Abstract
We present a patient with epilepsy who underwent left anterior temporal cortex resection, sparing the hippocampus, to stop drug-refractory seizures. Given that one year after surgery the patient showed verbal memory difficulties, we proposed a short (twelve weeks) and intensive (two times a week) training based on visual imagery strategies as the nonverbal memory abilities were preserved. Neuropsychological and fMRI assessments were performed before and after rehabilitation to evaluate the cognitive progress and cerebral modifications induced by this rehabilitation program. Our results showed that the rehabilitation program improved both scores for verbal memory and the everyday quality of life. Changes in cerebral activity highlighted by fMRI suggest that the program might have facilitated the development of compensatory strategies, as reflected by the shift of activation from the anterior to the posterior cerebral network during a verbal memory task. One year after the rehabilitation program, the patient reported using mental imagery in everyday life for routine and professional activities. Although supplementary evidence is necessary to increase the robustness of these findings, this case report suggests that an efficient rehabilitation program is feasible and (a) should be based on the individual cognitive profile and on the preserved cognitive abilities, (b) can be short but intensive, (c) can be applied even months after the lesion occurrence, and (d) can induce a positive effect which may be sustainable over time.
Keywords: Epilepsy surgery, Temporal lobe epilepsy, Verbal memory, Rehabilitation, Mental imagery, Neuropsychology
1. Introduction
Patients with drug-refractory temporal lobe epilepsy (TLE) may benefit from a therapeutic surgery [1] to stop seizures. Therapeutic surgery implies resection or functional disconnection of the epileptogenic zone (EZ) [2]. Given the role of the temporal lobe in long-term episodic memory (e.g., [3], [4]), a decline of this function may be observed after surgery. Several studies showed that left mesiotemporal regions are particularly involved in verbal memory, and right mesiotemporal regions in nonverbal memory [5], [6]. In line with these observations, patients with left mesio-TLE often show verbal memory impairment after surgery [7], [8]. It is important to mention that verbal memory decline has deeper impact on everyday life than nonverbal memory.
In order to remediate for memory decline after surgery, neuropsychological rehabilitation programs can be applied. These programs vary in terms of technique or method used [9], as well as in terms of duration and intensity of training [10], [11]. For instance, some rehabilitation methods include external memory supports or aids such as phone or agenda tools [12]. Other methods are based on specific learning strategies such as self-generation procedures that emphasize the active participation of the patient in learning processes [13] and sensory and semantic processes for encoding [14] and visual imagery [15]. In fact, little information is available in the epilepsy literature on the memory rehabilitation methods, but studies on other pathologies have provided several clues for the benefit of memory rehabilitation programs [16], [17].
Most rehabilitation programs are offered individually, based on individual characteristics [18] and considering the specific profile of preserved cognitive abilities of the patient, allowing the development of compensatory strategies [13]. For instance, visual imagery methods aim to allow patients with memory deficit to promote encoding and provide clues for recuperation by means of structured mental images (e.g., [19]). Overall, the general idea behind this rehabilitation method is to take advantage of preserved cognitive abilities to remediate the deficits. The use of this method supposes normal visual, attentional, and executive abilities and depends strongly on the individuals' ability to engage in mental imagery and structuring information. Another crucial point during the rehabilitation programs is the duration and the intensity of training. It has been shown that intensive training for short time periods (from 2 to 8 weeks) could be efficient in patients with epilepsy [20], [21].
Furthermore, as pointed out by Nordvik et al. [22], the majority of cognitive rehabilitation studies are performed without exploring their effect on the cerebral substrate. In this framework, fMRI could be a promising tool to detect changes in cerebral activity following the intensive training (and, therefore, to anticipate the potential benefit of the rehabilitation) and, in any case, could provide information on the mechanisms underlying the rehabilitation.
We report here the case of a female patient with epilepsy who performed a rehabilitation program for verbal memory dysfunction observed after left temporal lobe surgery. Based on her neuropsychological profile [9] used to identify her preserved cognitive abilities, we proposed a personalized, intensive (2 sessions/week) and short-term (three months) training program by using mental visual imagery as the central approach of this program. In order to evaluate the cerebral effect of the training program, the patient underwent fMRI for episodic memory before and after rehabilitation.
2. Case report: description and methods
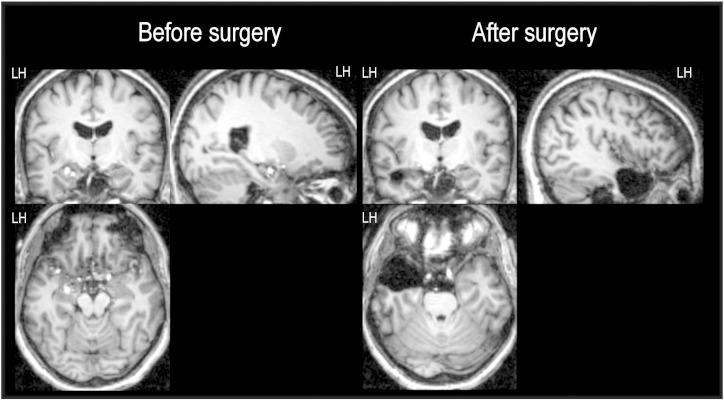
We examined a French female patient 57 years of age suffering from left mesiotemporal lobe epilepsy as assessed with video-EEG monitoring. Magnetic resonance imaging (MRI) revealed a left cavernoma located at the junction between the amygdala and the anterior part of the parahippocampal gyrus at the level of the uncus (see Fig. 1). Seizures had started at the age of 49 and remained drug-resistant despite different combinations of antiepileptic drugs. Seizures occurred once a month in clusters over a few days. Initially, seizures started with an epigastric discomfort and a “déjà vu” phenomenon. The semiology then changed and became characterized by difficulties in speech and, sometimes, speech arrest for about 20 s, without loss of contact. An amnestic postictal confusion lasting up to 30 min was also noticed. The patient was right-handed (Edinburgh Handedness Inventory, Oldfield [23]) and had a left hemisphere lateralization for language as determined by fMRI assessment described by Perrone-Bertolotti et al. [24]. Surgical treatment was considered to stop seizures and consisted of left anterior temporal resection sparing the hippocampus (see Fig. 1). Two years after surgery, the patient was seizure-free. Nevertheless, one year after surgery and despite the sparing of the hippocampus, she reported verbal memory complaints in everyday life. Specifically, she mentioned having difficulties in finding words during conversations and forgetting verbal information related to her work (she worked as a waitress).
Fig. 1.
MRI images before (left) and after (right) surgery. Before surgery, MRI reveals a left cavernoma located at the junction of the left uncus/amygdala. After surgery, MRI shows left medial temporal lobe resection (sparing hippocampus). The images are shown in neurological convention (left hemisphere to the left). Abbreviation: LH, left hemisphere.
The patient underwent three neuropsychological assessments, one before surgery (see [24] for details) and two after surgery (six months and one year postsurgery). The neuropsychological assessment performed six months after surgery was done to evaluate the patient’s global cognitive profile. The neuropsychological assessment performed one year after surgery was done when the patient sought evaluation for verbal memory difficulties. It was performed to refine the memory neuropsychological profile identified during the previous neuropsychological testing. Details of neuropsychological testing and associated results are provided in Table 1. The results revealed deficits for serial verbal memory tasks (Grober and Buschke test, ESR test 6 months later and also identified before surgery, see [24]), and the pattern of results suggested a persistent disorder of encoding and retrieval processing. This interpretation was based on the profile on these tasks (little help provided by cued items, impaired performances on word recognition phases, influence of depth of encoding). In addition, while the deficit was severe for verbal unstructured material such as these serial verbal memory tasks, the performances were in the low average range with the structured verbal material (i.e., story, verbal paired associates), as it provides conditions for efficient encoding and retrieval strategies [25]. Overall, the neuropsychological profile showed that the patient did not suffer from a deficit in verbal information storage but in encoding and in retrieval processing of verbal material. This is coherent with the surgical resection which spared the hippocampus. The forward and backward digit spans were within the average range, in favor of a preserved verbal short-term memory. Nonverbal long-term memory tests (recall and recognition for figures, faces, and door pictures) revealed scores which were all superior to the norm or in the high average range. Executive functions (flexibility in TMT and inhibition in Stroop) were preserved, but slowness of visual attention (D2) was detected. There was no deficit in naming (DO 80) nor in verbal fluency.
Table 1.
Neuropsychological assessment scores before the rehabilitation program and six months and one year (tests with an asterisk) after surgery.
| Neuropsychological assessment | Neuropsychological tests and scores | Norm comparisons |
|---|---|---|
| Long-term verbal memory | ||
| Grober and Buschke test | Immediate recall in encoding phase = 15/16 | 25th centilea |
| Third recall = 7/16 | − 2.29 SDb | |
| Third cued recall = 15/16 | 5th–25th centile | |
| Recognition = 15/16 | ||
| Delayed recall = 7/16 | − 2.50 SD | |
| Total recall after a delay = 13/16 | 1st–5th centile | |
| ESR test* | Immediate recall list 1 (superficial encoding) = 3/16 | − 1.29 SD |
| Recognition list 1 = 10/16 | − 5.37 SD | |
| Immediate recall list 2 (profound encoding) = 5/16 | − 3.15 SD | |
| Recognition list 2 = 16/16 | ||
| Recall after a delay (2 weeks) list 1 = 0/16 | − 1.13 SD | |
| Recognition list 1 = 6/16 | ||
| Recall after a delay (2 weeks) list 2 = 0/16 | − 1.82 SD | |
| Recognition list 2 = 6/16 | − 4.54 SD | |
| Associative learning words — MEM III | Immediate verbal paired associates = 8/32 | 7c |
| Verbal paired associates after a delay = 3/32 | 8 | |
| Story recall — BEM | Immediate recall = 8.5/12 | − 0.32 SD |
| Delayed recall = 8.5/12 | − 0.03 SD | |
| Verbal working memory — WAIS III | Digit forward = 6 digits | − 0.15 SD |
| Digit backward = 5 digits | 0.29 SD | |
| Long-term nonverbal memory | ||
| Doors test | (A) = 11/12 | 50th centile |
| (B) = 11/12 | > 90th centile | |
| Warrington recognition test for faces* | 16/18 | > 95th centile |
| Figure recall — BEM | Immediate recall = 10/12 | 0.72 SD |
| Delay recall = 10/12 | 1.09 SD | |
| Rey figure | 3-minute recall = 22/36 | 78th centile |
| Visual reproduction — MEM III* | Immediate recall = 98/104 | 18 |
| Recognition = 47/48 | 13 | |
| Copy = 101/104 | 15 | |
| Executive functions | ||
| Stroop | Interference = 117 s | − 0.03 SD |
| Interference vs. naming = 48 s | − 0.27 SD | |
| TMT | B = 86 s | − 0.08 SD |
| B–A = 67 s | 0.6 SD | |
| D2 | GZ = 312 | 8th centile |
| Language | ||
| Verbal fluency | Phonological fluency = 22 words | 0.18 SD |
| Semantic fluency = 26 words | − 0.78 SD | |
| DO 80 | 80/80 | |
Pathological performance if < 5th centile.
SD = standard deviation. Pathological performance if SD < 1.6.
Mean = 10, SD = 3. Pathological performance if < 5th centile.
Based on this profile that revealed impairment of verbal memory for unstructured verbal material but good abilities of visual memory and preserved memory abilities for structured material, we proposed a rehabilitation method based on the development of visual mental imagery strategies [25] in order to improve verbal memory abilities.
The rehabilitation program started one year after surgery for a duration of three months, consisting of twelve sessions (performed in three steps, see Table 2) with a frequency of 2 sessions per week. To develop this training, we considered the patient's preserved abilities; thus, the program consisted of mental imagery training based on the stacking method [26], which allows structuring this nonstructured material. The stacking method consists of associating several words included into an absurd mental image. For instance, by using the French words ‘elephant’, ‘moustache’, ‘scooter’, ‘telescope’, and ‘cap’, the patient has to create an absurd image, for instance: “an elephant with a moustache and a cap sitting on a scooter and looking through a telescope”.
Table 2.
The three steps of the verbal memory rehabilitation program.
| Step | Session number | Objectives | Tasks |
|---|---|---|---|
| First | S1 | To mobilize cognitive processes recruited by mental imagery. | Draw objects with increased complexity; describe them overtly; describe visual characteristics of images representing objects and perform mental manipulations of geometric forms; create semantic associations between words. |
| Familiarization with construction of a mental image and how to structure verbal material. | |||
| Second | S2–S3 | Explicit utilization of mental imagery strategy with verification and feedback on strategies. | Create integrative mental images by using two words (descriptions and drawings). |
| Create mentally absurd scenes including 4–5 words in each (descriptions and drawings). | |||
| Third | S4–S12 | Training of mental imagery strategy without feedback. | Encode lists of words (300 lists, number of words increased as a function of patient's performance). |
To quantify the efficiency of rehabilitation, we used the Selective Reminding Test [27]. This test consists of memorization of 15 words in 10 trials, followed by a free recall after 30 min. This test was used to compare long-term memory abilities of verbal unstructured information before and after rehabilitation.
Finally, to evaluate the effect of the rehabilitation program on the cerebral substrate of verbal memory, the patient underwent two fMRI (see Supplementary material for details of fMRI parameters and analysis) examinations, the first one before rehabilitation (at 1 year postsurgery) and the second one after rehabilitation (at 1 year and 3 months after surgery), including both encoding and recognition processing. First, the patient performed an incidental (encourage ecological encoding and avoiding the use of memorization strategies) memory-encoding task, which consisted of a word categorization task according to the French grammatical genre (feminine vs. masculine, i.e., the French word for butterfly is masculine — un papillon, and the French word for house is feminine — une maison). Thirty minutes after the encoding session, the patient performed a recognition task, which is used to indicate whether she could recall seeing each word during the encoding session or whether it was new (see Supplementary material for details). It is important to mention that the patient's performances during fMRI evaluation of verbal memory before and after rehabilitation were correct. Indeed, she correctly categorized words (incidental memory-encoding task) before (98%) and after (100%) the rehabilitation program, and no significant difference was found between sessions (F(1,118) = 1, p = 0.31). She also correctly recognized the items before (71%) and after (68%) the rehabilitation program, and no significant difference was found between sessions (F(1,118) = 0.1, p = 0.69).
3. Results and discussion
The aim of this study was to demonstrate that the use of a personalized rehabilitation program based on preserved visual memory and verbal memory on structured material may be appropriate to improve verbal memory abilities on nonstructured material, with changes in cerebral organization. Specifically, we proposed to a patient an intensive and personalized rehabilitation program based on mental imagery strategies, which required unimpaired nonverbal abilities, to compensate her memory deficit for encoding and retrieval verbal unstructured information.
As illustrated in Table 3, the patient presented an improvement of verbal memory scores. Indeed, gradual progression of performance during word recall along the rehabilitation sessions was observed. Furthermore, as shown in Table 4, the patient's performance on the Selective Reminding Test increased after the memory rehabilitation program. Indeed, before the training, the mean number of words recalled during the learning phase, the number of words recalled after a delay, and the percentage of retention after a delay (proportion between the last learning trial and delayed recall) were in the low range compared with data from normal population [27]. These scores were situated in the high average range after rehabilitation (from 8/15 words to 15/15 after rehabilitation as assessed after a 30-minute delay). This result suggests improvement of long-term memory abilities for nonstructured verbal information. Moreover, the patient reported a subjective improvement of the everyday quality of life.
Table 3.
Patient performance during recall of word lists along the training sessions.
| Session | Number of words per list | Number of lists proposed | Mean of correct recall | Mean of correct recall per session |
|---|---|---|---|---|
| 4 | 5 | 7 | 4.29 | 5.18 |
| 6 | 18 | 5.39 | ||
| 7 | 7 | 5.86 | ||
| 5 | 5 | 7 | 4 | 4.69 |
| 6 | 15 | 4.57 | ||
| 7 | 5 | 5.5 | ||
| 6 | 6 | 8 | 5 | 4.33 |
| 7 | 32 | 5 | ||
| 8 | 1 | 3 | ||
| 7 | 7 | 28 | 5.19 | 4.97 |
| 8 | 4 | 4.75 | ||
| 8 | 8 | 44 | 5.84 | 6.42 |
| 9 | 1 | 7 | ||
| 9 | 8 | 10 | 5.8 | 6.18 |
| 9 | 20 | 5.75 | ||
| 10 | 2 | 7 | ||
| 10 | 9 | 19 | 6.05 | 6.4 |
| 10 | 8 | 6.75 | ||
| 11 | 10 | 28 | 6.18 | 6.78 |
| 11 | 8 | 7.38 | ||
| 12 | 10 | 24 | 7.86 | 7.93 |
| 11 | 4 | 8 |
Table 4.
Performances on the Selective Reminding Test before and after rehabilitation.
| Before rehabilitation | Centilesa | After rehabilitation | Centilesa | |
|---|---|---|---|---|
| Mean number of words recalled on 10 trials (/15) | 8.6 | 5th–50th | 12.77 | > 50th |
| Number of words recalled after a delay (/15) | 8 | 5th | 15 | > 50th |
| % retention after a delay | 36.0% | 5th–50th | 86.9% | > 50th |
On normative data from [27].
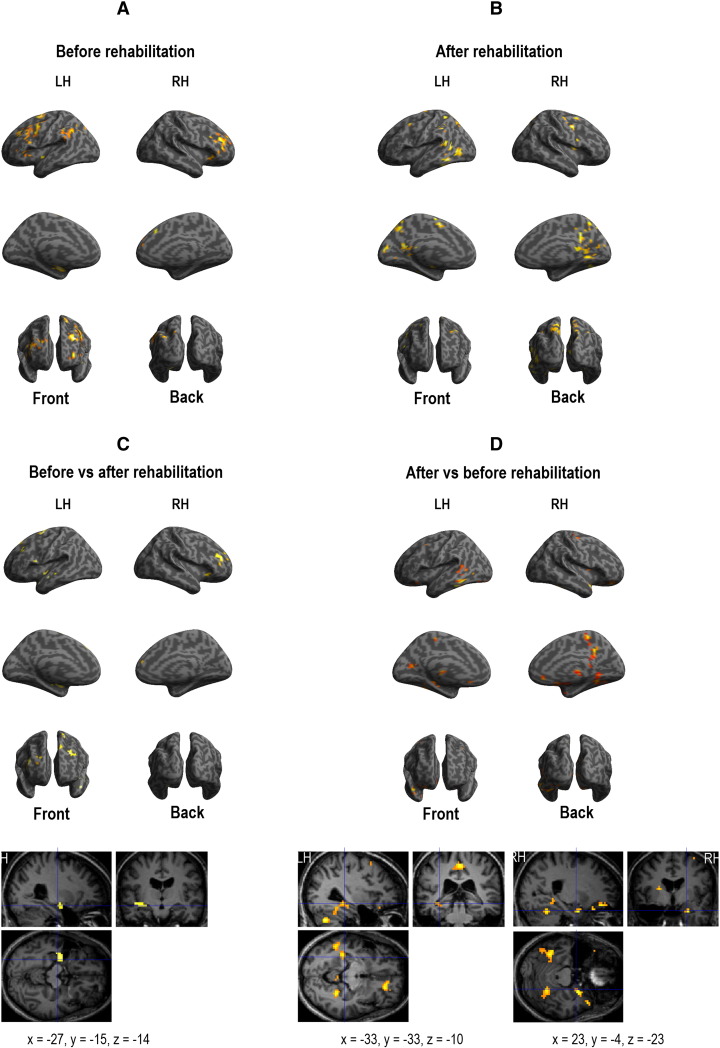
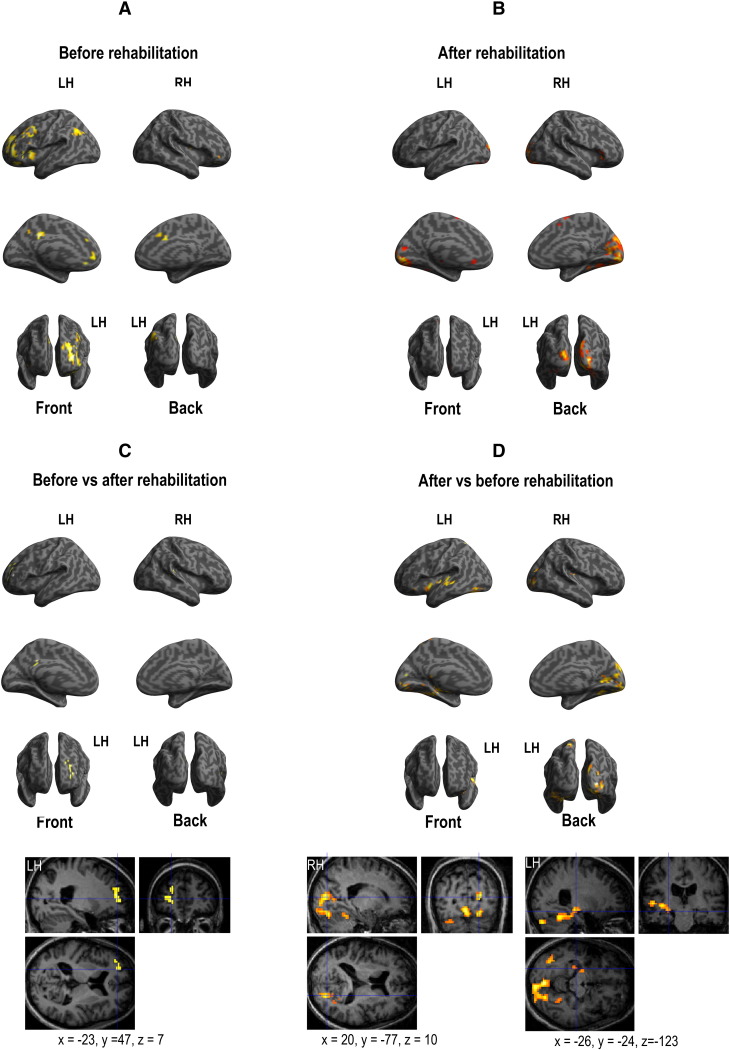
Functional MRI results suggest that two different strategies may be used by patients to perform the verbal encoding and recognition processes before and after surgery. Indeed, we showed that the rehabilitation program induced a shift of activation from the anterior to the posterior cerebral network (see Fig. 2, Fig. 3 for the specific cerebral network involved in each of the tasks, encoding and recognition, respectively).
Fig. 2.
Cerebral activation obtained with fMRI for successful verbal encoding task. Panel A shows before rehabilitation results. Panel B shows after rehabilitation results. Panel C shows the results provided by the direct comparison of “before vs. after rehabilitation sessions”. Panel D shows the results provided by the direct comparison of “after vs. before rehabilitation sessions”. All activations were projected onto a 3D-rendered canonical brain and onto 2D anatomical slices with axial, sagittal, and coronal orientations (MNI coordinates are also mentioned). Abbreviations: LH, left hemisphere; RH, right hemisphere.
Fig. 3.
Cerebral activation obtained with fMRI for successful verbal recognition task. Panel A shows before rehabilitation results. Panel B shows after rehabilitation results. Panel C shows the results provided by the direct comparison of “before vs. after rehabilitation sessions”. Panel D shows the results provided by the direct comparison of “after vs. before rehabilitation sessions”. All activations were projected onto a 3D-rendered canonical brain and onto 2D anatomical slices with axial, sagittal, and coronal orientations (MNI coordinates are also mentioned). Abbreviations: LH, left hemisphere; RH, right hemisphere.
The recruitment of anterior regions before rehabilitation suggests a significant involvement of attentional and executive processes, which may be required to compensate for verbal memory impairment, as observed during difficult tasks and also during decrease of memory efficiency with aging (e.g., [28]). After rehabilitation, the involvement of the posterior and medial cerebral regions may be explained by visual imagery strategies used to encode and to retrieve verbal information, as the patient had learnt during the rehabilitation program. Moreover, it is worth noting that fMRI tasks did not explicitly require a mental imagery strategy. This suggests that spontaneous visual imagery strategies are used by patients after the rehabilitation program and are efficient as indicated by the improvement of the neuropsychological scores.
4. Conclusion
Although these results were obtained with only one patient who presented with verbal memory impairment, they might suggest that an intensive (two times per week) and short (three months) rehabilitation program based on preserved cognitive abilities (here nonverbal memory) could be efficient to improve verbal memory abilities, even if it is applied months later after the curative surgery. One year after the rehabilitation program, the patient reported using the mental imagery in everyday life for routine and professional activities, suggesting that the positive effect could be sustainable over time. Supplementary evidence is clearly necessary to increase the robustness of these findings.
Conflict of interest
We have no conflict of interest to declare.
Acknowledgments
We thank the patient for her participation. This research has been funded by the FFRE Foundation La Fondation Française pour la Recherche sur l'Épilepsie).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebcr.2014.09.002.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Noachtar S., Borggraefe I. Epilepsy surgery: a critical review. Epilepsy Behav. 2009;15(1):66–72. doi: 10.1016/j.yebeh.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 2.Baxendale S. The impact of epilepsy surgery on cognition and behavior. Epilepsy Behav. 2008;12(4):592–599. doi: 10.1016/j.yebeh.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Scoville W.B., Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squire L.R., Stark C.E.L., Clark R.E. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 5.Powell H.W.R., Koepp M.J., Symms M.R., Boulby P.A., Salek-Haddadi A., Thompson P.J. Material-specific lateralization of memory encoding in the medial temporal lobe: blocked versus event-related design. Neuroimage. 2005;27(1):231–239. doi: 10.1016/j.neuroimage.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Wagner K., Frings L., Spreer J., Buller A., Everts R., Halsband U. Differential effect of side of temporal lobe epilepsy on lateralization of hippocampal, temporolateral, and inferior frontal activation patterns during a verbal episodic memory task. Epilepsy Behav. 2008;12(3):382–387. doi: 10.1016/j.yebeh.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Helmstaedter C., Elger C.E. Cognitive consequences of two thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996;37(2):171–180. doi: 10.1111/j.1528-1157.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 8.Helmstaedter C., Loer B., Wohlfahrt R., Hammen A., Saar J., Steinhoff B.J. The effects of cognitive rehabilitation on memory outcome after temporal lobe epilepsy surgery. Epilepsy Behav. 2008;12(3):402–409. doi: 10.1016/j.yebeh.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Ptak R., Van der Linden M., Schnider A. Cognitive rehabilitation of episodic memory disorders: from theory to practice. Front Hum Neurosci. 2010:4. doi: 10.3389/fnhum.2010.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koorenhof L., Baxendale S., Smith N., Thompson P. Memory rehabilitation and brain training for surgical temporal lobe epilepsy patients: a preliminary report. Seizure. 2012;21(3):178–182. doi: 10.1016/j.seizure.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Radford K., Lah S., Thayer Z., Miller L.A. Effective group-based memory training for patients with epilepsy. Epilepsy Behav. 2011;22(2):272–278. doi: 10.1016/j.yebeh.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Coyette F., Deroux C. Evaluation et prise en charge des troubles mnésiques Marseille. Solal; Marseille: 2003. L'utilisation des aides externes dans la prise en charge des troubles mnésiques; pp. 391–425. [Google Scholar]
- 13.Hendriks M.P.H. Neuropsychological compensatory strategies for memory deficits in patients with epilepsy. In: Pfâfflin M., Fraser R.T., Thorbecke R., Specht U., Wolf P., editors. Vol. 16. John Libbey; London: 2001. pp. 87–94. (Comprehensive care for people with epilepsy). [Google Scholar]
- 14.Bresson C., Lespinet-Najib V., Rougier A., Claverie B., N'Kaoua B. Verbal memory compensation: application to left and right temporal lobe epileptic patients. Brain Lang. 2007;102(1):13–21. doi: 10.1016/j.bandl.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Jones M.K. Imagery as a mnemonic aid after left temporal lobectomy: contrast between material-specific and generalized memory disorders. Neuropsychologia. 1974;12(1):21–30. doi: 10.1016/0028-3932(74)90023-2. [DOI] [PubMed] [Google Scholar]
- 16.Van der Linden M., Juillerat A.C. La revalidation neuropsychologique dans la maladie d'Alzheimer à un stade précoce: principes, méthodes et perspectives. Rev Neurol. 2004;160(4):64–70. doi: 10.1016/s0035-3787(04)70945-5. [DOI] [PubMed] [Google Scholar]
- 17.Grilli M.D., Glisky E.L. Self-imagining enhances recognition memory in memory-impaired individuals with neurological damage. Neuropsychology. 2010;24(6):698. doi: 10.1037/a0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam S., Allain P., Aubin G., Coyette F. Solar ed; Marseille: 2009. Actualités en rééducation neuropsychologique: études de cas. [Google Scholar]
- 19.Coyette F., Seron X., Meulemans T., Desgranges B., Adam S., Eustache F. Ealuation et prise en charge des troubles mnéiques. Solal; Marseille: 2003. Les stratégies d'imagerie mentale dans la réeducation des troubles de la mémoire; pp. 333–371. [Google Scholar]
- 20.Wedlund E.W., Nilsson L., Tomson T., Erdner A. What is important in rehabilitation for persons with epilepsy? Experiences from focus group interviews with patients and staff. Epilepsy Behav. 2013;28(3):347–353. doi: 10.1016/j.yebeh.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Pulvermüller F., Hauk O., Zohsel K., Neininger B., Mohr B. Therapy-related reorganization of language in both hemispheres of patients with chronic aphasia. Neuroimage. 2005;28(2):481–489. doi: 10.1016/j.neuroimage.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Nordvik J.E., KMh Walle, Nyberg C., Fjell A.M., Walhovd K.B., Westlye L.T. Bridging the gap between clinical neuroscience and cognitive rehabilitation: the role of cognitive training, models of neuroplasticity and advanced neuroimaging in future brain injury rehabilitation. NeuroRehabilitation. 2014;31(1):1–85. doi: 10.3233/NRE-131017. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Perrone-Bertolotti M., Zoubrinetzky R., Yvert G., Le Bas J.F., Baciu M. Functional MRI and neuropsychological evidence for language plasticity before and after surgery in one patient with left temporal lobe epilepsy. Epilepsy Behav. 2012;23(1):81–86. doi: 10.1016/j.yebeh.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Van der Linden M., Van der Kaa M.A. Reorganization therapy for memory impairments. In: Seron X., Deloche G., editors. Cognitive approaches in neuropsychological rehabilitation. Lawrence Erlbaum Associates; New York: 1989. pp. 105–158. [Google Scholar]
- 26.Coyette F., Coyette F., Van der Linden M. La réeducation en neuropsychologie: études de cas. Marseille. 1999. La réeducation des troubles de la mémoire: les stratégies de facilitation; pp. 89–101. [Google Scholar]
- 27.Rectem D., Pointrenaud J., Coyette F., Kalaft M., Van der Linden M. Une épreuve de rappel libre à 15 items avec remémoration sélective (RLS-15) In: Van der Linden M., Adam S., Agniel A., et les membres du GREMEM, editors. L’évaluation des troubles de la mémoire. Présentation de quatre tests de mémoire épisodique (avec leur étalonnage).; Marseille: Solal: 2014. pp. 69–84. [Google Scholar]
- 28.Davis S.W., Dennis N.A., Daselaar S.M., Fleck M.S., Cabeza R. Que PASA? The posterior–anterior shift in aging. Cereb Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.