Abstract
We describe a patient with an acute middle cerebral artery ischemic stroke developing subtle involuntary movements of the paretic upper limb with cortical origin during rt-PA perfusion. Despite the multiple potential pathophysiological mechanisms for the relationship between thrombolysis and epileptic activity, seizures during this procedure are scarcely reported. Our hypothesis is that subtle and transient clinical seizures, like those described in our patient, may not be detected or are misdiagnosed as nonepileptic involuntary movements. We aimed to draw attention to the recognition challenge of this paroxysmal motor behavior, highlighting this clinical and neurophysiological identification using video recording and back-average analysis of the EEG.
Keywords: Seizures, Cerebral infarct, Recombinant tissue-type plasminogen activator (rt-PA), EEG, Back-average analysis
1. Introduction
Early poststroke seizures (i.e. occurring within one week after stroke) are thought to result from cellular biochemical dysfunction leading to electrically irritable cerebral tissue. Intravenous thrombolysis (IVT) with recombinant tissue-type plasminogen activator (rt-PA) is the gold standard treatment for acute ischemic stroke, and, recently, Alvarez et al., [1] showed that this procedure may be independently associated with early seizures. However, and despite multiple potential pathophysiological mechanisms, seizures coincident with rt-PA administration are seldom reported. Here, we describe a patient with an acute middle cerebral artery (MCA) ischemic stroke who developed subtle involuntary movements of the paretic upper limb, with cortical origin as documented neurophysiologically, during rt-PA perfusion. We aimed to draw attention to the recognition challenge of this paroxysmal motor behavior, highlighting its clinical and neurophysiological identification using video recording and back-average analysis of the EEG.
2. Case report
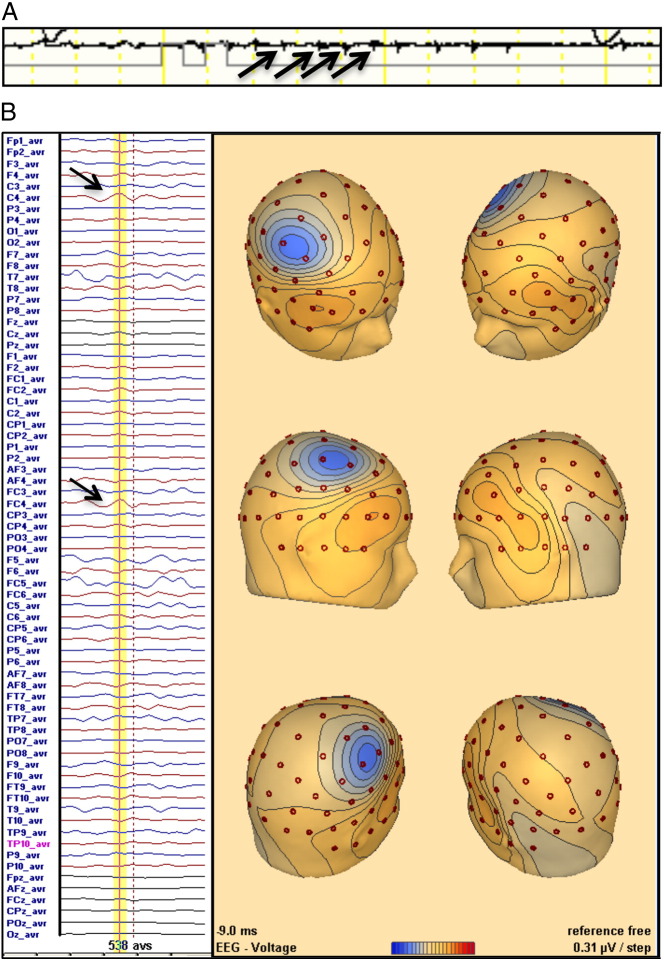
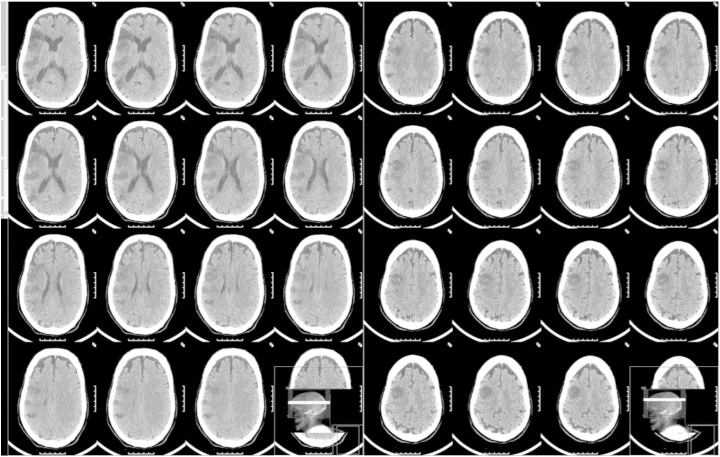
A 72-year-old male with a past history of hypertension, dyslipidemia, chronic kidney disease, and an ischemic stroke 15 years ago, with no poststroke seizures and from which he had completely recovered, presented to the emergency department with sudden onset of left central facial palsy, hemiparesis, homonymous hemianopsia, and right gaze deviation (NIHSS score = 10). Electrocardiogram showed atrial fibrillation, and blood analysis revealed acute renal failure (creatinine = 4 mg/dL, blood urea nitrogen = 134 mg/dL). Plain head computed tomography (CT) disclosed old occipital, parietal, and frontal ischemic lesions and a right medial cerebral artery (MCA) hyperdensity. Intravenous recombinant tissue-type plasminogen activator (rt-PA) was started 140 min after symptom onset. Twenty minutes after starting the infusion period, involuntary movements of the upper paretic limb were noticed. The movements involved either the distal or the proximal muscles, independently, and could be jerk-like, irregular, myoclonic-like, or slow and brief (Video). During rt-PA perfusion, a 72-channel EEG (International 10/10 System) with an EMG channel recording the left flexor digitorum superficialis (sample frequency of 1000 Hz) captured brief, repetitive, and almost periodic muscle activations (Fig. 1A). No epileptiform activity was apparent in the raw EEG data. Back-average analysis of the EEG time-locked with the onset of the recorded myoclonus (538 activations) was performed (BESA software, version 6.0), revealing a right frontocentral negative wave. This EEG transient preceded muscle activation by 30 ms (Fig. 1B). No antiepileptic drug was given, and the involuntary movements lasted approximately 40 min, stopping by the end of the rt-PA perfusion. The neurological deficit did not improve after thrombolysis. Transcranial Doppler showed no recanalization. Computed tomography at 24 h disclosed an acute MCA infarct scoring 5 on ASPECTS, with spared cortical areas within the infarct zone (Fig. 2). The patient partially recovered after 7 days (NIHSS score = 6). No further involuntary movements or clinically suspected seizures were observed despite transitory worsening of renal function during hospitalization. One year after stroke, the patient is alive and independent (NIHSS = 1 and mRS = 1), with no report of late poststroke seizures.
Fig. 1.
A) EMG channel recording the left flexor digitorum superficialis capturing brief, repetitive, and almost periodic muscle activations (arrows). B) EEG back-average analysis disclosing a negative transient (arrows) with a peak (yellow line) of 10 ms before EMG activations (dotted line) at right central electrodes (C4/FC4).
Fig. 2.
Plain head CT scan 24 h after thrombolysis disclosing an acute right MCA infarct, scoring 5 (I, M1, M2, M5, M6) on ASPECTS score, with spared cortical areas within the infarct zone.
3. Discussion
We report a patient with an acute MCA ischemic stroke who developed subtle involuntary movements of the paretic upper limb, with cortical origin as documented neurophysiologically, during rt-PA perfusion. Because cortical myoclonus and epileptogenic discharges are generated by neuronal hypersynchronous activities sharing the same physiopathogenic mechanisms, the recorded myoclonus can be considered an acute symptomatic seizure. Because of the subtleness of the movements, the clinical stability of the patient, and the absence of clear epileptiform activity on the immediate raw EEG analysis, no antiepileptic medication was given. Back-average analysis, enlightening the cortical origin of the myoclonus, was only performed after the acute phase.
Even though our patient had multiple risk factors for seizures (acute renal lesion, acute anterior stroke, cortical involvement), the close time relationship of this paroxysmal motor behavior with the therapeutic intervention raises the possibility of an association. It has been documented that seizures during rt-PA perfusion can occur even in the absence of a cerebral lesion, as described in 2 patients submitted to thrombolysis for acute myocardial infarction [2]. In fact, neurotoxic and epileptogenic properties [3] of rt-PA are known. Other postulated mechanisms for seizures during thrombolysis for ischemic stroke include secondary cortical infarct from distal embolization or reperfusion/hyperperfusion syndrome [4].
Despite the multiple potential pathophysiological mechanisms for the relationship between rt-PA and seizures, the frequency of seizures during thrombolysis is not well known. Besides a few reports of overt seizures occurring in close proximity to rt-PA perfusion [4], [2], most larger studies looking at seizure frequency in patients submitted to thrombolysis report a global incidence of seizures within 7 days after stroke and not specifically during the therapeutic procedure. In these studies, patients submitted to thrombolysis have similar frequency of early seizures when compared with patients without thrombolysis. Only one study concluded that thrombolysis is an independent risk factor for early seizures after stroke [1]. These discrepancies may be related to the retrospective collection of the data. Additionally, reporting bias due to increased clinical vigilance in the acute phase of patients undergoing thrombolysis cannot be excluded. Finally, it is also possible that subtle and transient clinical seizures, like those described in our patient, may not be detected or are misdiagnosed as nonepileptic involuntary movements.
The following is the Supplementary data to this article.
After rt-PA bolus, involuntary movements of the upper paretic limb were noticed. The movements involved either the distal or the proximal muscles, independently, and could be jerk-like, irregular, myoclonic-like, or slow and brief.
Statement and acknowledgment
This clinical case is included in the project “EEG in Cerebrovascular Disease” approved by the ethics committee of our hospital. All the persons included in this study gave their informed consent to their inclusion.
This project was supported by the 2012 Research Grant in Cerebrovascular Diseases of the Sociedade Portuguesa do Acidente Vascular Cerebral (SPAVC) sponsored by Tecnifar. The funding source had no involvement in the study design, collection, analysis, or interpretation of the data.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Alvarez V., Rossetti A.O., Papavasileiou V., Michel P. Acute seizures in acute ischemic stroke: does thrombolysis have a role to play? J Neurol. 2013;260(1):55–61. doi: 10.1007/s00415-012-6583-6. [DOI] [PubMed] [Google Scholar]
- 2.Hafeez F., Razzaq M.a., Levine R.L., Ramirez M.A.N. Reperfusion seizures: a manifestation of cerebral reperfusion injury after administration of recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2007;16(6):273–277. doi: 10.1016/j.jstrokecerebrovasdis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Iyer a.M., Zurolo E., Boer K., Baayen J.C., Giangaspero F., Arcella A. Tissue plasminogen activator and urokinase plasminogen activator in human epileptogenic pathologies. Neuroscience. 2010;167(3):929–945. doi: 10.1016/j.neuroscience.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Rodan L.H., Aviv R.I., Sahlas D.J., Murray B.J., Gladstone J.P., Gladstone D.J. Seizures during stroke thrombolysis heralding dramatic neurologic recovery. Neurology. 2006;67(11):2048–2049. doi: 10.1212/01.wnl.0000247231.25231.2e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After rt-PA bolus, involuntary movements of the upper paretic limb were noticed. The movements involved either the distal or the proximal muscles, independently, and could be jerk-like, irregular, myoclonic-like, or slow and brief.