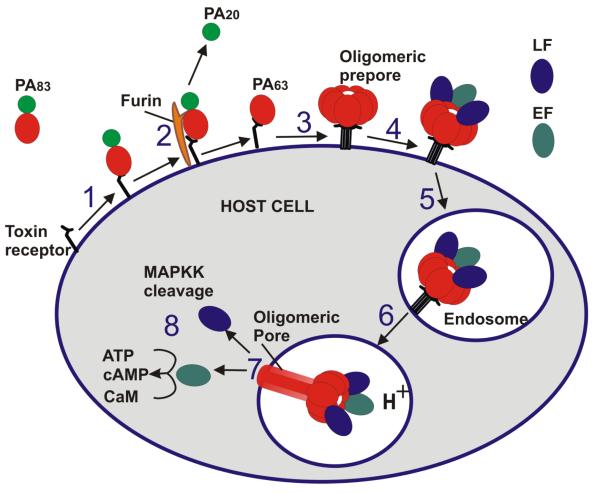
Bacillus anthracis, the causative agent of anthrax, is a large Gram-positive, rod-shaped, aerobic, spore-forming bacterial pathogen. The main virulence factors of B. anthracis are phagocytosis-inhibiting poly-D-glutamic acid capsule (9) and tripartite exotoxin (10, 11). The anthrax toxin is composed of two enzymatically active components: lethal factor (LF) and edema factor (EF) and one shared receptor binding and translocation component: protective antigen (PA). PA, LF, and EF, which are individually nontoxic, combine to form “classic” AB-type binary toxins (12): lethal toxin (LT = LF+PA) and edema toxin (ET = EF+PA), which are primarily responsible for the anthrax symptoms and lethality. Anthrax toxin-induced cell intoxication involves several stages shown in Figure 1. Full-length PA (PA83) binds to the cellular CMG2 and TEM8 receptors and, after being cleaved by extracellular furin protease to a 63-kDa form (PA63), undergoes oligomerization, forming either heptametic (13) or octameric (14) ring-shaped prepores. The prepore formation generates three (15) or four (14) LF and/or EF binding sites at the interface of two neighboring PA molecules. In addition, the oligomeric prepore formation causes receptor-mediated signaling that triggers endocytosis of the anthrax toxin complexes (16). Under the acidic endosomal environment, the oligomeric PA63 prepore undergoes substantial structural changes that allow it to embed into the endosomal membrane, where it forms a cation-selective channel (17). The protein wall of the oligomeric PA63 forms a single tunnel, a water-filled pore that connects solutions on both sides of the endosomal membrane. The elongated mushroom-like (of 125 Å diameter with 70 Å long cap and 100 Å long stem) membrane-spanning (PA63)7 structures were detected by the negative-stain electron microscopy (18). PA then is believed to act as an effective translocase, which, using the proton gradient across the endosomal membrane (pHendosome < pHcytosol), unfolds and translocates LF and EF into the host cell cytosol. The molecular details of the PA63 channel acting as the translocase have emerged as result of its intense studies in bilayer membranes (14, 19-29). Briefly, LF transport across the PA channel was directly observed by monitoring the resumption of ion current, originally reduced by channel occlusion by LF, after LF was translocated. Moreover, the translocation of LF was proven to be initiated by entry of its N-terminus into the PA63 channel (20) and driven through by either transmembrane potential (19) or by proton gradient (30) across the membrane. Importantly, the transport of LF through the PA63 channel, and, as a result, its toxicity were shown to be significantly suppressed by mutating phenylalanine residues at position 427 (21). The seven (or eight) F427 residues are believed to form a narrow constriction region inside the channel lumen (ϕ-clamp) that acts as a translocase active site crucial for the A components transport. Once in the cytosol, LF and EF exert their catalytic activities. LF is a Zn-metalloprotease which disturbs host cell signal transduction by cleaving mitogen-activated protein kinase kinases (MAPKKs) (31, 32) and Nlrp1 (33). EF is a calmodulin-dependent adenylyl cyclase (34), which aids in dissemination of B. anthracis in the host (35), and its neutralization was shown to significantly prolong survival time in a mouse model of spore infection (36). The newly identified key tissue targets responsible for the poisonous effects of lethal and edema toxins include two vital systems, the cardiovascular system (LT) and liver (ET) (37). Notably, targeting cardiomyocytes and vascular smooth muscle cells is required for LT-induced mortality, and action in hepatocytes occurs in ET-induced mortality. At the same time, endothelial cells that were often considered as a key object of LT-induced lethality were not found as primary targets for either LT or ET. The role of endothelial cells was assessed by generating endothelial-cell-specific CMG2 null mice (CMG2(EC−/−) and challenging them with 100 μg LT. The authors found that the CMG2(EC) −/− mice had similar sensitivity compared with their CMG2(EC)+/+ and CMG2+/+ littermates, whereas whole-body CMG2−/− mice were completely resistant to the toxin. Besides, endothelial-cell-specific CMG2-expressing mice (CMG2EC) survived two doses of 100 μg LT in contrast to their CMG2+/+ and CMG2+/− littermate (37). These recent findings are expected to contribute to our understanding of anthrax infection pathophysiology, which, provided that the documented human anthrax cases (38) are reexamined, would allow for widening the therapeutic window for the investigational anthrax antitoxins to work (37).
Figure 1.
A schematic model of Bacillus anthracis toxins cell entry. The numbers 1-8 indicate the eight main points at which antitoxins reviewed in the current article can act. Specifically, the potential agents can target PA-receptor binding (step 1), inhibit extracellular furin (step 2), prevent oligomeric PA63 prepore assembly (step 3), obstruct LF and EF binding (step 4), prevent endocytosis (step 5), impede oligomeric PA63 prepore-to-pore conversion (step 6), inhibit LF and EF PA-mediated translocation from endosomes (step 7), obstruct LF and EF enzymatic activity (step 8).
3. Novel strategies to design inhibitors of anthrax toxin
The events of 2001, when the existing vaccine was not available to the general public and only a limited number of antibiotics were approved by the FDA for the treatment of inhalational anthrax, had stimulated intense search for the new anthrax prophylactic and therapeutic approaches. Considering that humans are only occasionally become infected with B. anthracis, vaccination, undoubtedly being one of the most significant medical achievements of the 20th century, does not seem to provide a realistic solution to the problem. The controversial story of 2002 voluntary withdrawal of the LYMErix™, Lyme disease vaccine, which GlaxoSmithKline found to be economically unpractical to continue marketing, represents a gloomy example of the challenges that vaccine developers might face (39). Currently, there are several approved and recently discovered (40) antimicrobials with demonstrated ability to kill B. anthracis (for review see ref (41)). However, anthrax infection, especially in its inhalational form, is difficult to treat because flu-like symptoms appear only after B. anthracis have multiplied inside the human or animal host and started to produce the anthrax toxin (42, 43). Even though aggressive antibiotic therapy can inhibit bacterium growth, the infection can still be lethal because of the accumulation of the toxins (44). Moreover, engineered strains of B. anthracis resistant to the existing antimicrobials have been developed (10, 45, 46). Therefore, an effective post-exposure approach to anthrax treatment would most likely include synchronized blockage of bacterial growth by antibiotics and neutralization of anthrax toxin with antitoxins (10, 47).
Passive immunization with protective antibodies represents an attractive post-exposure option to combat anthrax infection (reviewed in ref (5)). Monoclonal antibodies (mAbs) directed against the major anthrax toxin components, PA, LF, and EF and against the capsule are at the most advanced stage of development. In December 2012, FDA and GlaxoSmithKline have announced the approval of raxibacumab mAbs for use as a treatment of inhalational anthrax in combination with antimicrobial agents (48). Raxibacumab efficiency was demonstrated in one study in monkeys and three studies in rabbits and its safety was evaluated in 326 healthy human volunteers. Because of the ethical issues with exposure of healthy human volunteers to aerosolized B. anthracis spores, raxibacumab was the first monoclonal antibody approved under the FDA’s animal efficacy rule, which allows approval of new pharmaceutical entities without testing for their efficacy in humans. Several different therapeutically suitable mAbs are currently under development.
On the other hand, using small-molecule therapeutic agents often offers several notable advantages when compared with antibodies. Small molecules are especially attractive as antitoxins, since their room temperature shelf-life far exceeds that of the current solution, antisera to toxins. Besides, small-molecule compounds are often orally bioavailable and therefore could be easily administered using ingestible tablets or capsules, which can later reach almost any target in the body, including the intracellular ones. Moreover, with regard to the rational drug design, the small-molecule drug candidate development represents an essentially different technological platform with a variety of classical and innovative approaches available.
Rational drug design involves knowledge of choices. As our understanding of anthrax tripartite toxin action has developed, the anthrax toxin components (PA, LF, and EF) have become targets of numerous efforts to design the effective antitoxins. Nearly every fundamental step of the toxin’s long intracellular journey have been explored and targeted. The potential antitoxin agents at investigational stages of development include: 1) targeting PA-receptor binding, 2) inhibiting extracellular furin, 3) preventing oligomeric PA63 prepore assembly, 4) preventing LF and EF binding, 5) preventing endocytosis, 6) targeting oligomeric PA63 prepore-to-pore conversion, 7) inhibiting LF and EF PA-mediated translocation from endosomes, 8) targeting LF and EF enzymatic activity (Fig. 1).
4. PA, LF, and EF as antitoxin design targets
Historically, PA, due to its principal role in anthrax pathophysiology, has been the key target in developing both preventive and therapeutic measures to combat anthrax. Because PA is the major antigen responsible for the toxin-neutralizing antibody production, it has been used as the principal component of the currently licensed and several investigational anthrax vaccines. “Protective antigen” name itself originates from the ability of this protein to elicit the protective antibodies in the anthrax vaccines. Moreover, because PA is the major anthrax toxin component responsible for the LF and EF intracellular delivery, it rapidly arose as an attractive target in a rational search for small- and macromolecule toxin inhibitors. Importantly, the PA-based inhibitors would most likely protect from both LT and ET. At the same time, numerous research efforts were concentrated on finding ways to disable anthrax toxins by specifically targeting LF or EF. LT traditionally has been considered as the more important factor in anthrax toxicity; however, due to the recently revealed possible role of ET in the anthrax infection of IV-heroin-users in Europe (49), disabling ET could also be critical (36).
4.1. Antitoxins targeting PA-receptor binding
PA binding to receptors on the cell surface is the first event in the multi-step anthrax toxin’s intracellular transit (Fig. 1). Provided effective diagnostics methods are available, any agents inhibiting this step may, in principle, show the benefits of early intervention. PA was found to bind to either one of the two cell-surface receptors, ANTXR1, which is a von Willebrand tumor endothelial marker 8 (TEM8) (50), or ANTXR2, which is capillary morphogenesis protein 2 (CMG2) (51). Besides, it was shown (52) that integrin beta 1, the cell surface von Willebrand A domain protein, is able to mediate anthrax toxin endocytosis. There are at least three different ways to target the receptor-binding step: 1) by blocking the receptor-binding domain of PA (53), 2) by blocking the host cell receptors (54, 55), 3) by using soluble TEM8 and CMG2 decoy proteins aimed to compete with the natural receptors for PA binding (56). One of the first successful attempts on disabling anthrax toxin by targeting the cell-surface receptors was reported by Kane’s group in 2006 (55). Using phage display technology, the authors identified several 12-residue and 7-residue ANTXR1 and ANTXR2 binding peptides. Interestingly, the downstream homology sequence (DHS) was shared by those identified peptides that were able to bind both receptors. Four polyvalent inhibitors were then synthesized by attaching multiple copies of each DHS-containing peptide to liposome scaffolds. The compounds were tested for their ability to neutralize anthrax toxin in RAW264.7 cell assays. The most potent polyvalent inhibitor, liposome functionalized with multiple copies of AWPLSQLDHSYN peptide, protected the cells from anthrax toxin intoxication with a half-maximal inhibitory concentration IC50 = 40 nM on a per-peptide basis, allowing for more than 50,000-fold enhancement of the single peptide activity. The ability of the polyvalent inhibitors to neutralize anthrax toxin in vivo was tested in Fisher 344 rats where 6 out of 6 animals survived when LT (a mixture of 30 μg of PA and 8 μg of LF) was coinjected intravenously with 400 nmol of the most effective polyvalent inhibitor (55).
More recently, two pilot screen studies were reported where a sensitive high-throughput fluorescence resonance energy transfer (FRET) assay was used to identify potent small-molecule inhibitors of PA interaction with CMG2 (57) and TEM8 (58). The assay was based on FRET detected during the interaction of a fluorescently labeled CMG2 or TEM8 with a fluorescently labeled PA and allowed identification of tannic acid and cisplatin, and edselen and thimerosal as, respectively, CMG2/PA and TEM8/PA interaction inhibitors. Importantly, even though the precise biological functions of the CMG2 and TEM8 receptors in the absence of anthrax toxin have not been fully understood, these proteins were first discovered due to their overexpression on endothelial cells undergoing angiogenesis or blood vessel growth (59). Targeting angiogenesis is an important strategy for current antitumor therapies, where both methods utilizing small-molecule CMG2 and TEM8 inhibitors and tailored forms of the anthrax toxin have potential for applications.
4.2. Antitoxins inhibiting extracellular furin
Proteolytic activation of PA to the functionally active PA63 by extracellular furin protease triggers PA63 oligomerization to form a receptor-bound heptameric or octameric prepore. Therefore, targeting host cell furin is a coherent therapeutic strategy against anthrax toxin. In particular, one of the potential blockers of Pseudomonas aeruginosa exotoxin A previously discovered using combinatorial chemistry techniques, small stable furin inhibitor hexa-D-arginine amide (D6R = (D-Arg)6–NH2) delayed anthrax toxin-induced toxemia both in vitro and in vivo (60). Thus, the lead furin inhibitors represent a potential group of compounds for further optimization and development as therapeutic agents against furin-dependent diseases, such as bacterial and viral infections, cancer, and Alzheimer’s disease (7). Further studies have shown that the polyarginine-rich peptides, in general, have an enhanced inhibitory activity against anthrax toxin specifically targeting furin protease. Thus nona-D-arginine (D9R= (D-Arg)9–NH2) demonstrates 30 times increase in activity compared to D6R (61). Interestingly, the peptides with arginine-rich sequences were also identified as inhibitors of metalloprotease LF (62, 63). In particular, In-2-LF, a peptide hydroxamate, blocked anthrax toxicity in RAW264.7 and J774.A1 cells (62). The common requirement for arginine-rich sequences between furin and LF inhibitors was addressed in a study where a potential inhibitory cross-reaction of LF and furin inhibitors was investigated (64). In-2-LF, in addition to inhibiting LF, also inhibited furin, whereas furin inhibitors D6R and D9R inhibited LF. The authors suggested that the combined treatment with both furin and LF inhibitors might represent a more effective approach to the blockage of anthrax toxemia than the use of either inhibitor alone.
More recently, sharply focused and specific antagonists of pathogen entry, nanomolar-range peptide inhibitors of host cell furin and furin-related proprotein convertases, were reported (65). The authors modified the extended furin cleavage sequence of avian influenza A H5N1 HA. The resulting competitive inhibitors were capable of efficiently obstructing furin proteolysis of anthrax PA83 and avian influenza H5N1 HA in vitro. Importantly, the peptides have also demonstrated efficacy in mice against both anthrax and Pseudomanas exotoxin. The authors later optimized their lead compounds focusing on N- and C-terminal modifications of the original RARRRKKRT scaffold inhibitory sequence (66). The resulting compounds did not interfere with the normal cellular function of furin, meanwhile showing a promising potential as selective anthrax inhibitors. Besides, a series of naturally occurring serine protease inhibitors was screened for the ability to rescue macrophages from LT-induced cytotoxicity. One endogenous protease inhibitor, a member of the inter-alpha-inhibitor protein (IαIp) complex, was highly protective in this macrophage lethality assay (67) and, when administered with antimicrobial therapy an hour or up to 24 hours after spore challenge with Bacillus anthracis Sterne strain, protected mice from lethality (68). IαIp is a large, multi-component, covalently linked glycopeptide-proteoglycan complex that functions as a trypsin-type protease inhibitor. To study the activity of furin inhibitors, an artificial neural network was used for constructing a quantitative structure-activity relationship model and exploring different methodologies for classifying and predicting the biological activity of these compounds (69).
4.3. Antitoxins preventing oligomeric PA63 prepore assembly
Considering the need for fast discovery of efficient anthrax antitoxins versus the long and costly FDA-regulated drug approval process, several groups focused their effort on screening for the drugs that are already approved for human use in treatment of different conditions. A table of the related FDA-approved substances that demonstrated a certain level of efficacy against anthrax toxin is provided in a review (8). Thus cisplatin, one of the most effective chemotherapeutic anticancer agents, protected LT-sensitive macrophages from lysis by inhibiting LF translocation to the cytosol (70). The authors report that cisplatin acts by modifying PA in a reversible non-covalent manner that does not interfere with its cell binding and processing but prevents the oligomer formation, which is required for LF binding. To confirm this conclusion, the authors tested cisplatin against two other toxins translocated by PA, the anthrax edema toxin and the cytotoxin FP59, a fusion of LF and exotoxin A from Pseudomonas aeruginosa, and demonstrated that cisplatin inhibited both toxins. Note that the cisplatin’s receptor-binding inhibitory properties were reported (57) as discussed earlier in present review. Interestingly, the administration of cisplatin-pretreated lethal doses of LT resulted in total protection of BALB/cJ mice and Fisher 344 rats, whereas the administration of cisplatin to mice before or after toxin administration was not protective. The authors explain these data by the cisplatin’s affinity towards thiols, leaving little drug to react with amino acid side chains of the anthrax toxin proteins, rapid cisplatin clearance from the plasma, and a very short half-life in blood. At the same time, identification of the PA residues modified by cisplatin, which leads to inhibition of oligomerization, may provide useful information for both structure/function studies of LF and further drug discovery efforts.
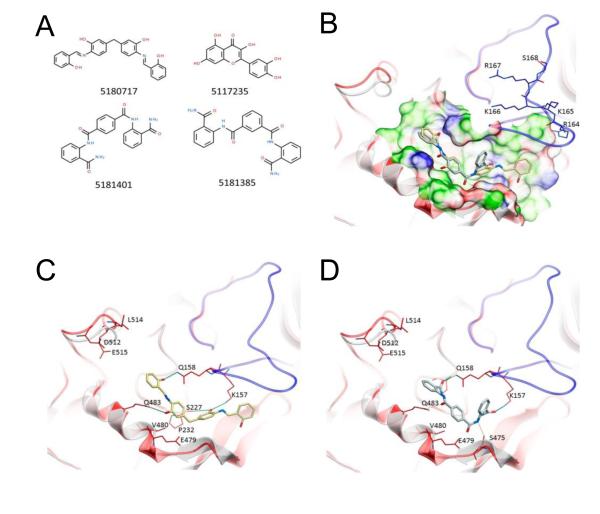
By mimicking key residues of PA63 needed for the heptamer-stabilizing intermolecular interactions, CAVEAT molecular design package was used to design two molecular scaffolds inhibiting the PA63 oligomerization (71). The authors reported that three out of seven designed and synthesized compounds showed modest anthrax toxin inhibition in murine J774A.1 macrophage cells. Most recently, using virtual ligand screening of a library of 10,000 members, Wein et al. have identified two lead small-molecule compounds (molecules 5180717 and 5181401 in Figure 2) capable to protect RAW264.7 and HT1080 cells, independently blocking both PA’s cleavage by furin and subsequent oligomerization (72). The compounds were originally designed in silico to bind to a pocket on an oligomerization face of PA and then, surprisingly, were shown to reduce the rate of furin cleavage of PA. The authors suggest several interesting structural models to explain this effect. These molecules are believed to be the most effective PA oligomerization-inhibiting compounds reported, and the paper represents the first study demonstrating dual-acting inhibitory effects of the designed blockers.
Figure 2.
Structures of small-molecule anthrax toxin inhibitors and their modes of binding to PA. (A) The compounds investigated in ref (72). (B) PA crystal structure 1T6B (red ribbon) superimposed on the crystal structure 3TEW (gray ribbon) with the ordered furin loop in 3TEW highlighted in blue. The furin-type protease cleaves after the sequence 164RKKR, which is shown in stick representation with carbon atoms colored blue. The predicted binding poses for inhibitors 5180717 and 5181401 are displayed in stick representation with carbon atoms colored yellow and gray, respectively. The binding pocket surface for 1T6B used for virtual screening is displayed (white = neutral surface, green = hydrophobic surface, red = hydrogen bonding acceptor potential, blue = hydrogen bond donor potential). (C, D) Predicted interactions of 5180717 (yellow stick) and 5181401 (gray stick), respectively, with PA. Both ligands make common hydrogen bonds with Q158, Q483, and K157. Reprinted with permission from ref (72). Copyright 2012. American Chemical Society.
4.4. Antitoxins obstructing LF and EF binding
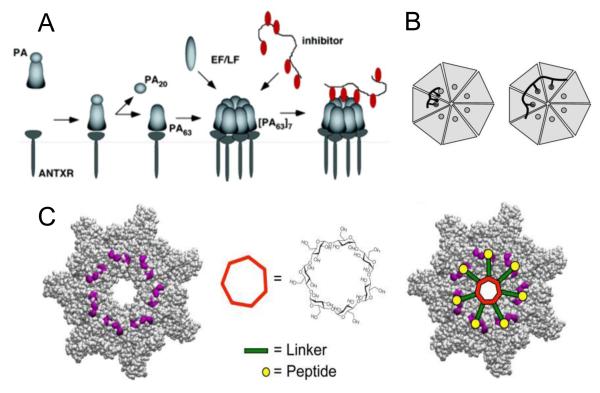
One way to disable anthrax toxin action is to block PA63 interaction with LF and EF (Figure 3). The dodecameric peptides able to bind to PA63 and weakly inhibit its interaction with enzymatic components of the anthrax toxins were identified from a phage-display library (73). However, when multiple copies of these peptides were covalently linked to a polyacrylamide backbone, the resulting polyvalent molecule strongly inhibited LF and EF binding to PA63 and protected cells and animals from the anthrax toxin action. The authors also emphasized that the use of a polymer with a flexible backbone (Fig. 3A) (74) would allow the drug designers to avoid the necessity of knowing the spatial relationships among the binding sites on the target, thus extending the applicability of the approach to targets in which these relationships are not yet understood. This approach was further advanced by designing functionalized liposomes of ~50 nm in size, which were allowed to interact with a cysteine-derivatized version of the earlier identified (73) PA63 binding peptides (75). Remarkably, peptide-functionalized liposomes inhibited the intoxication of RAW264.7 cells by LT at extremely low concentrations (IC50 = 20 nM on a per-peptide basis), whereas the monovalent peptide did not inhibit LT activity at concentrations as high as 250 μM. Moreover, the peptide-functionalized liposomes were active in vivo protecting rats from the lethal toxin action. The authors investigated the effect of altering the density of the PA63-binding peptides on the potency of the functionalized liposomes. Herewith, a distinct transition in IC50 values was observed over a narrow range of average distances between the peptides corresponding to their 3.5-5 nM bulk concentration thus demonstrating the importance of pattern matching in recognition of oligomeric PA63 by the polyvalent liposomes. Another approach to match the inhibitor/target pattern guided the synthesis of polyvalent inhibitors of the controlled molecular weight and ligand density and placement along an inert polymeric scaffold (76, 77) (Fig. 3B). Such control was achieved by using the reversible addition-fragmentation chain transfer polymerization (RAFT technique) (78).
Figure 3.
Inhibition of toxin assembly by polyvalent inhibitors. (A) After being cleaved by extracellular furin protease to a 63-kDa form, PA63 oligomerizes and binds either EF/LF or a peptide-based inhibitor (74). (B) Design of polyvalent inhibitors with control over the molecular weight and ligand spacing (76). The linear polyvalent inhibitors displaying peptides (black ovals) are shown bound to the PA63 heptamer at the peptide-binding sites (circles). The spacing between peptides on the linear scaffold is either too short (left panel) or sufficient (right panel) to allow polyvalent interactions. (C) Structure-based design of the heptavalent anthrax toxin inhibitors (80). Left: The structure of the LF-binding face of heptameric PA63. Residues 184, 187, 197, and 200, which form part of the peptide-binding site, are shown in purple. Middle: The structure of 7-fold symmetrical β-cyclodextrin, which was used as a scaffold for a heptameric inhibitor. Right: A scheme illustrating the binding of the heptavalent inhibitor, synthesized by the attachment of seven inhibitory peptides to the β-cyclodextrin via an appropriate PEG linker, to heptameric PA63. Reprinted with permission from refs (74, 76, 80). Copyright 2005, 2006, 2011. American Chemical Society.
In rational drug design of polyvalent inhibitors, once a biospecific ligand is identified, the next important step is a search for a suitable and inert scaffold to attach the ligands (79). To expand the scaffold diversity, the inhibitory peptides were also grafted to the core of β-cyclodextrins via polyethylene glycol (PEG) linkers (80) (Fig. 3C). These 7-fold symmetrical molecules were previously used as the scaffolds to design polyvalent PA63 channel blockers (81) (see paragraph 4.7 below). The PEG linker length was varied to find an optimal fit of the polyvalent inhibitor to PA63. To achieve an optimal inhibitor/target pattern match, experimental mutagenic studies were accompanied by computational docking experiments searching for a suitable binding site for the inhibitory peptide on the heptameric PA63. The root-mean square distance from the center of cyclodextrin core to the end of the PEG11 linker was estimated as 30 Å, which matched the distance from the center of the PA63 oligomer to the identified peptide-binding residues. The idea of the 7-fold symmetrical functional group placement allowed the authors to reach the IC50 values as low as 10 nM on a per-peptide basis in vitro by incubating RAW264.7 cells with the lethal toxin. The heptameric blocker showed a more than 100,000-fold increase in the activity compared with the monomeric peptide. The functionalized β-cyclodextrin-based blocker did not show any major decline in activity over a three-day period and was also effective in vivo protecting rats from intoxication by LT.
4.5. Antitoxins preventing endocytosis
AB-type toxins rely on target cell receptors to reach their desired intracellular targets. At the same time, PA binding to the extracellular domain of the anthrax toxin CMG2 and TEM8 receptors and its subsequent oligomerization was found to activate specific signaling events. Thus, anthrax toxin was shown to trigger tyrosine phosphorylation of its own receptors, which is required for toxin uptake because endocytosis of the mutant receptor lacking the cytoplasmic tyrosine residues was significantly slowed down (82). Herewith, receptor phosphorylation was found to depend on src-like kinases, which were activated because of toxin binding. The src-dependent phosphorylation of the receptor was critical for its later ubiquitination required for clathrin-mediated endocytosis (83). More specifically, to test the importance of tyrosine phosphorylation events in toxin uptake, the authors analyzed the effect of genistein, which is the general tyrosine phosphorylation inhibitor. Since the cleavage of the LF-target mitogen-activated protein kinase kinases MEK1 was not observed in genistein-treated cells, LF was not delivered to the cytoplasm. By blocking the activation of src-kinases, genistein inhibited endocytosis of the PA oligomeric complex. Even though genistein was used to investigate the mechanism of anthrax toxin endocytosis, the study suggests new possible targets for the rational design of anthrax antitoxins (82). In addition, it was found recently that B. anthracis toxins exploit the homeostatic actions of calpains to promote toxin entry into the targeted cells (84). Treatment of cells with a cell-permeable synthetic agent MDL28170, which broadly inhibits the activity of calpains, protected from anthrax toxin lethality.
Most recently, a high-throughput screening of 30,000 small molecule compounds was used to identify 4-bromobenzaldehyde N-(2,6-dimethylphenyl)semicarbazone (EGA) as the most active inhibitor of endosomal trafficking (85). The authors suggest obstruction of trafficking between early and late endosomes as the likely mechanism of the inhibition. Besides, EGA was shown to block the entry of pH-dependent bacterial toxins, such as P. aeruginosa ExoA and diphtheria toxin DT, which share similar cellular entry requirements with LT. EGA was also reported to inhibit the pH-dependent viral entry – the result showing EGA’s potential to become a powerful tool for the study of membrane trafficking.
4.6. Antitoxins targeting oligomeric PA63 prepore-to-pore conversion
Prepore to pore transformation is an important step in anthrax toxin intracellular transport, which requires essential structural reorganization in the PA oligomer (recently reviewed in ref (86)). Several small-molecule blockers impairing the PA heptamer prepore to pore conversion in cells expressing the CMG2 receptor were identified with a quantitative high-throughput screening (87). The method was based on a cell pathway assay that monitors receptor-mediated endocytosis of a modified, non-toxic anthrax toxin LF-β-lactamase/PA complex (88). Among the most effective compounds identified were the (1) novel chromeno[4,3-c]pyrazol-4(1H)-one (NCGC00084148-01), (2) lignan diphyllin, and (3) teniacide niclosamide. The inhibitors were shown to protect RAW264.7 macrophages from anthrax lethal toxin and CHO cells from the LF-Pseudomonas exotoxin fusion protein and diphtheria toxin. Interestingly, none of the compounds was shown to block cellular binding, proteolytic processing, heptamerization of PA, or PA-mediated LF-binding. Instead, the identified inhibitors influenced the heptamer prepore-to-pore conversion by inhibiting toxin internalization and/or trafficking to endosomes of increased acidity.
An isolated anti-PA monoclonal antibody, cAb29 was shown to effectively neutralize anthrax toxin (89). By systematic evaluation of the steps that take place during the PA-based intoxication, the authors found that cAB29 did not interfere with the initial steps of intoxication (receptor binding, proteolytic cleavage, oligomerization, prepore formation and LF binding) (90). However, the cAb29 binding to the prepore prevented its pH-triggered modification to the transmembrane pore thus blocking the LF/EF translocation into the cytosol.
4.7. Antitoxins blocking LF and EF PA-mediated translocation
After endocytosis, PA mediates the process of LF/EF translocation across the endosomal membrane inside the cell. A widely accepted traditional model of LF/EF transport into the cytosol of the host cell states that under acidic endosome conditions PA forms a cation-selective channel that unfolds and translocates LF and EF into the host cell cytosol. This model is supported by a number of careful experiments with single PA channels reconstituted in planar lipid membranes recently reviewed in ref (28, 91, 92). The reconstitution experiments demonstrate convincingly that PA channels are able to serve as trans-membrane conduits of the factors, with transport parameters depending on membrane voltage, pH gradient, and other conditions. An alternative mechanism, in which the anthrax toxin catalyzes the rupture of the endosomal membrane, thereby releasing the toxins into the cytoplasm, was also recently suggested (93). Regardless, it is important to note here that a variety of available and rationally design compounds aimed to specifically disrupt the channel’s translocation pathway were shown to be very effective in inhibiting anthrax toxin. In particular, dominant-negative mutants of protective antigen that co-assemble with the wild-type PA63 protein and block its ability to translocate the LF and EF components have been developed (94-97). Moreover, the dominant negative mutants of anthrax toxin were investigated as a replacement for PA in anthrax vaccines for both the post-exposure treatment and infection prophylactics (98). Interestingly, a single dominant-negative subunit with the key amino acid residues mutated was sufficient to significantly inhibit the translocase function of the PA63 heptamer. More specifically, mutations of Phe427 (or ϕ-clamp) to almost any residue except Trp, Tyr, or Leu disrupted the PA transport function (99), whereas some other Phe427 mutations affected the conformational transition of the prepore to the pore (100).
Aside from the PA-based dominant negative therapies, a number of small-molecule blockers designed to directly obstruct the anthrax toxin pore were reported. The PA63 channel is known to be preferentially selective to cations. Moreover, nearly any cationic compound tested with the planar lipid bilayer membrane technique was shown to reversibly interact with the PA63 channel’s lumen. The method of channel reconstitution into planar lipid bilayers, especially in its low-noise monolayer opposition arrangement (101), is a unique approach allowing for recording of the individual events of single molecule/single channel interactions. The approach turned out to be particularly useful in the design of the channel-blocking antitoxins recently reviewed in ref (102). It was found that the PA63 channel is permeable for and can be blocked by tetraalkylammonium ions (103-105). The inhibition of the channel conductance by small molecules carrying one or two positive charges was also reported (21, 106, 107).
Initially, the ability of the cationic compounds to block PA63 was explained by their Coulombic interactions with the binding sites formed by the negatively charged amino acid residues in the cis entry region of the channel. An alternative explanation involved interaction of cationic compounds with the ϕ-clamp, which was also shown to be crucial for the PA63 translocase activity (21). Across the studied library of molecules, the ϕ-clamp preferred aromatic moieties by 0.7 kcal/mol per aromatic ring. Several compounds with multiple aromatic rings were the ones that showed the promising nanomolar range of binding affinity towards the channel. The ϕ-clamp mutations were shown to significantly affect binding affinity of hydrophobic cations. The blocker/PA63 binding reaction was suggested to include cation-π interactions between aromatic residues and cations through their delocalized negative π-electron cloud.
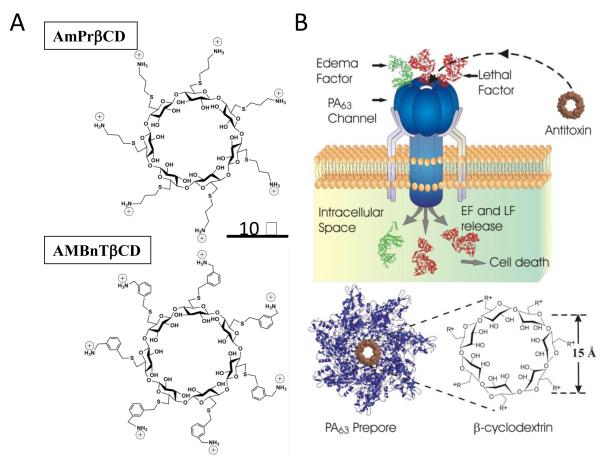
A considerable progress in inactivating anthrax toxin by the high-affinity blockage of the PA63 pore with cationic compounds was achieved using a rational design of 7-fold symmetrical positively charged derivatives of β-cyclodextrin (7+ βCD) schematically illustrated in Figure 4 (recently reviewed in ref (108)). The PA63 prepore internal diameter was estimated to be between 20 – 35 Å (13) with the PA63 pore’s constriction region not exceeding 12 Å (103-105). This finding directed the choice of the ~15-Å 7-fold symmetrical βCD molecules carrying seven positive charges covalently linked to the cyclodextrin’s core by hydrocarbon linkers of different nature and length. One of the first synthesized compounds, the so called AmPrβCD is shown in Fig. 4A, top. To optimize this molecule, the effect of the positively charged pendant groups and the length and nature of alkyl spacers on the activity of 7+βCDs was investigated (109-111). First, a group of hepta-6-thioaminoalkyl derivatives with alkyl spacers of increasing lengths was tested for their ability to both inhibit the lethal toxin cytotoxicity and to block ion current through the PA63 channels in planar lipid bilayers (109). The two methods showed that there is an optimal length of the alkyl spacers (3-8 CH2-linkers) between the amino-group and the cyclodextrin core. Short spacers (1 and 2 CH2-linkers) were less effective in inhibition of the channels, apparently limiting compound’s polyvalent properties. The molecules with longer spacers (9 and 10 CH2-linkers) exhibited higher toxicity to the RAW cells and caused bilayer lipid membrane rupture. Second, in order to check the influence of the nature of the positively charged groups on compound’s efficiency, a group of hepta-6-guanidine β-cyclodextrin derivatives, in which positive charges were distributed between the nitrogens of the guanidine moiety, was tested. The activity of these compounds was decreased only slightly compared to their aminoalkyl analogs. More importantly, hepta-6-arylamine βCD derivatives, which in addition to the pendant amino-groups contained one phenyl group per each thio-hydrocarbon linker, possessed noticeably greater binding affinity in vitro, in cell assays, and in vivo (109, 112). One of the derivatives, per-6-S-(3-aminomethyl)benzylthio-β-cyclodextrin (AMBnTβCD) (Fig. 4A, bottom) was chosen for further development. It was shown that AMBnTβCD blocked the pore of anthrax’s PA63 in planar lipid bilayers with KD = 0.13 ± 0.1 nM and protected cultured macrophage-like cells from anthrax lethal toxin with IC50 = 0.5 ± 0.2 μM (109, 111). These blockage and inhibition parameters are among the best published for the small-molecule anthrax toxin inhibitors. It was reported (102) that AMBnTβCD is several times more efficient than the most potent non-peptide blockers of ion-selective channels of excitable membranes, including the classical sodium channel blocker tetrodotoxin (113, 114), whose dissociation constant is close to 1 nM. Most importantly, AMBnTβCD completely protected Fisher F344 rats from intoxication with lethal toxin and, in combination with antibiotic ciprofloxacin, significantly increased the survival of mice in an infection model of anthrax (112). In addition to inhibition of anthrax toxin, positively charged β-cyclodextrin derivatives were also effective against clostridial binary toxins (115, 116).
Figure 4.
Blocking anthrax on a single-channel level. (A) Two sevenfold symmetrical synthetic molecules, AmPrβCD (top panel) and AMBnTβCD (bottom panel) were used as blockers of PA63 channels. (B) A heptameric mushroom-like channel of PA63 believed to be a translocation pathway for lethal and edema factor (LF and EF) inside the cell under attack (top). The idea was to design complementary heptameric low-molecular weight compounds – cationic cyclodextrins (bottom) that enter the channel and block it as molecular plugs. The cartoon is a simplified illustration of the LF and EF penetration into the mammalian cell. In reality, the process is much more complex. Reprinted with permission from refs (116, 162). Copyright 2010, 2012. Biophysical Society.
To examine the nature of the physical forces involved in the pore/blocker binding reaction, voltage and salt dependences of the rate constants of binding of the two structurally different β-cyclodextrins (AmPrβCD and AMBnTβCD) to the PA63 channels were recently investigated (116). The authors found that the blocking efficiency directly correlated with the presence of aromatic groups carried by the cationic molecules and that F427A was the prime site for the pore/blocker interaction. By estimating the relative contributions of the applied transmembrane field, long-range Coulomb, and salt-concentration-independent, short-range forces, it was reported that the latter represent the leading interaction, which accounts for the high efficiency of the blockage. The particular origin of these forces was unclear. At this moment, guided by the structural features of the efficient cyclodextrin-based blockers, one can only speculate that in addition to hydrophobic interactions many others such as aromatic-aromatic π-π and cation-π interactions, hydrogen bonding, van der Waals interactions, etc. might be involved.
It was also reported that even though the 7-fold symmetry of the blocker molecules complementing the heptameric structure of PA63 was important, it was not an absolute requirement for the effective blockage. Both 6-fold symmetrical αCD carrying 6 positive charges and 8-fold symmetrical γCD carrying 8 positive charges were able to block the PA63 channel in planar lipid membranes (111). The activity of the 8+γCDs tested with cell assays was equal or even higher compared with their 7+βCD analogs, whereas binding of 6+αCD was not strong enough to make these compounds protective against anthrax toxin. It was suggested that the inhibitory activity of the 8-fold symmetrical γCDs could be related to the reported observation of the PA63 octameric pores (14). However, both positively charged βCDs and γCDs were able to block any single PA63 channel incorporated into planar lipid bilayers. This indicates that it is the polyligand nature of these cyclical compounds rather than the exact pore/blocker symmetry match that determines their enhanced efficiency. These findings demonstrate the value of the 7+βCD and other polyvalent inhibitors for designing drugs against anthrax toxins, especially when an appropriate lead optimization and pharmacokinetic studies are conducted.
Note, that in a number of studies cells were shown to be protected from lethal and edema toxin action by weak bases, such as ammonium chloride or antimalarial drug chloroquine as well as by proton pump inhibitors, such as bafilomycin A (117-120). It is interesting that chloroquine, which is known to reversibly block PA63 (106), is also discussed in the literature in regards to its ability to block endosomal acidification by raising endosomal pH (121). Moreover, a group of the FDA-approved small-molecule compounds that presumably block LF or EF transport by changing endosomal pH was reported (87, 122). It would be interesting to evaluate to what degree the other pore-blocking cationic compounds are able to influence the endosomal pH which is known to be critical for both PA63 pore formation and LF/EF translocation. At the same time, we cannot exclude a possibility that some of the cationic compounds may interact with the PA63 prepore outside the cell. Some indications of such interactions were reported for the β-cyclodextrin-based inhibitors (123).
4.8. Antitoxins targeting LF enzymatic activity
While the antitoxins discussed so far were mostly targeting PA, the molecules described in this and the following sections are specific for the causative agents that disrupt cell functioning, namely anthrax toxin’s catalytic moieties. PA as a target has certain shortcomings that shift the focus of the current antitoxin research towards LF. In contrast to less stable PA, LF remains active in cells and in animal tissues for days, as manifested by continued cleavage of MAPPKs proteins by the toxin (124). Moreover, PA was recently shown to translocate LF not only into the cytosol but also into the lumen of endosomal intraluminal vesicles that can later fuse and release LF into the cytosol. As a result, LF can survive in the vesicles for days being fully protected from proteolytic degradation (125). Therefore, efficacy of the post-exposure treatment with PA-targeting drugs can be time-dependent, being more effective at the early stages of infection. Since the discovery of one of the first LF inhibitors in 2002 (62), numerous research efforts to search for the “ideal” (126) LF inhibitor were undertaken. Because of the great number of these studies, which are reviewed elsewhere (7, 127-129), the examples below are not exhaustive and rather serve the specific scope of the current review. While LF and EF inhibitors can act at any appropriate stage of the toxin’s pathway, for instance by preventing LF/EF interaction with PA, most of the designed compounds perform intracellularly by inhibiting the metalloprotease activity of LF or the adenylase cyclase activity of EF.
For that purpose, the rational design efforts to target the catalytic moieties of the anthrax toxin mostly focused on small-molecule (non-peptide) compounds that are able to pass the transmembrane barrier and find their intracellular target. Peptide-based compounds are less likely to reach the required inhibitory concentration in the cytosol. Thus, three small organic LF inhibitors with Ki values in the 0.5-5 μM range were identified using LF X-ray crystal structure and structure-based molecular diversity screening combined with three-dimensional database searching and molecular modeling (130). The hydroxamate LF inhibitor able to co-crystalize with LF at the LF-active state was identified (131). The molecular interaction between the inhibitor and LF led to inhibition of LF catalytic activity in a cell-based assay and complete protection in a lethal mouse toxemia model. Moreover, a 50% improvement in survival was reached in mice challenged with B. anthracis Sterne vegetative cells and in rabbits challenged with B. anthracis Ames spores. When the compound was used in combination with a specific antimicrobial agent ciprofloxacin, 100% protection against B. anthracis spore challenge was demonstrated. At the same time, ciprofloxacin alone provided only 50% protection.
Several chemically distinct classes of small and peptide-based polycationic molecules have been identified as LF metalloprotease inhibitors. For example, a small library of ~500 compounds of several chemical classes has been screened to identify molecules that would specifically bind to the anionic sites on LF and block the substrate cleavage (132). Using a fluorescence-based assay, a number of positively charged inhibitory molecules including polyamines, aminoglycosides, and cationic peptides were identified and characterized. In these studies, spermine was shown to inhibit LF with the Ki value of 0.9 μM. Besides, a detailed kinetic investigation of aminoglycoside neomycin B and neamine binding was reported, which showed that these molecules act as mixed-type noncompetitive inhibitors of LF (133). The paper offers interesting discussion aimed to explain the discrepancy between the data on molecular details governing the inhibition of the lethal factor protease by aminoglycosides reported in literature, namely, strictly competitive (134) versus noncompetitive (133) binding. Understanding the difference between the two mechanisms can indeed have important implications in the rational design of LF inhibitors. It is questionable if these cationic compounds can reach a sufficient endosomal concentration to influence LF transport by changing the endosomal pH, as it was claimed for chloroquine, or act by blocking the PA channel, similarly to what was reported for the positively charged cyclodextrins and other small-molecule cationic compounds.
LF was also neutralized in vivo by a group of α-defensins produced by the human neutrophils (135). The mechanism of protection involved inhibition of cleavage and restoring the signaling function of MAPKK. The cyclic octadecapeptides, encoded θ-defensins by the modified α-defensin genes of nonhuman primates were shown to inhibit the anthrax infection in two ways. In addition to being active against B. anthracis cells, θ-defensins inhibited LT by binding to it with high affinity in the experiments with RAW264.7 cells (136). Another approach to inhibit LT was developed in a study where the importance of the N-end rule for LT-mediated caspase-1 activation and cell death was established (137). The authors also showed that bestatin methyl ester, an aminopeptidase inhibitor protected against LT both in vitro and in vivo and that different inhibitors of the protein degradation pathway act synergistically in protecting against LT.
A new pharmacophore map assembly designed to rapidly identify and prioritize promising LF inhibitor scaffolds from virtual compound libraries was reported (138). The authors utilized structural information from five available LF enzyme-inhibitor complexes deposited into the Protein Data Bank and considered all three key subsites of the LF catalytic binding region. The usefulness of the model was confirmed experimentally. In is known that among hundreds of the small molecule LF inhibitors designed only a limited number of compounds were active in cell protection assays. The authors performed a validation study on 546 compounds previously tested and successfully identified 72% of the existing nanomolar-level inhibitors, while rejecting 100% of the weakly active (> 100 μM) compounds.
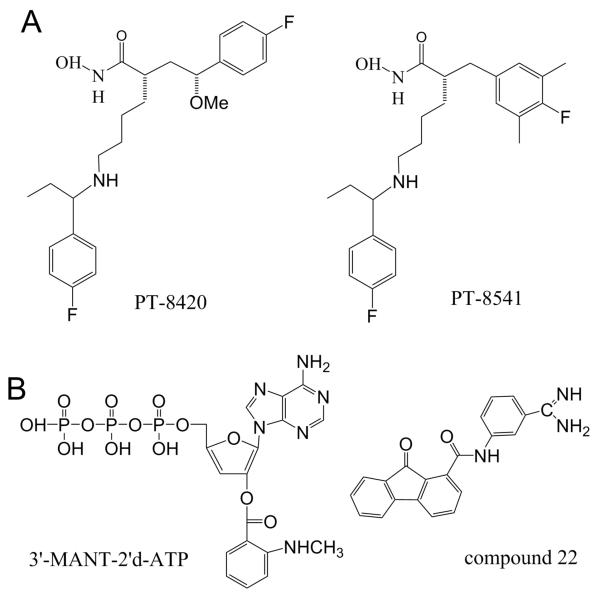
A group of small-molecule LF blockers with subnanomolar inhibitory constants was described in a series of four consecutive publications. Part 1 and part 2 report the identification of several core structures capable of inhibiting LF with Ki values of about 10 nM and in vivo efficacy in a rat LT model of anthrax (139, 140). The identified lead structures were further optimized to provide sub-nanomolar Ki values (part 3) (141) (Figure 5A). The efficacy evaluation was performed by comparing the potential for toxicity, physicochemical properties, in vitro ADME profiles, and relative efficacy in the rat LT model. Based on these results, the authors then reported that these blockers protected against lethality caused by anthrax infection in mice when combined with subprotective doses of either antibiotics or neutralizing monoclonal antibodies targeting edema toxin (124). Importantly, the identified inhibitors also provided protection against lethal infection when administered as a monotherapy. The two doses (10 mg/kg) administered at 2 h and 8 h after spore infection were sufficient to provide a significant survival benefit in infected mice. Besides, administration of the blockers early in infection inhibited vegetative bacteria dissemination to the organs in the first 32 hours following infection. Importantly, the authors demonstrated the direct correlation between ability to target LF after it has entered cells and survival outcome, emphasizing the importance of LF as the target.
Figure 5.
Small molecule inhibitors of LF and EF. (A) Structure of PT-8420 and PT-8541 - small molecule compounds that were identified and characterized for their ability to protect animals from death in a rat model of LT intoxication with IC50 values of 0.33 and 0.04 nM, respectively (141). (B) Small-molecule EF inhibitors. Left: Structure of compound 22, one of the (M)ANT nucleotides inhibiting with IC50= 10 nM (157). Right: Structure for an optimized EF inhibitor, compound 22 (159) inhibiting EF-induced secretion of cAMP by cultured cells with IC50 = 2.71 μM.
4.9. Antitoxins targeting EF enzymatic activity
Because the 5:1 LF:EF ratio reported for anthrax infections models (142-144), EF, a calmodulin (CaM)-activated adenylyl cyclase toxin, is believed to reach lethal doses in the very late stages of infection (37). This circumstance shifted the focus of the search for antitoxins towards the more universal PA and more active LF; however, a number of EF blockers were also identified and reported. Advancements in the development of EF inhibitors have been discussed in a series of recent review articles (145, 146) including the most recent one (147). Thus, EF was neutralized by a number of mAbs (53, 148-150). In particular, chimpanzee/human mAb, EF13D neutralized EF in vitro in the subnanomolar range and its effectiveness was shown in mice (148). EF13D was shown to bind to a conformational epitope on EF and to inhibit CaM-mediated activation of EF by outcompeting CaM for binding to EF and replacing the pre-bound CaM in the EF/CaM complex. The critical amino acid residues responsible for mediating EF13D/EF interactions were then identified providing an insight into the molecular basis of the EF13D activity (150).
Several small-molecule inhibitors of EF were discovered (151-156). The investigational EF neutralizing drugs target the catalytic site of EF, the EF/CaM interaction site, and the allosteric sites. While the compounds targeting the catalytic site were among the most potent reported, adjusting their selectivity towards EF was problematic (147). Many of the discovered compounds were also interacting with mammalian membranous adenylyl cyclases, mACs. Using the structure of the catalytic site of EF, 24 commercially available small molecular weight chemicals were screened to identify inhibitors of adenylyl cyclase activity of EF (151). A quinazoline compound, ethyl 5-aminopyrazolo[1,5-a]quinazoline-3-carboxylate was found to bind competitively to the adenine portion of the ATP binding site on EF with Ki ~ 20 μM. Adefovir diphosphate (PMEApp), a clinically approved drug for chronic hepatitis B virus infection, was found to inhibit EF with a much higher affinity in vitro, Ki ~ 27 nM (156). PMEApp demonstrated the affinity for the EF catalytic site that is four orders of magnitude higher than the affinity of ATP for EF. To determine molecular basis for this difference, the authors identified the molecular structure of the EF-CaM in complex with PMEApp, which revealed that the catalytic site of EF forms better van der Waals contacts and more hydrogen bonds with PMEApp than with ATP.
The most potent EF inhibitor, 3′-(N-methyl)anthraniloyl-2′-deoxyadenosine-5′-triphosphate (Ki = 10 nM) (157, 158), was one of the investigated (M)ANT-nucleotides, which carries a fluorescent (N-methyl)anthraniloyl-group to bind different nucleotide-binding proteins (147) (Figure 5B, left) . However, an additional problem that the developers of the ET inhibitors has faced was related to the high hydrophilicity and, therefore, low membrane permeability of the inhibitors determined by the nucleotide-based structure of the compounds. Based on this and similar crystal structures of EF alone or complexed with substrates and small molecule inhibitors, a structure-based method to search for potential non-nucleotide EF inhibitors was described (152). A 3D-pharmacophore that fits the active site of EF was constructed from fragments (so called fragment-based 3D-pharmacophore and in silico screening) to identify small-molecule EF inhibitors. For this purpose, a library of small-molecule fragments was docked into the EF-active site and ranked according to their docking scores. As a result, 19 best-scored compounds were tested in a cell assay for their ability to reduce cAMP secretion induced by EF, and it was found that four structurally different compounds inhibited EF in the low micromolar range. Recently, the same group published a study aiming to redesign one of the several initial “hits”, (3-[(9-oxo-9H-fluorene-1-carbonyl)-amino]-benzoic acid) or DC-5 (also refereed as compound 1 and FIV-50), to search for derivatives that would have similar or better activity, improved aqueous solubility, and reduced toxicity (159). Molecular docking was used to predict the potency and solubility of the new derivatives. The positions important for compound’s activity were identified using the structure-activity relationship, SAR analysis of the bioassay results. As a result, pharmacological properties of the lead DC-5 compounds were significantly improved by modification in the benzoic acid ring and switch from a hydrogen-bond donor group (-COOH) to a hydrogen-bond acceptor group (-C(=NH)NH2) (Figure 5B, right). Besides, a number of thiophen ureidoacid inhibitors, with the most efficient one inhibiting the EF activity at Ki ~ 10 μM, were identified using rational structure-based design that strategically targeted allosteric pockets (153). The binding of CaM to EF induced the closed-to-open state transition that reorganized the EF catalytic site into the active configuration that can convert ATP into cAMP. The transitions of the EF allosteric site between known active and inactive conformations were used for virtual screening to search for the candidate EF inhibitors. This approach has allowed identifying a targetable pocket that underwent major reorganization during the EF activation transition. We refer the reader for the details of both DC-5- and thiophen ureidoacid-based inhibitor design to two recent reviews authored by the groups that performed the studies (145, 146).
5. Expert opinion
The events of September 11, 2001 and the following intentional dissemination of the “anthrax letters” led to the twelve years of continuing research efforts to develop medical countermeasures to protect humans from anthrax bioterrorism (4). Significant research attention was focused on gaining understanding of Bacillus anthracis through the details of anthrax pathogenesis and host responses, as well as on developing vaccines, antitoxins, and antimicrobial agents. Mass vaccination, being among one of the most significant achievements of the 20th century, seems to be economically impractical with certain types of infectious agents. Antibiotics for a long time have been a favored treatment for bacterial infections. However, antibiotic therapy is most effective only at the early stages of anthrax infection. The infection can still be lethal because of the accumulation of the tripartite anthrax toxin even when bacteria have been cleared from the host. Besides, strains of Bacillus anthracis that are resistant to the effect of antimicrobial agents have been engineered. What makes the situation even worse, at the early stages of inhalational anthrax infection the flu-like symptoms of the disease could be difficult to differentiate from other illnesses. After all, few doctors in the US and other western countries have ever seen a single anthrax patient. These and similar considerations urged the government and the scientific community to search for new effective anthrax antitoxin agents. Numerous research publications focusing on targeting every possible stage of the anthrax toxin pathway were soon to follow. However, 12 years later, the options to combat anthrax toxin lethality are still limited. With the only anthrax toxin inhibiting therapy (PA-targeting with a monoclonal antibody, raxibacumab) approved for treatment of inhalational anthrax in 2012, in our view, the situation is still insecure. First of all, the FDA’s animal rule for drug approval, which clears compounds without validated efficacy studies on humans, creates high level of uncertainty (160). This is especially true when a well-characterized animal model for predicting the response in humans does not exist. Second, Moayeri et al. reported that unlike PA, which is known to be unstable, LF remains active in cells and in animal tissues for days, as manifested by continued cleavage of MAPKKs proteins by the toxin during this time (124). For instance, antibody therapies against PA, LF, or EF administered later than 24 h after infection were shown to rarely provide protection in a mice model. Therefore, the efficacy of the post-exposure treatment of the individuals with anti-PA therapeutics can be time-dependent, requiring coordinated use of membrane-permeable small-molecule inhibitors that would block the LF and EF enzymatic activity intracellularly.
One of the interesting approaches that we would like to highlight explores the idea of attaching multiple functional groups onto an inert scaffold and creating the so-called “multivalent interaction” between the polyvalent therapeutic agent and the target. These cooperative interactions can be significantly stronger than the corresponding monovalent interactions of the same ligands (79). The examples include potent biospecific peptide-based or small-molecule ligands (or functional groups) attached to a variety of scaffolds formed by the liposomes (75), polymers (76), or cyclodextrins (80, 81) . The strength of specific binding of some of these molecules has been manipulated with a series of rational chemical and structural modifications. In a number of these studies, the polyvalent blockers were designed with a specific universal target in mind: the ion-conductive transmembrane pores formed by channel-forming components of the binary bacterial toxins (115). Focus on this universal target might lead to development of new pharmacological strategies that, in line with the “Next-generation research” agenda put forward by the National Institute of Allergy and Infection Diseases, NIH, are based on the development of broad-spectrum drugs versus the traditional “one bug-one drug” approach.
There are still serious gaps between the current drug discovery approaches that academic labs and small biotech companies can offer and the trial and error methods that pharmaceutical giants traditionally use. While the former focus on the innovative approaches and greater-than-ever understanding of the disease, the latter concentrate on the drug-delivery and side-effect aspects, such as compound’s solubility, permeability, removal rate, and toxicity. For instance, we found only several mentioning of the classic Lipinski’s rule of five (161), which allows evaluation of druglikeness of a small-molecule compound, in the academic research devoted to the development of anthrax toxin inhibitors. Without paying attention to these impediments, rational drug discovery programs will find themselves in a disadvantage while competing with big pharma’s dollars. The good news is that the situation may be expected to improve. The research on the rational design of PA channel-blocking antitoxins offers several interesting examples. For instance, polyvalent cationic β-cyclodextrins-based inhibitors may possess higher toxicity than their uncharged analogous. However, it was demonstrated that most of the attractive interactions responsible for the high binding strength of the compounds to their PA target are due to the short-range forces other than Coulombic (116). This finding opens new prospects in optimizing these lead compounds. Then again, the recently designed cationic aromatic-rich PA-blocking chloroquine-related azolopyridinium salts (107) may offer an advantage due to their low toxicity and ability to permeate through lipid bilayer part of biological membranes in mammalian organisms.
To conclude, we believe that a successful anthrax toxin therapy of the future will include combination polytherapies, where the action of the effective antimicrobials will be enhanced by the antitoxin agents specifically designed to target the A (LF, EF) and B (PA) components of the toxin, similar to what is used in the current tuberculosis, leprosy, cancer, malaria, and HIV/AIDS treatments. The polytherapies may also involve the innovative approaches where a possible dual role of the designed antitoxins would be exploited. For instance, the ability of liposomes and cyclodextrin-based nanoparticles to deliver low-soluble drugs was widely studied and utilized. The polyvalent liposome and cyclodextrin antitoxins can therefore be investigated and optimized to act as the double-functioning agents designed to simultaneously disable toxin components and to deliver other small-molecule compounds, either covalently attached or dissolved in their inner cavities, to the desired target. We hope that these strategies will be among the main subjects of future research.
Article Highlights.
The intentional dissemination of Bacillus anthracis spores in 2001 via the “anthrax letters” led to the twelve years of continuing efforts to develop anthrax toxin inhibitors.
As our understanding of anthrax tripartite toxin action has advanced, the toxin components (PA, LF, and EF) became targets of numerous attempts to design effective antitoxins with nearly every step of the toxin pathway explored and targeted.
Many of the reviewed antitoxins were discovered with the use of innovative rational approaches with special attention paid to the physicochemical properties of lead small-molecule candidates and the physical forces involved in the drug/target interaction.
Polyvalent interactions can be significantly stronger than the corresponding monovalent interactions of the same ligand thus representing a promising strategy to explore in drug discovery.
Despite recent significant advances in the rational drug design, tailor-made antitoxins have yet to reach the market.
Bibliography
Papers of special merit have been highlighted as of interest (•) or special interest (••) to readers
- 1.Ravina E. The evolution of drug discovery. from traditional medicines to modern drugs. Wiley-VCH; Weinheim, Germany: 2011. [Google Scholar]
- 2.Krogsgaard-Larsen P, Stromgaard K, Madsen U. Drug design and discovery. CRC Press Taylor & Francis Group; 2010. [Google Scholar]
- 3.Schneider G, Baringhaus K- Molecular design: Concepts and applications. Wiley-VCH; Weinheim, Germany: 2008. [Google Scholar]
- 4.Bouzianas DG. Medical countermeasures to protect humans from anthrax bioterrorism. Trends Microbiol. 2009 Nov;17:522–8. doi: 10.1016/j.tim.2009.08.006. [DOI] [PubMed] [Google Scholar]
- • 5.Chen Z, Moayeri M, Purcell R. Monoclonal antibody therapies against anthrax. Toxins (Basel) 2011 Aug;3:1004–19. doi: 10.3390/toxins3081004. A comprehensive review of the post-exposure passive immunization strategies to disable the anthrax infection.
- 6.Beierlein JM, Anderson AC. New developments in vaccines, inhibitors of anthrax toxins, and antibiotic therapeutics for bacillus anthracis. Curr Med Chem. 2011;18:5083–94. doi: 10.2174/092986711797636036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 7.Bouzianas DG. Current and future medical approaches to combat the anthrax threat. J Med Chem. 2010 Jun 10;53:4305–31. doi: 10.1021/jm901024b. A comprehensive review of the most important medical countermeasures against inhalational anthrax.
- • 8.Artenstein AW, Opal SM. Novel approaches to the treatment of systemic anthrax. Clin Infect Dis. 2012 Apr;54:1148–61. doi: 10.1093/cid/cis017. The article providing a table of the FDA-approved substances with a certain level of efficacy against anthrax toxin.
- 9.Fouet A. The surface of bacillus anthracis. Mol Aspects Med. 2009 Dec;30:374–85. doi: 10.1016/j.mam.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Young JA, Collier RJ. Anthrax toxin: Receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–65. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 11.Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009 Dec;30:439–55. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 12.Barth H, Aktories K, Popoff MR, et al. Binary bacterial toxins: Biochemistry, biology, and applications of common clostridium and bacillus proteins. Microbiol Mol Biol Rev. 2004 Sep;68:373–402. doi: 10.1128/MMBR.68.3.373-402.2004. A widely cited classical review on different aspects of the binary toxins’ biochemistry and applications.
- 13.Petosa C, Collier RJ, Klimpel KR, et al. Crystal structure of the anthrax toxin protective antigen. Nature. 1997 Feb 27;385:833–8. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 14.Kintzer AF, Thoren KL, Sterling HJ, et al. The protective antigen component of anthrax toxin forms functional octameric complexes. J Mol Biol. 2009 Sep 25;392:614–29. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogridge J, Cunningham K, Collier RJ. Stoichiometry of anthrax toxin complexes. Biochemistry. 2002 Jan 22;41:1079–82. doi: 10.1021/bi015860m. [DOI] [PubMed] [Google Scholar]
- 16.Pilpa RM, Bayrhuber M, Marlett JM, et al. A receptor-based switch that regulates anthrax toxin pore formation. PLoS Pathog. 2011 Dec 7;:e1002354. doi: 10.1371/journal.ppat.1002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaustein RO, Koehler TM, Collier RJ, et al. Anthrax toxin: Channel-forming activity of protective antigen in planar phospholipid bilayers. Proc Natl Acad Sci U S A. 1989 Apr;86:2209–13. doi: 10.1073/pnas.86.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama H, Janowiak BE, Brzozowski M, et al. GroEL as a molecular scaffold for structural analysis of the anthrax toxin pore. Nat Struct Mol Biol. 2008 Jul;15:754–60. doi: 10.1038/nsmb.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Udho E, Wu Z, et al. Protein translocation through anthrax toxin channels formed in planar lipid bilayers. Biophys J. 2004 Dec;87:3842–9. doi: 10.1529/biophysj.104.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Finkelstein A, Collier RJ. Evidence that translocation of anthrax toxin’s lethal factor is initiated by entry of its N terminus into the protective antigen channel. Proc Natl Acad Sci U S A. 2004 Nov 30;101:16756–61. doi: 10.1073/pnas.0405754101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 21.Krantz BA, Melnyk RA, Zhang S, et al. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2005 Jul 29;309:777–81. doi: 10.1126/science.1113380. The article was the first to describe the phenylalanine clamp importance in PA-facilitated transport of LF and EF and to highlight the role of hydrophobic interactions in designing small-molecule pore inhibitors.
- 22.Basilio D, Juris SJ, Collier RJ, et al. Evidence for a proton-protein symport mechanism in the anthrax toxin channel. J Gen Physiol. 2009 Mar;133:307–14. doi: 10.1085/jgp.200810170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basilio D, Kienker PK, Briggs SW, et al. A kinetic analysis of protein transport through the anthrax toxin channel. J Gen Physiol. 2011 Jun;137:521–31. doi: 10.1085/jgp.201110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basilio D, Jennings-Antipov LD, Jakes KS, et al. Trapping a translocating protein within the anthrax toxin channel: Implications for the secondary structure of permeating proteins. J Gen Physiol. 2011 Apr;137:343–56. doi: 10.1085/jgp.201010578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janowiak BE, Finkelstein A, Collier RJ. An approach to characterizing single-subunit mutations in multimeric prepores and pores of anthrax protective antigen. Protein Sci. 2009 Feb;18:348–58. doi: 10.1002/pro.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thoren KL, Worden EJ, Yassif JM, et al. Lethal factor unfolding is the most force-dependent step of anthrax toxin translocation. Proc Natl Acad Sci U S A. 2009 Dec 22;106:21555–60. doi: 10.1073/pnas.0905880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pentelute BL, Sharma O, Collier RJ. Chemical dissection of protein translocation through the anthrax toxin pore. Angew Chem Int Ed Engl. 2011 Mar 1;50:2294–6. doi: 10.1002/anie.201006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feld GK, Brown MJ, Krantz BA. Ratcheting up protein translocation with anthrax toxin. Protein Sci. 2012 May;21:606–24. doi: 10.1002/pro.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MJ, Thoren KL, Krantz BA. Charge requirements for proton gradient-driven translocation of anthrax toxin. J Biol Chem. 2011 Jul 1;286:23189–99. doi: 10.1074/jbc.M111.231167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krantz BA, Finkelstein A, Collier RJ. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J Mol Biol. 2006 Feb 3;355:968–79. doi: 10.1016/j.jmb.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Duesbery NS, Webb CP, Leppla SH, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998 May 1;280:734–7. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 32.Vitale G, Bernardi L, Napolitani G, et al. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem J. 2000 Dec 15;352(Pt 3):739–45. [PMC free article] [PubMed] [Google Scholar]
- 33.Levinsohn JL, Newman ZL, Hellmich KA, et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leppla SH. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79:3162–6. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumetz F, Jouvion G, Khun H, et al. Noninvasive imaging technologies reveal edema toxin as a key virulence factor in anthrax. Am J Pathol. 2011 Jun;178:2523–35. doi: 10.1016/j.ajpath.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leysath CE, Chen KH, Moayeri M, et al. Mouse monoclonal antibodies to anthrax edema factor protect against infection. Infect Immun. 2011 Nov;79:4609–16. doi: 10.1128/IAI.05314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 37.Liu S, Zhang Y, Moayeri M, et al. Key tissue targets responsible for anthrax-toxin-induced lethality. Nature. 2013 Sep;May;501:63–8. doi: 10.1038/nature12510. The most recent study identifying the key tissue targets responsible for the poisonous effects of lethal and edema toxins.
- 38.Guarner J, Jernigan JA, Shieh WJ, et al. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am J Pathol. 2003 Aug;163:701–9. doi: 10.1016/S0002-9440(10)63697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nigrovic LE, Thompson KM. The lyme vaccine: A cautionary tale. Epidemiol Infect. 2007 Jan;135:1–8. doi: 10.1017/S0950268806007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang KH, Nam SJ, Locke JB, et al. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew Chem Int Ed Engl. 2013 Jul 22;52:7822–4. doi: 10.1002/anie.201302749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouzianas DG. Potential biological targets of bacillus anthracis in anti-infective approaches against the threat of bioterrorism. Expert Rev Anti Infect Ther. 2007 Aug;5:665–84. doi: 10.1586/14787210.5.4.665. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett JG, Inglesby TV, Jr, Borio L. Management of anthrax. Clin Infect Dis. 2002 Oct 1;35:851–8. doi: 10.1086/341902. [DOI] [PubMed] [Google Scholar]
- 43.Bull JJ, Parrish CR. Microbiology. A binding contract for anthrax. Science. 2002 Jul 12;297:201–2. doi: 10.1126/science.1074590. [DOI] [PubMed] [Google Scholar]
- 44.Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–71. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 45.Gilligan PH. Therapeutic challenges posed by bacterial bioterrorism threats. Curr Opin Microbiol. 2002 Oct;5:489–95. doi: 10.1016/s1369-5274(02)00359-4. [DOI] [PubMed] [Google Scholar]
- 46.Rainey GJ, Young JA. Antitoxins: Novel strategies to target agents of bioterrorism. Nat Rev Microbiol. 2004 Sep;2:721–6. doi: 10.1038/nrmicro977. [DOI] [PubMed] [Google Scholar]
- 47.Friedlander AM. Tackling anthrax. Nature. 2001 Nov 8;414:160–1. doi: 10.1038/35102660. [DOI] [PubMed] [Google Scholar]
- • 48.Fox JL. Anthrax drug first antibacterial mAb to win approval. Nat Biotechnol. 2013 Jan;31:8. doi: 10.1038/nbt0113-8. The article highlighting a recent approval of raxibacumab monoclonal antibodies for use in inhalational anthrax treatment.
- 49.Russell L, Pedersen M, Jensen AV, et al. Two anthrax cases with soft tissue infection, severe oedema and sepsis in danish heroin users. BMC Infect Dis. 2013 Sep 3;13:408. doi: 10.1186/1471-2334-13-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradley KA, Mogridge J, Mourez M, et al. Identification of the cellular receptor for anthrax toxin. Nature. 2001 Nov 8;414:225–9. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 51.Scobie HM, Rainey GJ, Bradley KA, et al. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003 Apr 29;100:5170–4. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martchenko M, Jeong SY, Cohen SN. Heterodimeric integrin complexes containing beta1-integrin promote internalization and lethality of anthrax toxin. Proc Natl Acad Sci U S A. 2010 Aug 31;107:15583–8. doi: 10.1073/pnas.1010145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Little SF, Leppla SH, Cora E. Production and characterization of monoclonal antibodies to the protective antigen component of bacillus anthracis toxin. Infect Immun. 1988 Jul;56:1807–13. doi: 10.1128/iai.56.7.1807-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bann JG, Cegelski L, Hultgren SJ. LRP6 holds the key to the entry of anthrax toxin. Cell. 2006 Mar 24;124:1119–21. doi: 10.1016/j.cell.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Basha S, Rai P, Poon V, et al. Polyvalent inhibitors of anthrax toxin that target host receptors. Proc Natl Acad Sci U S A. 2006 Sep 5;103:13509–13. doi: 10.1073/pnas.0509870103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai C, Che J, Xu L, et al. Tumor endothelium marker-8 based decoys exhibit superiority over capillary morphogenesis protein-2 based decoys as anthrax toxin inhibitors. PLoS One. 2011;6:e20646. doi: 10.1371/journal.pone.0020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers MS, Cryan LM, Habeshian KA, et al. A FRET-based high throughput screening assay to identify inhibitors of anthrax protective antigen binding to capillary morphogenesis gene 2 protein. PLoS One. 2012;7:e39911. doi: 10.1371/journal.pone.0039911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cryan LM, Habeshian KA, Caldwell TP, et al. Identification of small molecules that inhibit the interaction of TEM8 with anthrax protective antigen using a FRET assay. J Biomol Screen. 2013 Jul;18:714–25. doi: 10.1177/1087057113478655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cryan LM, Rogers MS. Targeting the anthrax receptors, TEM-8 and CMG-2, for anti-angiogenic therapy. Front Biosci (Landmark Ed) 2011 Jan 1;16:1574–88. doi: 10.2741/3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarac MS, Peinado JR, Leppla SH, et al. Protection against anthrax toxemia by hexa-D-arginine in vitro and in vivo. Infect Immun. 2004 Jan;72:602–5. doi: 10.1128/IAI.72.1.602-605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kacprzak MM, Peinado JR, Than ME, et al. Inhibition of furin by polyarginine-containing peptides: Nanomolar inhibition by nona-D-arginine. J Biol Chem. 2004 Aug 27;279:36788–94. doi: 10.1074/jbc.M400484200. [DOI] [PubMed] [Google Scholar]
- 62.Tonello F, Seveso M, Marin O, et al. Screening inhibitors of anthrax lethal factor. Nature. 2002 Jul 25;418:386. doi: 10.1038/418386a. vnd.skype.click2call.chrome.5.7. [DOI] [PubMed] [Google Scholar]
- 63.Turk BE, Wong TY, Schwarzenbacher R, et al. The structural basis for substrate and inhibitor selectivity of the anthrax lethal factor. Nat Struct Mol Biol. 2004 Jan;11:60–6. doi: 10.1038/nsmb708. [DOI] [PubMed] [Google Scholar]
- • 64.Peinado JR, Kacprzak MM, Leppla SH, et al. Cross-inhibition between furin and lethal factor inhibitors. Biochem Biophys Res Commun. 2004 Aug 27;321:601–5. doi: 10.1016/j.bbrc.2004.07.012. This article examines the cross-inhibition between arginine-reach furin and lethal factor inhibitors suggesting that the combined treatment may represent a more effective approach to block anthrax toxemia than the use of either inhibitor alone.
- 65.Shiryaev SA, Remacle AG, Ratnikov BI, et al. Targeting host cell furin proprotein convertases as a therapeutic strategy against bacterial toxins and viral pathogens. J Biol Chem. 2007 Jul 20;282:20847–53. doi: 10.1074/jbc.M703847200. [DOI] [PubMed] [Google Scholar]
- 66.Remacle AG, Gawlik K, Golubkov VS, et al. Selective and potent furin inhibitors protect cells from anthrax without significant toxicity. Int J Biochem Cell Biol. 2010 Jun;42:987–95. doi: 10.1016/j.biocel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Opal SM, Artenstein AW, Cristofaro PA, et al. Inter-alpha-inhibitor proteins are endogenous furin inhibitors and provide protection against experimental anthrax intoxication. Infect Immun. 2005 Aug;73:5101–5. doi: 10.1128/IAI.73.8.5101-5105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Opal SM, Lim YP, Cristofaro P, et al. Inter-alpha inhibitor proteins: A novel therapeutic strategy for experimental anthrax infection. Shock. 2011 Jan;35:42–4. doi: 10.1097/SHK.0b013e3181e83204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Worachartcheewan A, Nantasenamat C, Naenna T, et al. Modeling the activity of furin inhibitors using artificial neural network. Eur J Med Chem. 2009 Apr;44:1664–73. doi: 10.1016/j.ejmech.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 70.Moayeri M, Wiggins JF, Lindeman RE, et al. Cisplatin inhibition of anthrax lethal toxin. Antimicrob Agents Chemother. 2006 Aug;50:2658–65. doi: 10.1128/AAC.01412-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rubert Perez C, Lopez-Perez D, Chmielewski J, et al. Small molecule inhibitors of anthrax toxin-induced cytotoxicity targeted against protective antigen. Chem Biol Drug Des. 2012 Mar;79:260–9. doi: 10.1111/j.1747-0285.2011.01285.x. [DOI] [PubMed] [Google Scholar]
- • 72.Wein AN, Williams BN, Liu S, et al. Small molecule inhibitors of bacillus anthracis protective antigen proteolytic activation and oligomerization. J Med Chem. 2012 Sep 27;55:7998–8006. doi: 10.1021/jm300804e. The first study which demonstrated dual-acting inhibitory effects of the rationally designed antitoxins.
- • 73.Mourez M, Kane RS, Mogridge J, et al. Designing a polyvalent inhibitor of anthrax toxin. Nat Biotechnol. 2001 Oct;19:958–61. doi: 10.1038/nbt1001-958. One of the first reports on the polyvalent anthrax toxin inhibitors.
- 74.Gujraty K, Sadacharan S, Frost M, et al. Functional characterization of peptide-based anthrax toxin inhibitors. Mol Pharm. 2005 Sep-Oct;2:367–72. doi: 10.1021/mp050040f. [DOI] [PubMed] [Google Scholar]
- 75.Rai P, Padala C, Poon V, et al. Statistical pattern matching facilitates the design of polyvalent inhibitors of anthrax and cholera toxins. Nat Biotechnol. 2006 May;24:582–6. doi: 10.1038/nbt1204. [DOI] [PubMed] [Google Scholar]
- 76.Gujraty KV, Joshi A, Saraph A, et al. Synthesis of polyvalent inhibitors of controlled molecular weight: Structure-activity relationship for inhibitors of anthrax toxin. Biomacromolecules. 2006 Jul;7:2082–5. doi: 10.1021/bm060210p. [DOI] [PubMed] [Google Scholar]
- 77.Gujraty KV, Yanjarappa MJ, Saraph A, et al. Synthesis of homopolymers and copolymers containing an active ester of acrylic acid by RAFT: Scaffolds for controlling polyvalent ligand display. J Polym Sci A Polym Chem. 2008 Sep 25;46:7246–57. doi: 10.1002/pola.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rai PR, Saraph A, Ashton R, et al. Raftlike polyvalent inhibitors of the anthrax toxin: Modulating inhibitory potency by formation of lipid microdomains. Angew Chem Int Ed Engl. 2007;46:2207–9. doi: 10.1002/anie.200604317. [DOI] [PubMed] [Google Scholar]
- 79.Vance D, Shah M, Joshi A, et al. Polyvalency: A promising strategy for drug design. Biotechnol Bioeng. 2008 Oct 15;101:429–34. doi: 10.1002/bit.22056. [DOI] [PubMed] [Google Scholar]
- • 80.Joshi A, Kate S, Poon V, et al. Structure-based design of a heptavalent anthrax toxin inhibitor. Biomacromolecules. 2011 Mar 14;12:791–6. doi: 10.1021/bm101396u. An article promoting the idea of polyvalency as a perspective drug design strategy.
- • 81.Karginov VA, Nestorovich EM, Moayeri M, et al. Blocking anthrax lethal toxin at the protective antigen channel by using structure-inspired drug design. Proc Natl Acad Sci U S A. 2005 Oct 18;102:15075–80. doi: 10.1073/pnas.0507488102. The first study to demonstrate PA pore blocking by cationic -cyclodextrins.
- 82.Abrami L, Kunz B, van der Goot FG. Anthrax toxin triggers the activation of src-like kinases to mediate its own uptake. Proc Natl Acad Sci U S A. 2010 Jan 26;107:1420–4. doi: 10.1073/pnas.0910782107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abrami L, Bischofberger M, Kunz B, et al. Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors. PLoS Pathog. 2010 Mar 5;6:e1000792. doi: 10.1371/journal.ppat.1000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 84.Jeong SY, Martchenko M, Cohen SN. Calpain-dependent cytoskeletal rearrangement exploited for anthrax toxin endocytosis. Proc Natl Acad Sci U S A. 2013 Oct 15;110:E4007–15. doi: 10.1073/pnas.1316852110. A comprehensive review of the essential structural reorganization in the PA oligomer during pore formation.
- 85.Gillespie EJ, Ho CL, Balaji K, et al. Selective inhibitor of endosomal trafficking pathways exploited by multiple toxins and viruses. Proc Natl Acad Sci U S A. 2013 Nov 4;110:E4904–12. doi: 10.1073/pnas.1302334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bann JG. Anthrax toxin protective antigen--insights into molecular switching from prepore to pore. Protein Sci. 2012 Jan;21:1–12. doi: 10.1002/pro.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu PJ, Hobson JP, Southall N, et al. Quantitative high-throughput screening identifies inhibitors of anthrax-induced cell death. Bioorg Med Chem. 2009 Jul 15;17:5139–45. doi: 10.1016/j.bmc.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hobson JP, Liu S, Rono B, et al. Imaging specific cell-surface proteolytic activity in single living cells. Nat Methods. 2006 Apr;3:259–61. doi: 10.1038/nmeth862. [DOI] [PubMed] [Google Scholar]
- 89.Rosenfeld R, Marcus H, Ben-Arie E, et al. Isolation and chimerization of a highly neutralizing antibody conferring passive protection against lethal bacillus anthracis infection. PLoS One. 2009 Jul 24;4:e6351. doi: 10.1371/journal.pone.0006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mechaly A, Levy H, Epstein E, et al. A novel mechanism for antibody-based anthrax toxin neutralization: Inhibition of prepore-to-pore conversion. J Biol Chem. 2012 Sep 21;287:32665–73. doi: 10.1074/jbc.M112.400473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collier RJ. Membrane translocation by anthrax toxin. Mol Aspects Med. 2009 Dec;30:413–22. doi: 10.1016/j.mam.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thoren KL, Krantz BA. The unfolding story of anthrax toxin translocation. Mol Microbiol. 2011 May;80:588–95. doi: 10.1111/j.1365-2958.2011.07614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nablo BJ, Panchal RG, Bavari S, et al. Anthrax toxin-induced rupture of artificial lipid bilayer membranes. J Chem Phys. 2013 Aug 14;139:065101. doi: 10.1063/1.4816467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 94.Sellman BR, Mourez M, Collier RJ. Dominant-negative mutants of a toxin subunit: An approach to therapy of anthrax. Science. 2001 Apr 27;292:695–7. doi: 10.1126/science.109563. An early work investigating dominant negative PA mutants as antitoxins.
- 95.Singh Y, Khanna H, Chopra AP, et al. A dominant negative mutant of bacillus anthracis protective antigen inhibits anthrax toxin action in vivo. J Biol Chem. 2001 Jun 22;276:22090–4. doi: 10.1074/jbc.M010222200. [DOI] [PubMed] [Google Scholar]
- 96.Yan M, Collier RJ. Characterization of dominant-negative forms of anthrax protective antigen. Mol Med. 2003 Jan-Feb;9:46–51. [PMC free article] [PubMed] [Google Scholar]
- 97.Cao S, Guo A, Liu Z, et al. Investigation of new dominant-negative inhibitors of anthrax protective antigen mutants for use in therapy and vaccination. Infect Immun. 2009 Oct;77:4679–87. doi: 10.1128/IAI.00264-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aulinger BA, Roehrl MH, Mekalanos JJ, et al. Combining anthrax vaccine and therapy: A dominant-negative inhibitor of anthrax toxin is also a potent and safe immunogen for vaccines. Infect Immun. 2005 Jun;73:3408–14. doi: 10.1128/IAI.73.6.3408-3414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janowiak BE, Fischer A, Collier RJ. Effects of introducing a single charged residue into the phenylalanine clamp of multimeric anthrax protective antigen. J Biol Chem. 2010 Mar 12;285:8130–7. doi: 10.1074/jbc.M109.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun J, Lang AE, Aktories K, et al. Phenylalanine-427 of anthrax protective antigen functions in both pore formation and protein translocation. Proc Natl Acad Sci U S A. 2008 Mar 18;105:4346–51. doi: 10.1073/pnas.0800701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montal M, Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69:3561–6. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 102.Nestorovich EM, Bezrukov SM. Obstructing toxin pathways by targeted pore blockage. Chem Rev. 2012 Oct 11; doi: 10.1021/cr300141q. A recent review focusing on the rational drug design of pore-blocking antitoxins.
- 103.Blaustein RO, Finkelstein A. Diffusion limitation in the block by symmetric tetraalkylammonium ions of anthrax toxin channels in planar phospholipid bilayer membranes. J Gen Physiol. 1990 Nov;96:943–57. doi: 10.1085/jgp.96.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blaustein RO, Finkelstein A. Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. effects on macroscopic conductance. J Gen Physiol. 1990 Nov;96:905–19. doi: 10.1085/jgp.96.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blaustein RO, Lea EJ, Finkelstein A. Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. single-channel analysis. J Gen Physiol. 1990 Nov;96:921–42. doi: 10.1085/jgp.96.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orlik F, Schiffler B, Benz R. Anthrax toxin protective antigen: Inhibition of channel function by chloroquine and related compounds and study of binding kinetics using the current noise analysis. Biophys J. 2005 Mar;88:1715–24. doi: 10.1529/biophysj.104.050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beitzinger C, Bronnhuber A, Duscha K, et al. Designed azolopyridinium salts block protective antigen pores in vitro and protect cells from anthrax toxin. PLoS One. 2013 Jun 20;8:e66099. doi: 10.1371/journal.pone.0066099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karginov VA. Cyclodextrin derivatives as anti-infectives. Curr Opin Pharmacol. 2013 Oct;13:717–25. doi: 10.1016/j.coph.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karginov VA, Nestorovich EM, Yohannes A, et al. Search for cyclodextrin-based inhibitors of anthrax toxins: Synthesis, structural features, and relative activities. Antimicrob Agents Chemother. 2006 Nov;50:3740–53. doi: 10.1128/AAC.00693-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diaz-Moscoso A, Mendez-Ardoy A, Ortega-Caballero F, et al. Symmetry complementarity-guided design of anthrax toxin inhibitors based on beta-cyclodextrin: Synthesis and relative activities of face-selective functionalized polycationic clusters. ChemMedChem. 2011 Jan 3;6:181–92. doi: 10.1002/cmdc.201000419. [DOI] [PubMed] [Google Scholar]
- 111.Yannakopoulou K, Jicsinszky L, Aggelidou C, et al. Symmetry requirements for effective blocking of pore-forming toxins: Comparative study with alpha-, beta-, and gamma-cyclodextrin derivatives. Antimicrob Agents Chemother. 2011 Jul;55:3594–7. doi: 10.1128/AAC.01764-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moayeri M, Robinson TM, Leppla SH, et al. In vivo efficacy of beta-cyclodextrin derivatives against anthrax lethal toxin. Antimicrob Agents Chemother. 2008 Jun;52:2239–41. doi: 10.1128/AAC.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dietrich PS, McGivern JG, Delgado SG, et al. Functional analysis of a voltage-gated sodium channel and its splice variant from rat dorsal root ganglia. J Neurochem. 1998 Jun;70:2262–72. doi: 10.1046/j.1471-4159.1998.70062262.x. [DOI] [PubMed] [Google Scholar]
- 114.Catterall WA, Perez-Reyes E, Snutch TP, et al. International union of pharmacology. XLVIII. nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005 Dec;57:411–25. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 115.Nestorovich EM, Karginov VA, Popoff MR, et al. Tailored ss-cyclodextrin blocks the translocation pores of binary exotoxins from C. botulinum and C. perfringens and protects cells from intoxication. PLoS One. 2011;6:e23927. doi: 10.1371/journal.pone.0023927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bezrukov SM, Liu X, Karginov VA, et al. Interactions of high-affinity cationic blockers with the translocation pores of B. anthracis, C. botulinum, and C. perfringens binary toxins. Biophys J. 2012 Sep 19;103:1208–17. doi: 10.1016/j.bpj.2012.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Friedlander AM. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986 Jun 5;261:7123–6. [PubMed] [Google Scholar]
- 118.Gordon VM, Leppla SH, Hewlett EL. Inhibitors of receptor-mediated endocytosis block the entry of bacillus anthracis adenylate cyclase toxin but not that of bordetella pertussis adenylate cyclase toxin. Infect Immun. 1988 May;56:1066–9. doi: 10.1128/iai.56.5.1066-1069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finkelstein A. The channel formed in planar lipid bilayers by the protective antigen component of anthrax toxin. Toxicology. 1994 Feb 28;87:29–41. doi: 10.1016/0300-483x(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 120.Dal Molin F, Tonello F, Ladant D, et al. Cell entry and cAMP imaging of anthrax edema toxin. EMBO J. 2006 Nov 15;25:5405–13. doi: 10.1038/sj.emboj.7601408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Artenstein AW, Opal SM, Cristofaro P, et al. Chloroquine enhances survival in bacillus anthracis intoxication. J Infect Dis. 2004 Nov 1;190:1655–60. doi: 10.1086/424853. [DOI] [PubMed] [Google Scholar]
- 122.Sanchez AM, Thomas D, Gillespie EJ, et al. Amiodarone and bepridil inhibit anthrax toxin entry into host cells. Antimicrob Agents Chemother. 2007 Jul;51:2403–11. doi: 10.1128/AAC.01184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Backer MV, Patel V, Jehning BT, et al. Inhibition of anthrax protective antigen outside and inside the cell. Antimicrob Agents Chemother. 2007 Jan;51:245–51. doi: 10.1128/AAC.00983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 124.Moayeri M, Crown D, Jiao GS, et al. Small-molecule inhibitors of lethal factor protease activity protect against anthrax infection. Antimicrob Agents Chemother. 2013 Sep;57:4139–45. doi: 10.1128/AAC.00941-13. The article highlighting the importance of small molecule lethal factor inhibitors acting intracellularly at the later stages of infection.
- • 125.Abrami L, Brandi L, Moayeri M, et al. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013 Nov 13; doi: 10.1016/j.celrep.2013.10.019. The recent article shows that LF can persist in intraluminal endosomal vesicles for days, protected from proteolytic degradation.
- 126.Montecucco C, Tonello F, Zanotti G. Stop the killer: How to inhibit the anthrax lethal factor metalloprotease. Trends Biochem Sci. 2004 Jun;29:282–5. doi: 10.1016/j.tibs.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 127.Dalkas GA, Papakyriakou A, Vlamis-Gardikas A, et al. Low molecular weight inhibitors of the protease anthrax lethal factor. Mini Rev Med Chem. 2008 Mar;8:290–306. doi: 10.2174/138955708783744083. [DOI] [PubMed] [Google Scholar]
- 128.Turk BE. Discovery and development of anthrax lethal factor metalloproteinase inhibitors. Curr Pharm Biotechnol. 2008 Feb;9:24–33. doi: 10.2174/138920108783497604. [DOI] [PubMed] [Google Scholar]
- 129.Tonello F, Montecucco C. The anthrax lethal factor and its MAPK kinase-specific metalloprotease activity. Mol Aspects Med. 2009 Dec;30:431–8. doi: 10.1016/j.mam.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 130.Panchal RG, Hermone AR, Nguyen TL, et al. Identification of small molecule inhibitors of anthrax lethal factor. Nat Struct Mol Biol. 2004 Jan;11:67–72. doi: 10.1038/nsmb711. [DOI] [PubMed] [Google Scholar]
- 131.Shoop WL, Xiong Y, Wiltsie J, et al. Anthrax lethal factor inhibition. Proc Natl Acad Sci U S A. 2005 May 31;102:7958–63. doi: 10.1073/pnas.0502159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goldman ME, Cregar L, Nguyen D, et al. Cationic polyamines inhibit anthrax lethal factor protease. BMC Pharmacol. 2006 Jun 8;6:8. doi: 10.1186/1471-2210-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 133.Kuzmic P, Cregar L, Millis SZ, et al. Mixed-type noncompetitive inhibition of anthrax lethal factor protease by aminoglycosides. FEBS J. 2006 Jul;273:3054–62. doi: 10.1111/j.1742-4658.2006.05316.x. The study offering a biophysical insight into the molecular details of lethal factor inhibition by aminoglycosides reported in literature, differentiating between strictly competitive and noncompetitive binding.
- 134.Fridman M, Belakhov V, Lee LV, et al. Dual effect of synthetic aminoglycosides: Antibacterial activity against bacillus anthracis and inhibition of anthrax lethal factor. Angew Chem Int Ed Engl. 2005 Jan 7;44:447–52. doi: 10.1002/anie.200462003. [DOI] [PubMed] [Google Scholar]
- 135.Kim C, Gajendran N, Mittrucker HW, et al. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc Natl Acad Sci U S A. 2005 Mar 29;102:4830–5. doi: 10.1073/pnas.0500508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang W, Mulakala C, Ward SC, et al. Retrocyclins kill bacilli and germinating spores of bacillus anthracis and inactivate anthrax lethal toxin. J Biol Chem. 2006 Oct 27;281:32755–64. doi: 10.1074/jbc.M603614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wickliffe KE, Leppla SH, Moayeri M. Killing of macrophages by anthrax lethal toxin: Involvement of the N-end rule pathway. Cell Microbiol. 2008 Jun;10:1352–62. doi: 10.1111/j.1462-5822.2008.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chiu TL, Amin EA. Development of a comprehensive, validated pharmacophore hypothesis for anthrax toxin lethal factor (LF) inhibitors using genetic algorithms, pareto scoring, and structural biology. J Chem Inf Model. 2012 Jul 23;52:1886–97. doi: 10.1021/ci300121p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiao GS, Kim S, Moayeri M, et al. Antidotes to anthrax lethal factor intoxication. part 1: Discovery of potent lethal factor inhibitors with in vivo efficacy. Bioorg Med Chem Lett. 2010 Nov 15;20:6850–3. doi: 10.1016/j.bmcl.2010.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim S, Jiao GS, Moayeri M, et al. Antidotes to anthrax lethal factor intoxication. part 2: Structural modifications leading to improved in vivo efficacy. Bioorg Med Chem Lett. 2011 Apr 1;21:2030–3. doi: 10.1016/j.bmcl.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiao GS, Kim S, Moayeri M, et al. Antidotes to anthrax lethal factor intoxication. part 3: Evaluation of core structures and further modifications to the C2-side chain. Bioorg Med Chem Lett. 2012 Mar 15;22:2242–6. doi: 10.1016/j.bmcl.2012.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sirard JC, Mock M, Fouet A. The three bacillus anthracis toxin genes are coordinately regulated by bicarbonate and temperature. J Bacteriol. 1994 Aug;176:5188–92. doi: 10.1128/jb.176.16.5188-5192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mabry R, Brasky K, Geiger R, et al. Detection of anthrax toxin in the serum of animals infected with bacillus anthracis by using engineered immunoassays. Clin Vaccine Immunol. 2006 Jun;13:671–7. doi: 10.1128/CVI.00023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Molin FD, Fasanella A, Simonato M, et al. Ratio of lethal and edema factors in rabbit systemic anthrax. Toxicon. 2008 Dec 1;52:824–8. doi: 10.1016/j.toxicon.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 145.Laine E, Martinez L, Ladant D, et al. Molecular motions as a drug target: Mechanistic simulations of anthrax toxin edema factor function led to the discovery of novel allosteric inhibitors. Toxins (Basel) 2012 Aug;4:580–604. doi: 10.3390/toxins4080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schein CH, Chen D, Ma L, et al. Pharmacophore selection and redesign of non-nucleotide inhibitors of anthrax edema factor. Toxins (Basel) 2012 Nov 8;4:1288–300. doi: 10.3390/toxins4111288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 147.Seifert R, Dove S. Inhibitors of bacillus anthracis edema factor. Pharmacol Ther. 2013 Nov;140:200–12. doi: 10.1016/j.pharmthera.2013.07.002. The most recent review on the edema factor inhibitors
- 148.Chen Z, Moayeri M, Zhao H, et al. Potent neutralization of anthrax edema toxin by a humanized monoclonal antibody that competes with calmodulin for edema factor binding. Proc Natl Acad Sci U S A. 2009 Aug 11;106:13487–92. doi: 10.1073/pnas.0906581106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Winterroth L, Rivera J, Nakouzi AS, et al. Neutralizing monoclonal antibody to edema toxin and its effect on murine anthrax. Infect Immun. 2010 Jun;78:2890–8. doi: 10.1128/IAI.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Makiya M, Dolan M, Agulto L, et al. Structural basis of anthrax edema factor neutralization by a neutralizing antibody. Biochem Biophys Res Commun. 2012 Jan 6;417:324–9. doi: 10.1016/j.bbrc.2011.11.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Soelaiman S, Wei BQ, Bergson P, et al. Structure-based inhibitor discovery against adenylyl cyclase toxins from pathogenic bacteria that cause anthrax and whooping cough. J Biol Chem. 2003 Jul 11;278:25990–7. doi: 10.1074/jbc.M301232200. [DOI] [PubMed] [Google Scholar]
- 152.Chen D, Misra M, Sower L, et al. Novel inhibitors of anthrax edema factor. Bioorg Med Chem. 2008 Aug 1;16:7225–33. doi: 10.1016/j.bmc.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laine E, Goncalves C, Karst JC, et al. Use of allostery to identify inhibitors of calmodulin-induced activation of bacillus anthracis edema factor. Proc Natl Acad Sci U S A. 2010 Jun 22;107:11277–82. doi: 10.1073/pnas.0914611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Taha HM, Schmidt J, Gottle M, et al. Molecular analysis of the interaction of anthrax adenylyl cyclase toxin, edema factor, with 2′(3′)-O-(N-(methyl)anthraniloyl)-substituted purine and pyrimidine nucleotides. Mol Pharmacol. 2009 Mar;75:693–703. doi: 10.1124/mol.108.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Taha H, Dove S, Geduhn J, et al. Inhibition of the adenylyl cyclase toxin, edema factor, from bacillus anthracis by a series of 18 mono- and bis-(M)ANT-substituted nucleoside 5′-triphosphates. Naunyn Schmiedebergs Arch Pharmacol. 2012 Jan;385:57–68. doi: 10.1007/s00210-011-0688-9. [DOI] [PubMed] [Google Scholar]
- 156.Shen Y, Zhukovskaya NL, Zimmer MI, et al. Selective inhibition of anthrax edema factor by adefovir, a drug for chronic hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004 Mar 2;101:3242–7. doi: 10.1073/pnas.0306552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suryanarayana S, Wang JL, Richter M, et al. Distinct interactions of 2′- and 3′-O-(N-methyl)anthraniloyl-isomers of ATP and GTP with the adenylyl cyclase toxin of bacillus anthracis, edema factor. Biochem Pharmacol. 2009 Aug 1;78:224–30. doi: 10.1016/j.bcp.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pinto C, Lushington GH, Richter M, et al. Structure-activity relationships for the interactions of 2′- and 3′-(O)-(N-methyl)anthraniloyl-substituted purine and pyrimidine nucleotides with mammalian adenylyl cyclases. Biochem Pharmacol. 2011 Aug 15;82:358–70. doi: 10.1016/j.bcp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen D, Ma L, Kanalas JJ, et al. Structure-based redesign of an edema toxin inhibitor. Bioorg Med Chem. 2012 Jan 1;20:368–76. doi: 10.1016/j.bmc.2011.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 160.Dolgin E. Animal rule for drug approval creates a jungle of confusion. Nat Med. 2013 Feb;19:118–9. doi: 10.1038/nm0213-118. An informative “News” article discussing nuances of the FDA’s “animal rule”.
- • 161.Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. A fundamental and influential medicinal chemistry publication where the Lipinski’s “Rule of 5” was first described.
- 162.Nestorovich EM, Karginov VA, Berezhkovskii AM, et al. Blockage of anthrax PA63 pore by a multicharged high-affinity toxin inhibitor. Biophys J. 2010 Jul 7;99:134–4. doi: 10.1016/j.bpj.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]