The Rationale for CT Screening
Lung cancer is the leading cause of cancer death in the United States. In 2007, it is estimated that 160,390 lung cancer deaths will occur in the US, representing 29% of all cancer deaths (1). Roughly 87% of lung cancers are attributed to cigarette smoking (2). Although cancer risk is attenuated by smoking cessation, the risk is not eliminated, and lung cancer now occurs with equal frequency in current and former smokers (3). Moreover, other factors clearly influence risk, including age; family history; chronic obstructive pulmonary disease; pulmonary fibrosis; and exposures to environmental radon, asbestos, and certain other occupational agents (4). Realistically, lung cancer will likely remain at epidemic proportions for decades to come.
Non-small cell lung cancer (NSCLC), which accounts for 75–80% of all lung cancers, is typically diagnosed when disease is locally advanced or there are systemic metastases (5), explaining the dismal overall five-year survival of 15% (6). In contrast, the five-year survival of individuals with surgically resected, early stage NSCLC approaches 75% (6,7). These differences have fueled the impetus to find a screening test that can detect NSCLC in its early preclinical stages, when surgical resection is most likely to prolong life and potentially reduce lung cancer mortality. Although earlier randomized controlled trials of lung cancer screening using chest radiography and sputum cytology failed to show reduced lung cancer mortality, computed tomography (CT) is a much more sensitive test for detecting small lung nodules, and has generated considerable enthusiasm as a potential contemporary screening tool for lung cancer.
Results of Observational Trials with Low Dose Helical CT
There are now published results of several cohort studies (8–18) investigating CT screening in groups at varying risk of lung cancer. The first published American study was the Early Lung Cancer Action Program (ELCAP) (8,9). In this observational study, 1000 individuals aged 60 years or greater with a minimum 10 pack year smoking history underwent prevalence screening; 841 underwent at least one incidence (annual repeat) screen. At the prevalence screen, 233 nodules and 31 lung cancers were detected—27 lung cancers based on the detection of a nodule and four, which were excluded from analysis, based on the detection of mediastinal or airway lesions. Twenty-three of the 27 nodular lung cancers (85%) were stage I. On annual follow-up, there were seven incidence cancers as well as two interval cancers not detected by screening; of these nine cancers, five (56%) were stage I NSCLC. In this study, both CT and chest radiographs (CXR) were obtained on participants; three times more nodules and four times more lung cancers were detected by CT than by CXR (8).
Swensen et al. recently reported their five-year results on combined CT and sputum screening in 1520 high risk individuals aged 50 years or older with a smoking history of greater than 20 pack years (10). This cohort underwent four incidence (annual repeat) screenings; compliance rates were 95% or greater through the first three incidence screens and 80% for the final incidence screen at year 5. They observed 68 primary lung cancers in 66 participants (4% of participants and 2% of 3356 nodules), consisting of 31 prevalence, 34 incidence, and three interval cancers. Two cancers were detected only by sputum cytology. Of prevalence cancers, 71% were stage I; 49% of combined incidence and interval cancers were Stage I. There was one post-surgical mortality in a lung cancer patient. Thirteen participants underwent 15 surgeries for ultimately benign disease. Although no surgical deaths were reported in individuals with ultimately benign disease, the rates of unnecessary thoracotomies has raised concerns about the potential for screening-related harms.
Three major cohort studies using different clinical protocols have investigated low dose helical CT screening in Japan (11 – 13). Sobue et al. performed both CT and combined CXR with sputum cytology on 1611 participants (13). In this study, CT detected 4-fold more lung cancers than CXR across all screenings. Two studies performed unselected population screening in smokers and non-smokers aged 40 years and older (11 – 12). In these two studies, lung cancer prevalence rates were low (averaging 0.4%); however, roughly equal numbers of lung cancers were seen in smokers and non-smokers. The prevalence rates of lung cancer in non-smoking females averaged 0.44% and most were indolent bronchioloalveolar cell carcinomas or well differentiated adenocarcinomas; these detection rates represent a 10 to 15-fold increase over annual mortality rates in Japanese females. Detection rates of lung cancer in smoking males using CT screening were also increased by 2 to 15-fold (11).
Diederich et al. reported their findings with low dose screening CT in a cohort study of 817 smokers aged 40 and older in Europe (14, 15). Of the original cohort, 668 participants (81.8%) underwent at least one of five annual incidence (follow-up) screens. At prevalence screen, they observed 858 indeterminate nodules in 408 participants (50%), of which > 98% were deemed benign based on follow-up or biopsy. There were 12 prevalence lung cancers in 11 participants (1.3% prevalence) of which 7 were stage I. At incidence screens, 174 new nodules were observed; over 93% were deemed benign. A total of 21 suspicious incidence nodules—11 growing nodules and 10 new nodules—underwent additional diagnostic evaluation. Incidence lung cancers were diagnosed in 11 (incidence of 0.86%), benign disease in 8, and in 2 nodules histology was indeterminate because biopsy was refused (one patient with bladder cancer; one patient with growth of a nodule from 4 to 6 mm over 36 months). Five interval cancers were diagnosed between annual screens, only one of which had abnormalities visible on retrospective review of screening CT, in the form of a hilar mass. Overall, they reported that 26.7% of invasive procedures were for benign disease, including one thoracoscopy and one thoracotomy.
Diederich’s findings were consonant with prior observational studies in that: [1] A large number of nodules are seen at prevalence (baseline) and incidence screens using low dose CT, of which less than 10% will represent lung cancer. [2] Using standardized diagnostic algorithms, the number of invasive procedures can be minimized. [3] Nodules with documented growth should undergo additional diagnostic testing, although benign disease will ultimately be found even in growing nodules. The conclusions of Diederich and colleagues differed from the American and Japanese trials in that: [1] They observed that one third of lung cancers diagnosed after the baseline exam were interval cancers (between screenings) due to symptoms; and [2] Their lung cancers were not disproportionately adenocarcinomas, as reported elsewhere; rather, their patients had relatively greater numbers of both squamous cell and small cell carcinomas. From these data, the number of early stage lung cancers is higher with CT screening; however, the impact of screening on lowering the number of late stage cancers is not clear.
Findings From Preliminary Randomized Controlled Trials
Preliminary data from four randomized controlled trials (RCTs) using CT screening in the intervention arm have been published (16–21). Three of these trials were conducted in advance of large multi-center national trials to determine the feasibility of enrollment into a randomized trial by generalists (16–18). The Depiscan, conducted in France, involved 765 subjects randomized to receive either low dose CT or CXR at baseline and annually for two years. Complete clinical and imaging baseline data were obtained in 81% of patients. One or more nodules were seen in 336 participants (45.2%) in the CT arm versus 21 participants (7.4%) in the CXR arm. Eight lung cancers were detected in the LDCT arm vs. one in the CXR arm. Trial limitations included a 19% rate of non-compliance by participants who withdrew consent and only modest participation of the enrolling generalists (only 41% of 232 investigators enrolled participants).
In 2003, the NELSON trial was launched, a Dutch-Belgian trial that randomized roughly 16,000 high risk participants to receive either annual CT screening (at years 1, 2 and 4) versus a control arm receiving no screening (19, 20). The NELSON is the only trial in which the control arm receives no screening intervention, and has 80% power to show a lung cancer mortality reduction of at least 25% by 10 years after randomization. The data from the NELSON will be pooled with the Danish RCT; preliminary data are pending.
Gohagan et al., representing the Lung Screening Study (LSS) Research Group, reported the final results of the Lung Screening Study, in which 3318 individuals at high risk of lung cancer were equally randomized to receive either low dose CT or CXR for two annual screenings (21). The study was not powered to address differential lung cancer mortality between the two arms, but rather, whether a large scale multicenter randomized trial was feasible in the United States. Compliance rates at baseline and year 1 screenings were 96% and 86% for CT and 93% and 80% for CXR, indicating that a RCT was possible in the United States. Positive screens were seen in 20% and 10% of baseline CT and CXR screened participants, respectively, and in 18% and 7% of incidence screens. Over the study period, there were 40 CT-detected lung cancers and 20 CXR-detected lung cancers; stage I cancers comprised 48% of all CT-detected cancers and 40% of CXR-detected cancers.
Newest Data From Cohort Studies
Two recent studies have further confounded the subject of the benefit of CT screening. The first was the report of the International-ELCAP (I-ELCAP), a multi-national, observational study undertaken in the United States, Europe, Japan, and Israel in which 31,567 participants underwent prevalence screens and 27,456 incidence screens (22). Each site determined its own eligibility criteria, which included smokers, never smokers, and individuals with exposures to various environmental and occupational carcinogens. Lung cancer was diagnosed in 484 participants: 405 at prevalence screen, 74 at incidence screen, and 5 interval cancers (e.g., symptom-detected). Of these, 412 were clinical stage I; pathologic stage was not reported. Participants were followed for a median of 3.3 years; 2 participants were followed for 10 years. Using Kaplan-Meyer estimates, the 10-year lung cancer-specific survival for all participants was 80% (95% confidence interval, 74 to 85); among the 302 participants who underwent resection within 1 month of diagnosis, the estimated lung cancer-specific 10-year survival was 92% (95% confidence interval, 88–95). Eight untreated lung cancer patients died within five years of diagnosis. Based on these survival statistics, the investigators concluded that such screening could “prevent some 80% of deaths” from lung cancer.
Subsequent to the I-ELCAP report, Bach and colleagues described their findings in 3246 asymptomatic, high risk individuals who underwent at least three annual CT screenings after a median follow-up of 3.9 years (23). They compared observed lung cancer rates, stages, surgical resections, and deaths with what would have occurred absent screening based on a set of validated prediction models (24, 25). They observed 144 individuals with lung cancer compared with 44.5 predicted cases (relative risk, 3.2), among whom 42 had advanced stage disease versus 33.4 predicted. There were 109 individuals who underwent lung resection, representing a 10-fold increase over the expected number of surgeries. Lung cancer mortality was unchanged—38 lung cancer deaths were observed; 38.8 were predicted. They concluded that CT screening might increase the rate of lung cancer detection and treatment without reducing the number of advanced lung cancers or lung cancer mortality, calling into question the benefit of CT screening in face of possible risk. The authors rightfully acknowledged that their data were preliminary, predicated on a small sample and on the validity of their risk model and assumptions. Nonetheless, their findings contrast with those of the I-ELCAP and underscore a quintessential concern in the discussion of lung cancer screening: the I-ELCAP reported increased survival; Bach et al reported no difference in mortality. At the heart of screening debate is the question of which endpoint provides adequate validation of the efficacy of CT screening for lung cancer.
Survival versus Mortality Endpoints in Screening
At face value, increased lung cancer survival would seem to be synonymous with decreased lung cancer mortality. Yet, such is not the case. Lung cancer survival is appropriately an endpoint in controlled trials that compare different treatment interventions. Under these conditions, the diagnosis of the disease precedes the intervention and all patients have the disease. In the case of screening, the individuals undergoing the screening intervention are asymptomatic, relatively few actually have the disease, and the screening intervention precedes the diagnosis. These differences place screening interventions in a profoundly different paradigm than treatment interventions because some healthy individuals will become patients due to a positive screen, resulting in collective psychological, economic, and medical jeopardy. Moreover, this paradigm introduces confounding variables that make disease-specific survival misrepresentative of screening efficacy: the biases of lead-time, length, and overdiagnosis. Each of these can prolong survival without actually enhancing longevity, to produce an artificial semblance of benefit where none may exist (27–29).
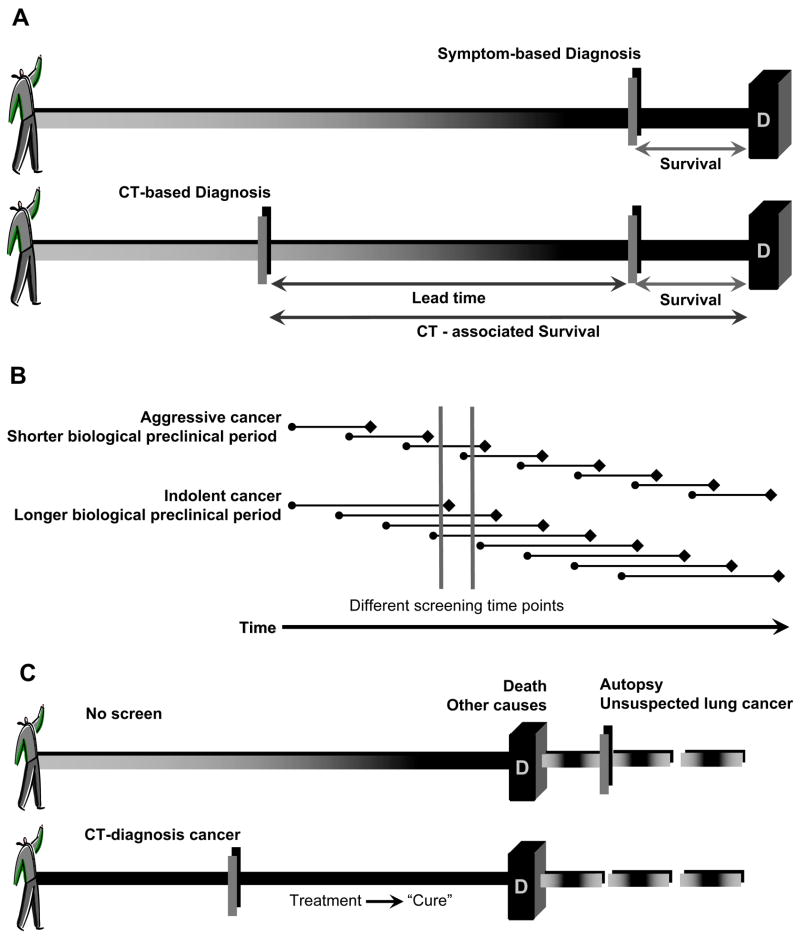
Lead-time bias refers to the fact that with screening, earlier detection will increase survival time even if death is not delayed (Fig 1A). Survival is measured from the time of lung cancer diagnosis or the initiation of treatment, events that are usually temporally proximate. To the extent that the time of diagnosis is advanced (lead time) by screening relative to symptom-diagnosed lung cancer, and assuming that early treatment intervention is not lethal, survival will increase even if death is not delayed. In point of fact, the early detection of lung cancer may favorably alter the natural course of disease by delaying death (e.g., increase longevity). The phenomena of lead-time and increased longevity are indistinguishable by survival statistics, particularly in a cancer with so heterogeneous a biology as lung cancer, because the rate of disease progression is not known.
Figure 1.
The effects of the biases of lead time, length, and overdiagnosis on increased survival, independent of improved longevity or mortality reduction. (A) Lead time from the early detection of a lung cancer by screening will prolong survival independent of any delay in death. (B) Screen-detected lung cancers tend to be more indolent than symptom-detected or intervals cancers diagnosed between screenings. The survival benefit associated with these favorable cancers is ascribed to the screening test, even if screening does nothing to reduce the number of advanced stage cancers or lung cancer deaths. (C) The pseudodisease detected because of overdiagnosis bias markedly increases survival, regardless of whether screening or associated treatments are effective.
The second bias that confounds the use of survival as a measure of efficacy is length bias. The purpose of lung cancer screening is to detect malignancy when it is preclinical, prior to the development of overt symptoms, providing the opportunity for treatment intervention that will prevent or delay death. There is clear evidence that lung cancer is not the product of one single molecular construct: there is considerable variation in lung cancer growth rates, as has been amply demonstrated by several observed phenomena: by calculations of tumor doubling time using CT (30, 31), by the propensity of a subset of pathologic stage IA lung cancers to recur following presumed curative surgery (32, 33), and by the indolent growth characteristics of certain subtypes of adenocarcinoma (34, 35). Effectively, some lung cancers are biologically indolent and grow slowly; some lung cancers are biologically aggressive and grow and metastasize rapidly. Length bias refers to the tendency of screening tests to preferentially select for more indolent lung cancers because of their longer preclinical phase (Fig 1B). Aggressive cancers are less likely to be screen-detected because they have a shorter preclinical phase. As such, the survival of individuals with screen-detected lung cancers will predictably be longer than that of individuals with symptom-detected lung cancers of the same stage. Similarly, if two screening tests are compared, the more sensitive screening test should detect more favorable (indolent) cancers than the less sensitive screening test.
The third bias peculiar to screening interventions is overdiagnosis bias, which can be considered an extreme form of length bias (Figure 1C). Overdiagnosis bias refers to the screening detection of cancers that would not have contributed to the death of an individual (pseudodisease) (27–29). Overdiagnosis can occur when screening detects a potentially lethal lung cancer, the course of which is superseded by competing co-morbidities, such as chronic obstructive pulmonary disease and cardiovascular disease, conditions that are prevalent in lung cancer patients and may result in death prior to demise from lung cancer. Overdiagnosis also occurs when screening detects a lung cancer that satisfies histologic criteria of malignancy, but that is so biologically indolent that it will never contribute to death. This concept is well established as a consideration in prostate carcinoma screening (36–38), but has been relatively ignored in lung cancer because of the high lethality of most symptom-detected cancers.
It is rarely possible to document pseudodisease in a living individual; once detected by screening, treatment is initiated and long term survival is ascribed to the successful treatment. In the I-ELCAP study, 8 participants with clinical stage I cancers who did not receive treatment died within five years of diagnosis (26). While it is tempting to infer from this that pseudodisease is not a significant factor in lung cancer, it is not possible to draw meaningful conclusions, both because the numbers are so small and because the factors that prevented primary treatment intervention—access to healthcare, co-morbidity, emotional state—also likely influenced survival.
The documentation of pseudodisease in lung cancer derives from necropsy studies in which unsuspected (surprise) lung cancers were observed in individuals who died of other causes. In a review of necropsies performed at Yale New Haven Hospital over a 30 year period, surprise lung cancers represented 13% of all lung cancers observed (39). Over the 30 years, the total number of surprise lung cancers increased; moreover, the proportion of resectable lung cancers in the surprise cases rose from 35% in the first decade to 70% in the third and final decade. The investigators postulated that necropsy documents a large reservoir of undetected lung cancers in the general population and that future advances in diagnostic technology would most certainly result in increased numbers of detected early stage lung cancers during life and would improve survival rates, particularly in women (39).
As if to confirm this premise, the most compelling evidence for overdiagnosis from the current screening studies is the observation of roughly equivalent rates of lung cancer among smokers and non-smokers in Japanese population CT screening, where lung cancer detection rates with CT in non-smoking females exceed annual mortality rates by several fold (11). Some proportion of these excess screen-detected cancers are tumors that would never contribute to the death of the individual.
When analyzing statistical trends in lung cancer, and especially in screening trials, it is important to distinguish lung cancer mortality from case fatality rates.
Lung cancer-specific mortality is defined as:
Lung cancer-specific case fatality, often called lung cancer death rate, is defined as:
Lung cancer-specific mortality rates consider the number of lung cancer deaths among all individuals under surveillance and address the impact of the screening intervention across the entire population to which screening is applied. In contrast, case fatality rates consider lung cancer deaths exclusively in individuals with lung cancer. Because case fatality rates consider only individuals with lung cancer, these rates are subject to the same biases as survival statistics. Mortality rates address the essential question of screening impact across the entire population that undergoes screening. Mortality rates are meaningful in the context of a contemporaneous control arm, a population that has not undergone the intervention of interest.
Finally, CT screening for lung cancer is a special case of screening. Unlike screening mammography that images only breast tissue, cervical Papanikolaou smears in which cervical epithelial cells are analyzed, or colonoscopy in which the colonic epithelium is directly visualized, chest CT scans span a wide anatomic range from the lower cervical region to the upper abdomen/retroperitoneum. Imagers are obliged to report any potentially significant findings observed, whether related to potential lung cancer or not. These additional observations may themselves incur downstream diagnostic tests that involve additional risk and potential death (40). Studies that follow and report medical outcomes on only the small percentage of individuals with suspected or diagnosed lung cancer cannot inform the benefits or risks of CT screening across the entire population being screened.
Summarizing our Present Knowledge Base
There is much to be learned from the multiple single arm observational studies and the preliminary experience of the RCTs of CT screening for lung cancer. We have learned that low dose helical CT is a sensitive imaging tool and detects many more lung nodules than CXR, on the order of 2 to 6-fold. Variations in the rates of nodule detection on screening CT are due primarily to the lower size threshold used by various investigators to define a “lung nodule” as well as the spatial quality of the CT scan, determined primarily by slice thickness. The majority of CT-detected nodules, typically over 90%, will be benign, even in a targeted, high risk population.
CT screening detects roughly 2 to 4 times more lung cancers than CXR. The increase in lung cancer detection is associated with a higher proportion of stage I disease and a relative oversampling of adenocarcinoma. However, the impact of CT screening on the absolute number of late stage cancers is not known. A true stage shift requires that the increase in screen-detected early stage cancers occur in parallel with a decrease in the number (not percentage) of advanced lung cancers. Absent this, it is unlikely that CT screening will confer benefit. In the study by Diederich et al., one third of lung cancers were interval cancers not detected by screening, the implication being that aggressive cancers may escape screen-detection (15).
CT detects smaller lung cancers than are normally visible on CXR. The average size of CT-detected prevalence cancers has ranged from 9– 16.5 mm (10, 31, 15). Some investigators have reported that CT-detected incidence cancers are smaller than prevalence nodules (26), albeit biologically more aggressive. A key premise by CT screening proponents is that the smaller tumors detected by CT have a different prognosis than larger lesions. In support of this, Visnivesky et al. analyzed 7620 patients with stage I NSCLC who had undergone resection from the Surveillance, Epidemiology, and End Results (SEER) registry 2003 to determine survival statistics (41). They found that smaller tumor size at diagnosis was associated with improved survival within stage I NSCLC. Specifically, the 12-year survival rate for patients with tumors 5–15 mm diameter was 69% (95% confidence interval [CI], 64 to 74%) and 63% for tumors 16 to 25 mm diameter (95% CI, 60 to 67%). Similarly, Flieder et al. retrospectively reviewed data on 503 patients with completely resected invasive NSCLC with invasive primary tumors ≤ 3 cm. They observed that primary NSCLC > 2.0 cm diameter is twice as likely to have nodal metastases as tumors ≤ 2 cm, lending support for the notion that small lesions represent early stage disease (42).
Although other investigators have not observed a significant relationship between T1 lesion size and stage distribution (43), the preponderance of data suggest small, but significant survival benefits for tumors within the T1 category ≤ 2 cm. This has provided the impetus for staging revisions in the seventh edition of the TNM Classification of Malignant Tumors due in 2009, which will propose that T1 lesions be subclassified as lesions ≤ 2 cm and > 2 cm (44). What is not known from these data is the efficacy of CT screening in reducing “underdiagnosis,” meaning the detection of small primary lesions prior to metastases (advanced disease). SEER data suggest that up to 40% of lung cancers measuring ≤ 15 mm diameter present with mediastinal or distant metastases at diagnosis (45). Experimental studies have shown that a 1 cm tumor sheds 3–6 million tumor cells into the blood every 24 hours (46). Tumor genetics, epigenetic phenomena, and tumor angiogenesis may be more influential than lesion size in determining cancer biology and metastatic potential.
In summary, it is expected that survival will increase in individuals with CT screen-detected lung cancers. The increased survival may be due to any of the biases inherent to screening: lead time, length, and overdiagnosis, or may be a consequence of true longevity (delaying the time of death). The essential concerns about the current data on CT screening are that: (1) CT screening may be detecting biologically favorable lesions, some of which would remain subclinical during life, promoting unnecessary diagnostic and treatment interventions that themselves incur morbidity and mortality; (2) CT screening may not result in true stage shift, e.g., reduce the number of advanced stage lung cancers, and therefore do nothing to significantly reduce mortality in small cancers destined to become advanced; and (3) the balance of benefit and risk have yet to be determined across the entire population subjected to screening.
Despite the lack of evidence of a mortality benefit from CT screening, the observational studies have informed several of our concepts of best practices. One notable example is the evaluation of the positive screen. Screening is beneficial to the extent that it detects disease sufficiently early that intervention can increase survivability or prevent death. Diagnostic pathways to further evaluate CT-detected indeterminate nodules have been largely empirical. However, these algorithms have been refined through the experience of several different groups of investigators with the goals of achieving the most efficient discrimination between malignant and benign disease while minimizing risks. With minor variation, different investigators have converged on very similar diagnostic pathways (26, 47–49). This is itself encouraging, and suggests that should CT be found to confer a legitimate mortality benefit, its implementation for public policy can be accomplished in concert with rationale diagnostic algorithms to manage indeterminate nodules detected by screening.
The current studies have also shown the considerations important to implementing standardized protocols across multiple sites for low dose helical CT. The earliest screening studies were done using thick-section (10 mm) CT scans (8). With CT screening, the detection task is to be able to reliably detect and follow the morphology of small, subcentimeter lung nodules, which mandates high spatial resolution on the order of 2 mm contiguous slice thicknesses. Moreover, the whole chest should be imaged at suspended full inspiration in a single breath-hold with minimal cardio-respiratory motion and using low radiation exposures (50). Over time, the acquisition protocols for helical CT across different groups of investigators have converged on common techniques. Moreover, with the increasing potential of software designed for computer aided nodule detection and volumetric analysis, helical CT technology lends itself to such image analysis in that additional high-resolution image datasets can be constructed from a single acquisition. The optimal datasets for computational analysis must be of high spatial quality, on the order of 1 mm contiguous intervals or better. These datasets serve automated image analysis purposes well, but are cumbersome for human interpretation needs. The standardization of acquisition parameters across different scanner platforms and the adoption of standardized image interpretation guidelines will be critical to the validity and quality of CT screening data and its analysis across different cohorts.
The National Lung Screening Trial (NLST)
Blueprints for a randomized trial that could validate the efficacy of CT screening for lung cancer were initially drafted in the late 1990s, partially in response to a series of workshops hosted by the National Cancer Institute (NCI). What ultimately became the NLST was the product of considerable collaboration and input by several researchers in both the extramural research community and the NCI. The NLST is a lung cancer screening trial sponsored by the National Cancer Institute (NCI) (51). Now in its fifth year, this randomized controlled trial (RCT) will compare the efficacy between two different screening tests: low dose helical CT and CXR. The trial has randomized 53,476 individuals at high risk of lung cancer in a 1:1 ratio to receive either annual CT or CXR for three screens (Figure 2). By the end of the trial, medical outcomes will have been collected for four to six years from randomization, depending on the time of enrollment. All trial-related data are reviewed at least twice annually by an independent data and safety monitoring board; interim analyses are also performed annually to determine potential trends that might reflect differential benefits or risks between the two arms. The final analysis of the NLST is expected some time in 2009.
Figure 2.
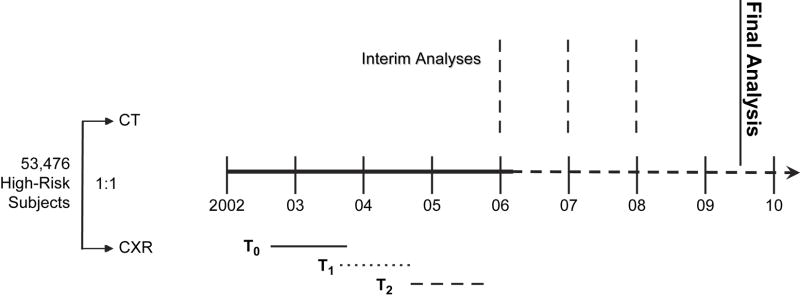
NLST Trial Schema. T0 is the baseline screen; T1 is Year 1 incidence screen; and T2 is Year 2 incidence screen.
The primary endpoint of the NLST is lung cancer-specific mortality; for which the trial has 90% power to detect a 20% difference between the two screening arms. Secondary endpoints include all cause mortality, differences in stage distribution of lung cancers at diagnosis, medical resource utilization (including complications of downstream diagnostic testing) in participants with positive screening tests, and the overall performance of the two screening tests. Additional secondary aims are being studied in a subset of the NLST participants who were enrolled at sites sponsored by the American College of Radiology Network (ACRIN) and include: (a) quality of life issues in screenees and anxiety in participants with positive screening tests; (b) medical resource utilization in participants across all categories of screening result; and (c) the impact of lung cancer screening on smoking behaviors and beliefs. Specimens of blood, urine, and sputum have also been collected at each of the three screening time points in over 10,000 ACRIN participants; these specimens will be used to validate biomarkers or panels of biomarkers of early lung cancer that appear promising based on preliminary tests (52).
The eligibility criteria for the NLST were designed to investigate individuals at highest risk of lung cancer, and include: asymptomatic male and female cigarette smokers between the ages of 55 and 74 years with a minimum cigarette smoking history of 30 pack years (total years smoked × packs per day). Current and former smokers were eligible, although the latter must have quit within 15 years prior to enrollment. Participants could not have been previously diagnosed with primary lung cancer; and could not have been treated for, or have evidence of, another cancer within the preceding five years (excluding non-melanoma skin cancers). Exclusion criteria were intended to ensure that participants can tolerate potentially curative lung resection.
NLST Screening Interpretations
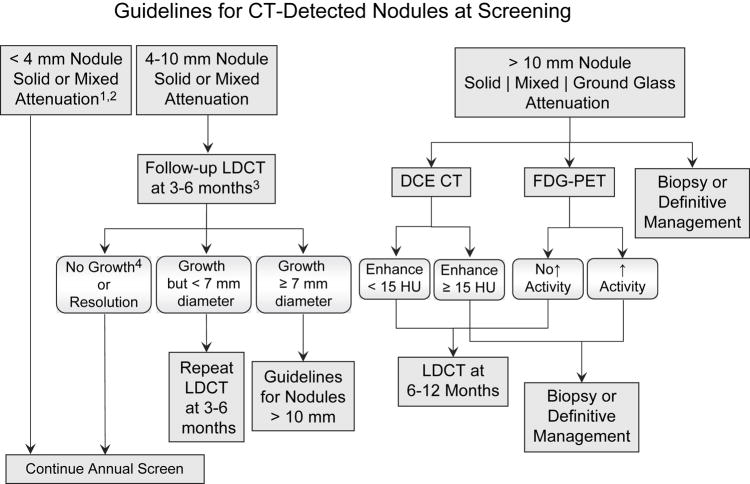
For both CT and CXR arms, there are three types of screening results as follows: (a) Positive screen, based on the presence of one or more nodules ≥ 4 mm diameter or other abnormality potentially related to lung cancer; (b) Negative screen with no, or minor, abnormalities not requiring follow-up; and (c) Negative screen with significant finding(s) unrelated to potential lung cancer. For all positive screening results, radiologists are obliged to provide some form of diagnostic recommendation. Recommendations for the follow-up of positive CT screening examinations are based on guidelines that take into consideration the size and attenuation characteristics of CT-detected nodules (Figure 3).
Figure 3.
Guidelines for diagnostic follow-up of CT-detected nodules in the NLST.
1 Pure ground glass nodules < 10 mm can be followed-up with LDCT at 6–12 months.
2 At T2 (final year screen) new nodules < 4 mm can be follow-up with repeat LDCT at 3–4 months.
3 The timing of repeat LDCT will vary according to nodule size. Larger nodules should be followed-up sooner than smaller nodules.
4 No growth is defined as < 15% increase in overall diameter OR no increase in solid component LDCT = low dose helical CT; DCE-CT = dynamic contrast enhanced CT; FDG-PET = 18 fluoro-deoxy-glucose positron emission tomography
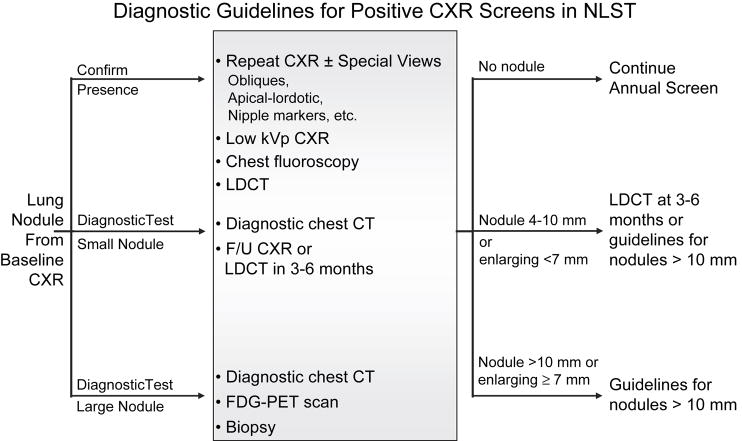
The interpretation task with CXR screening is often two-fold: to determine whether there is an abnormality and to further evaluate a visualized abnormality (Figure 4). The NLST protocol provided diagnostic recommendations as guidelines; in all instances, the decision regarding the diagnostic recommendation for a given screening result was left to the discretion of the radiologist based on their best judgment and local institutional practices. Screening examinations in which significant findings unrelated to potential lung cancer also mandate some form of recommendation, although no guidelines were provided, given the diverse range of possible findings and variations in practices across institutions.
Figure 4.
Diagnostic Guidelines for positive CXR screening tests.
Specific choices depend upon the nature of the detected abnormality and institutional preferences. CXR = chest radiograph; LDCT = low dose CT; FDG-PET = 18 fluoro deoxy glucose positron emission tomography.
NLST Outcomes Assessment
The intention of longitudinal assessment in the NLST is to determine the consequences, both positive and negative, that result from screening the population at risk. Participants are contacted at least annually to determine their health status. In participants with positive screens, all diagnostic tests, complications of tests, and test results are obtained. In participants with the diagnosis of lung cancer, the histology, grade, and stage of cancer are documented, as are the diagnostic tests used to establish the diagnosis, complications of diagnostic evaluation, treatments, treatment complications, and cancer progression or recurrence. Subsets of participants with negative screens, both with and without significant other findings, are also followed to tabulate all health encounters so that differential medical resource utilization between the two screening arms can be determined.
Deaths are documented through annual follow-up with participants or their contacts, or by querying the National Health Index data linked to death certificates. In all trial decedents, death certificates are obtained and coded using the International Classification of Diseases-10 Revision (ICD-10). These codes, combined with screening results and the timing of death relative to screening or downstream diagnostic tests, factor into an algorithm to determine those trial decedents whose deaths should be independently evaluated by experts. An endpoint verification committee then reviews medical records and available documentation to establish cause of death independent of the death certificate. The intent of the endpoint verification committee is to minimize biases that may over- or underestimate the benefit of screening.
Beyond CT Screening-Molecular Biomarkers
The potential of CT lies in its capacity to detect small lung cancers in the parenchyma. However, 15–20% of lung cancers in previous screening trials have been detected only by sputum cytology (53, 54). In particular, early squamous cell carcinomas in the large central airways escape CT detection, as do premalignant lesions like severe dysplasia and carcinoma-in-situ (CIS). These precursor lesions have been shown to progress to invasive squamous cell carcinoma in over 50% of cases in some studies (55). Indeed, absent severe dysplasia, the progression of low-grade dysplasia to CIS and invasive squamous cell carcinoma is not negligible, suggesting that each of these preneoplastic lesions must be independently considered to have malignant clones (56). Although atypical adenomatous hyperplasia (AAH)—now classified as a precursor lesion of lung adenocarcinoma—is detectable on CT and is known to have many of the same molecular alterations as adenocarcinoma (57, 58), the progression of AAH to adenocarcinoma has not been directly demonstrated, and it is not known with what frequency AAH transforms into invasive cancer. Helical CT is only now providing the means by which to study this lesion; prior to CT, most investigations of AAH involved specimens discovered incidentally in tissue resected by patients with adenocarcinoma (59).
Molecular studies show that lung cancers have multiple genetic and epigenetic alterations, numbering greater than twenty per cancer (60). Many of the alterations in gene expression and chromosome structure seen in lung cancer have also been demonstrated in preneoplastic lesions, including hyperproliferation and loss of cell cycle control; abnormalities in the p53 pathway, the ras genes and genes in the genomic regions of 3p14.2; aberrant gene promoter hypermethylation; angiogenesis; and altered expression of multiple proteins (61). These observations support the concept of lung carcinogenesis as a multistep process developing from normal epithelium and involving successive genetic and epigenetic abnormalities, typically through exposure to tobacco-related carcinogens.
Although sputum cytology itself has not satisfied the criteria necessary for an effective screening tool, sputum, blood and urine are attractive for screening strategies because they are readily attainable without invasive procedures and lend themselves to the exploration of potential molecular biomarkers. If reproducibly detected, biomarkers from these biospecimens could identify individuals destined to develop lung cancer and could be applied to lung cancer prevention strategies rather than treatment strategies.
A number of molecular analyses have been applied to sputum. Among these are DNA evaluation techniques, such as fluorescence in situ hybridization (FISH) using probes for several genes known to be associated with lung cancer, including c-myc, EGFR, 5p15, and CEP6 (62). These molecular biomarkers are relatively insensitive for the detection of lung cancer when considered alone (sensitivity 41%; specificity 94%); but become more sensitive when combined with sputum cytology (sensitivity 83%; specificity 80%).
The RAS family of protooncogenes (HRAS, KRAS) encode proteins that regulate key signal-transduction pathways involved in normal cellular differentiation, proliferation, and survival. Activating mutations in the KRAS genes are common in lung cancer of both adenocarcinoma and squamous cell histologies. A significant number of individuals with squamous cell carcinoma have KRAS mutations in sputum. However, these mutations are also found in individuals without subsequent lung cancer, and their significance as biomarkers of early disease has not been validated (63).
Among the more promising sputum biomarkers is the detection of aberrant methylation of DNA promoter genes, which occurs early in the development of lung cancer, and is associated with silencing of the transcription of genes involved in several aspects of normal cell function (Figure 5). Belinsky et al recently reported that promoter hypermethylation in sputum is associated with lung cancer and may precede its clinical diagnosis (64).
Figure 5.
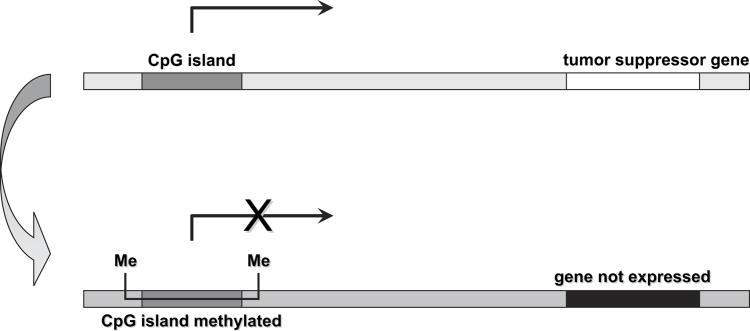
The effect of hypermethylation of tumor-suppressor gene promoters. CpG islands are regions of the gene rich in cytosine-guanine dinucleotides. Most CpG islands are found in the proximal promoter region of nearly half of all genes in the mammalian genome and are normally unmethylated. In the human genome, methylation (Me) occurs only at CpG islands. Hypermethylation of the promoter regions of tumor suppressor genes occurs early in the development of lung cancer and silences the transcription of the gene. Silencing by hypermethylation affects genes involved in all aspects of normal cell function and is a critical trigger for malignant transformation and cancer progression.
Similarly, promoter DNA hypermethylation changes have been observed in blood samples from patients with lung cancer (65). The levels of circulating cell-free DNA in blood are generally higher in patients with cancer than in healthy controls. The source of the increased DNA is assumed to be apoptotic or necrotic tumor cells that release DNA into the circulation. The circulating DNA in lung cancer patients exhibits many of the genetic and epigenetic changes typical of the primary tumor. For example, K-ras and p53 gene mutations have been found in between 20–30% of lung cancer patients (66). These mutations in blood are identical to those in the primary tumor, and provide solid evidence that nucleic acids are released into the circulation by lung cancers.
Proteomic studies in lung cancer have taken two forms: (a) protein profiling, in which patterns of protein expression are used to identify malignancy without knowledge of the specific proteins, and (b) techniques to identify individual proteins that may serve as biomarkers. Many of the molecular markers for NSCLC are growth factors and their receptors, such as the epidermal growth factor receptor (EGFR) and c-ErB-2 (Her-2/neu) and serum cytokines such as vascular endothelial growth factor (VEGF), stem cell factor (SCF), and hepatocyte growth factor/scatter factor (HGF/SF) (67). The field of proteomic biomarker discovery is rapidly accelerating, enabled by recent advances in high-throughput technologies and bioinformatics platforms. Substantial challenges, including instrument standardization, sample handling, reproducibility, and validation of preliminary results in larger prospective studies will need to be met in order to see the translation of these molecular approaches to the clinical practice of lung cancer screening.
Conclusions
CT screening is highly sensitive for the detection of lung nodules. Among individuals at high risk of lung cancer, up to 10% of CT-detected nodules detected at prevalence screening will ultimately prove to be lung cancer. CT detects more lung cancers than does chest radiography; a high percentage of these cancers will be early stage when diagnosed. The survival of CT screen-detected lung cancers is increased relative to historical controls—which are typically symptom-detected. While these observations are provocative, the demonstration of prolonged survival is itself insufficient evidence of screening benefit. When applied to screening, survival statistics are subject to the biases of lead time, length, and overdiagnosis. Moreover, there is no evidence to date that CT screening will reduce the number of aggressive, advanced stage lung cancers. Because of these biases, prolonged survival can occur absent a meaningful decrease in lung cancer-specific mortality. The results of the ongoing large-scale randomized controlled trials, such as the NLST and NELSON trial are essential in order to determine whether lung cancer-specific and all cause mortality differs between those who undergo CT screening and those who do not.
Beyond benefit, these trials must show that screening benefit outweighs risk—across all individuals screened, not only those with lung cancer. Chest CT provides a window to the evaluation of multiple organs and conditions. The collective consequences of all screening observations, the diagnostic follow-up associated with these imaging findings, the complications of downstream tests, and the potential to treat patients who neither require nor will benefit from treatments must be weighed against the unbiased measure of mortality benefit (68).
Concurrently, molecular research is accelerating at extraordinary pace. Although there has yet to be found a single biomarker, or panel of biomarkers, that predicts lung cancer, it is reasonable to anticipate that this may occur in the foreseeable future. How molecular medicine and contemporary imaging will converge to subdue the scourge of lung cancer remains to be written. Certainly, both the prospects and the stakes are high; the hope for a screening test that will reduce lung cancer mortality must be balanced with the necessary scientific skepticism to keep the journey honest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures 2007. Atlanta: American Cancer Society; 2007. pp. 1–51. [Google Scholar]
- 2.Strauss GM. Textbook of Pulmonary Diseases. 6. Philadelphia, PA: Lippincott-Raven Publishers; 1998. Bronchogenic carcinoma. [Google Scholar]
- 3.Ebbert JO, Yang P, Vachon CM, et al. Lung cancer risk reduction after smoking cessation: observations from a prospective cohort of women. J Clin Oncol. 2003;21:921–926. doi: 10.1200/JCO.2003.05.085. Artinian #12. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Glynn TG. Epidemiology of lung cancer. Radiol Clin North Am. 2000;38:453–570. doi: 10.1016/s0033-8389(05)70176-7. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Fry WA, Phillips JL, Merick HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: a national cancer data base report. Cancer. 1999;86:1667–1876. doi: 10.1002/(sici)1097-0142(19991101)86:9<1867::aid-cncr31>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 8.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 9.Henschke CI, Naidich DP, Yankelevitz DF, et al. Early Lung Cancer Action Project. Initial findings on repeat screening. Cancer. 2001;92:153–159. doi: 10.1002/1097-0142(20010701)92:1<153::aid-cncr1303>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five year prospective experience. Radiology. 2005;235:259–265. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 11.Sone S, Li F, Yang Z-G, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer. 2001;84:25–32. doi: 10.1054/bjoc.2000.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nawa T, Nakagawa T, Kusano S, et al. Lung cancer screening using low-dose spiral CT. Chest. 2002;2122:15–22. doi: 10.1378/chest.122.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-Lung Cancer Association Project. J Clin Oncol. 2002;20:911–920. doi: 10.1200/JCO.2002.20.4.911. [DOI] [PubMed] [Google Scholar]
- 14.Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222:773–781. doi: 10.1148/radiol.2223010490. [DOI] [PubMed] [Google Scholar]
- 15.Diederich S, Thomas M, Semik M, et al. Screening for early lung cancer with low-dose spiral computed tomography: results of annual follow-up examinations in asymptomatic smokers. Eur Radiol. 2004;14:691–702. doi: 10.1007/s00330-003-2200-5. [DOI] [PubMed] [Google Scholar]
- 16.Garg K, Keith RL, Byers T, Kelly K, Kerzner AL, Lynch DA, Miller YE. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology. 2002 Nov;225(2):506–10. doi: 10.1148/radiol.2252011851. [DOI] [PubMed] [Google Scholar]
- 17.Blanchon T, Bréchot JM, Grenier PA, et al. for the “Dépiscan” Group. Baseline results of the Depiscan study: A French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR) Lung Cancer. 2007 Oct;58(1):50–8. doi: 10.1016/j.lungcan.2007.05.009. Epub 2007 Jul 12. [DOI] [PubMed] [Google Scholar]
- 18.Gohagan JK, Marcus PM, Fagerstrom RM, Pinsky PF, et al. The Lung Screening Study Research Group. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer. 2005;47:9–15. doi: 10.1016/j.lungcan.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 19.van Iersel CA, de Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, Prokop M, Habbema JD, Oudkerk M, van Klaveren RJ. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON) Int J Cancer. 2007 Feb 15;120(4):868–74. doi: 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]
- 20.van Iersel CA, de Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, Prokop M, Habbema JD, Oudkerk M, van Klaveren RJ. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON) Int J Cancer. 2007 Feb 15;120(4):868–74. doi: 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]
- 21.Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the lung screening study, a randomized feasibility stuffy of spiral CT versus chest X-ray screening for lung cancer. Lung cancer. 2005;47:9–15. doi: 10.1016/j.lungcan.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 22.The International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Eng J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 23.Bach PB, Jett JR, Pastorino U, et al. Computed tomography screening and lung cancer outcomes. JAMA. 2007;297:953–961. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- 24.Bach PB, Elkin EB, Pastorino U, et al. Benchmarking lung cancer mortality rates in current and former smokers. Chest. 2004;126:1742–1749. doi: 10.1378/chest.126.6.1742. [DOI] [PubMed] [Google Scholar]
- 25.Cronin K, Gail MH, Zou Z, et al. Validation of a model of lung cancer risk prediction among smokers. J Natl Cancer Inst. 2006;98:637–640. doi: 10.1093/jnci/djj163. [DOI] [PubMed] [Google Scholar]
- 26.Henschke CI New York Early Lung Cancer Action Project Investigators. CT screening for lung cancer: diagnoses resulting from the New York Early Lung Cancer Action Project. Radiology. 2007;243:239–249. doi: 10.1148/radiol.2431060467. [DOI] [PubMed] [Google Scholar]
- 27.Black WC, Welch HG. Screening for disease. AJR. 1997;168:3–11. doi: 10.2214/ajr.168.1.8976910. [DOI] [PubMed] [Google Scholar]
- 28.Patz EF, Goodman PC, Bepler G. Screening for lung cancer. NEJM. 2000;343:1627–1633. doi: 10.1056/NEJM200011303432208. [DOI] [PubMed] [Google Scholar]
- 29.Reich JM. Assessing the efficacy of lung cancer screening. Radiology. 2006;238:398–401. doi: 10.1148/radiol.2382050668. [DOI] [PubMed] [Google Scholar]
- 30.Hasagawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73:1252–1259. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 31.Lindell RM, Hartman TE, Swensen SJ, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007;242:555–562. doi: 10.1148/radiol.2422052090. [DOI] [PubMed] [Google Scholar]
- 32.Gu C-D, Osaki T, Oyama T, et al. Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer. Impact on recurrence and survival. Ann Surg. 2002;1:133–139. doi: 10.1097/00000658-200201000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Porject: Proposals for the revision of the TNM stage grouping in the forthcoming (seventh) edition of the TNM Classification of malignant tumors. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi M, Morikawa A, Kawasaki M, Matsuno Y, Yamada T, Hirohashi S, Kondo H, Shimosato Y. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Kusumoto M, Watanabe S, Tsuchiya R, Asamura H. Radiologic classification of small adenocarcinoma of the lung: Radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg. 2006;81:413–420. doi: 10.1016/j.athoracsur.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 36.Stenman UH, Abrahamsson PA, Aus G, et al. Prognostic value of serum markers for prostrate cancer. Scand J Urol Nephrol Suppl. 2005;216:64–81. doi: 10.1080/03008880510030941. [DOI] [PubMed] [Google Scholar]
- 37.Telesca D, Etzioni R, Gulati R. Estimating lead time and overdiagnosis associated with PSA screening from prostate cancer incidence trends. Biometrics. 2007 May 14; doi: 10.1111/j.1541-0420.2007.00825.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 38.Bangma CH, Roemeling S, Schroder FH. Overdiagnosis and overtreatment of early detected prostate cancer. World J Urol. 2007;25:3–9. doi: 10.1007/s00345-007-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan CK, Wells CK, McFarlane MJ, Feinstein AR. More lung cancer but better survival. Implications of secular trends in “necropsy surprise” rates. Chest. 1989;96:291–296. doi: 10.1378/chest.96.2.291. [DOI] [PubMed] [Google Scholar]
- 40.Casarella WJ. A patient’s viewpoint on a current controversy. Radiology. 2002;224:927. doi: 10.1148/radiol.2243020024. [DOI] [PubMed] [Google Scholar]
- 41.Wisnivesky JP, Yankelevitz D, Henschke CI. The effect of tumor size on curability of stage I non-small cell lung cancers. Chest. 2004;126:761–765. doi: 10.1378/chest.126.3.761. [DOI] [PubMed] [Google Scholar]
- 42.Flieder DB, Port JL, Korst RJ, et al. Tumor size is a determinant of stage distribution in T1 non-small cell lung cancer. Chest. 2005;128:2304–2308. doi: 10.1378/chest.128.4.2304. [DOI] [PubMed] [Google Scholar]
- 43.Heyneman LE, Herndon JE, Goodman PC, et al. Stage distribution in patients with a small (3 cm) primary nonsmall cell lung cancer. Cancer. 2100;92:3051–3055. doi: 10.1002/1097-0142(20011215)92:12<3051::aid-cncr10106>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 44.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groups in the forthcoming (seventh) edition of the TNM Classification of malignant tumors. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 45.Pastorino U. Early detection of lung cancer. Respiration. 2006;73:5–13. doi: 10.1159/000090990. [DOI] [PubMed] [Google Scholar]
- 46.Swartz MA, Kristensen CA, Melder RJ, et al. Cell shed from tumours show reduced clonogenicity, resistance to apoptosis, and in vivo tumorigenicity. Br J Cancer. 1999;81:756–759. doi: 10.1038/sj.bjc.6690760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomized lung cancer screening trial. Lung Cancer. 2006;54:177–184. doi: 10.1016/j.lungcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 48.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005 Nov;237(2):395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 49.Aberle DR, Black WC, Goldin JG, Patz E, Gareen I, Gatsonis C. ACRIN Protocol 6654 (NLST): Contemporary Screening for the Detection of Lung Cancer. [Last Accessed 10-15-2007]. http://www.acrin.org/pdf_file2.html?file=protocol_docs/A6654partial_summary.pdf.
- 50.Cagnon CH, Cody DD, McNitt-Gray MF, Seibert JA, Judy PF, Aberle DR. Description and implementation of a quality control program in an imaging-based clinical trial. Acad Radiol. 2006;13:1431–1441. doi: 10.1016/j.acra.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 51.NLST: National Lung Screening Trial . [Last accessed 10-25-2007]. http://www.cancer.gov/nlst/what-is-nlst.
- 52.American College of Radiology Imaging Network. Protocol 6654. Contemporary screening for the detection of lung cancer. [Last accessed 10-15-2007]. http://www.acrin.org/pdf_file2.html?file=protocol_docs/A6654partial_summary.pdf.
- 53.Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low dose spiral CT versus radiography. Radiology. 1996;210:798–802. doi: 10.1148/radiology.201.3.8939234. [DOI] [PubMed] [Google Scholar]
- 54.Fontana RS, Sanderson DR, Taylor WF, et al. Lung cancer screening: The Mayo Program. J Occupat Med. 1986;28:746–750. doi: 10.1097/00043764-198608000-00038. [DOI] [PubMed] [Google Scholar]
- 55.Bota S, Auliac JB, Paris C, et al. Follow-up of bronchial precancerous lesions and carcinoma-in-situ using fluorescence endoscopy. Am J Respir Crit Care Med. 2001;164:1688–1693. doi: 10.1164/ajrccm.164.9.2012147. [DOI] [PubMed] [Google Scholar]
- 56.Breuer RH, Pasic A, Emit EF, et al. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Research. 2005;11:537–543. [PubMed] [Google Scholar]
- 57.Morandi L, Asioli S, Cavassa A, Pession A, Damiani S. Genetic relationships among atypical adenomatous hyperplasia, bronchioloalveolar carcinoma and adenocarcinoma of the lung. Lung cancer. 2007;56:35–42. doi: 10.1016/j.lungcan.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Kitamura H, Kameda Y, Ito T, et al. Atypical adenomatous hyperplasia of the lung. Implications for the pathogenesis of peripheral lung adenocarcinoma. Am J Clin Pathol. 1999;111:610–622. doi: 10.1093/ajcp/111.5.610. [DOI] [PubMed] [Google Scholar]
- 59.Yokose T, Ito Y, Ochiai A. High prevalence of atypical adenomatous hyperplasia of the lung in autopsy specimens from elderly patients with malignant neoplasms. Lung Cancer. 2000;29:125–130. doi: 10.1016/s0169-5002(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 60.Sekido Y, Fong KM, Minna JD. Molecular genetics of lung cancer. Annu Rev Med. 2003;54:73–87. doi: 10.1146/annurev.med.54.101601.152202. [DOI] [PubMed] [Google Scholar]
- 61.Greenberg AK, Lee MS. Biomarkers for lung cancer: clinical uses. Curr Opin Pulm Med. 2007;13:249–255. doi: 10.1097/MCP.0b013e32819f8f06. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy TC, Hirsch FR. Using molecular markers in sputum for the early detection of lung cancer: A review. Lung Cancer. 2004;45:S21–S27. doi: 10.1016/j.lungcan.2004.07.996. [DOI] [PubMed] [Google Scholar]
- 63.Aviel-Ronen S, Blackhall F, Shepherd F, et al. K-ras mutations in nonsmall cell lung carcinoma: a review. Clin Lung Cancer. 2006;30:211–214. doi: 10.3816/CLC.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 64.Belinsky SA, Liechty KC, Gentry FD, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66:3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- 65.Russo AL, Thiagalingam A, Pan H, et al. Differential DNA hypermethylation of critical genes mediates the stage-specific tobacco smoke-induced neoplastic progression of lung cancer. Clin Cancer Res. 2005 Apr 1;11(7):2466–2470. doi: 10.1158/1078-0432.CCR-04-1962. [DOI] [PubMed] [Google Scholar]
- 66.Bremnes RM, Sirera R, Camps C. Circulating tumor-dervied DNA and RNA markers in blood: a tool for early detection, diagnostics, and follow-up? Lung Cancer. 2006;49:1–12. doi: 10.1016/j.lungcan.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Bharti A, Ma PC, Salgia R. Biomarker discovery in lung cancer—promises and challenges of clinical proteomics. Mass Spec Rev. 2007;26:451–466. doi: 10.1002/mas.20125. [DOI] [PubMed] [Google Scholar]
- 68.Lee CI, Forman HP. CT screening for lung cancer: implications on social responsibility. AJR. 2007;188:297–298. doi: 10.2214/AJR.07.5212. [DOI] [PubMed] [Google Scholar]