Abstract
Background
The National Lung Screening Trial was conducted to determine whether three annual screenings (rounds T0, T1, and T2) with low-dose helical computed tomography (CT), as compared with chest radiography, could reduce mortality from lung cancer. We present detailed findings from the first two incidence screenings (rounds T1 and T2).
Methods
We evaluated the rate of adherence of the participants to the screening protocol, the results of screening and downstream diagnostic tests, features of the lung-cancer cases, and first-line treatments, and we estimated the performance characteristics of both screening methods.
Results
At the T1 and T2 rounds, positive screening results were observed in 27.9% and 16.8% of participants in the low-dose CT group and in 6.2% and 5.0% of participants in the radiography group, respectively. In the low-dose CT group, the sensitivity was 94.4%, the specificity was 72.6%, the positive predictive value was 2.4%, and the negative predictive value was 99.9% at T1; at T2, the positive predictive value increased to 5.2%. In the radiography group, the sensitivity was 59.6%, the specificity was 94.1%, the positive predictive value was 4.4%, and the negative predictive value was 99.8% at T1; both the sensitivity and the positive predictive value increased at T2. Among lung cancers of known stage, 87 (47.5%) were stage IA and 57 (31.1%) were stage III or IV in the low-dose CT group at T1; in the radiography group, 31 (23.5%) were stage IA and 78 (59.1%) were stage III or IV at T1. These differences in stage distribution between groups persisted at T2.
Conclusions
Low-dose CT was more sensitive in detecting early-stage lung cancers, but its measured positive predictive value was lower than that of radiography. As compared with radiography, the two annual incidence screenings with low-dose CT resulted in a decrease in the number of advanced-stage cancers diagnosed and an increase in the number of early-stage lung cancers diagnosed. (Funded by the National Cancer Institute; NLST ClinicalTrials.gov number, NCT00047385.)
THE NATIONAL LUNG SCREENING TRIAL (NLST) was a randomized trial of lung-cancer–specific mortality among participants in an asymptomatic high-risk cohort who underwent screening with the use of low-dose helical computed tomography (CT) as compared with screening with the use of single-view posteroanterior chest radiography. The NLST showed a 20% relative reduction in mortality from lung cancer with three rounds of low-dose CT screening (rounds T0, T1, and T2) as compared with radiography.1 In this article, we present more detailed findings from the two incidence screenings (rounds T1 and T2), including information on rates of positive screening tests, performance characteristics of the screening tests, diagnostic follow-up of positive screening results, numbers and characteristics of the lung cancers detected, and first-line treatments. Detailed findings from the prevalence screening (T0) are reported separately.2
METHODS
TRIAL OVERSIGHT
The design and eligibility criteria of the NLST, as well as demographic characteristics of the NLST participants, have been described in detail previously.3,4 Enrollment occurred from August 2002 through April 2004, and screening occurred from August 2002 through September 2007. Participants were followed for events that occurred through December 31, 2009. A total of 53,454 participants who were at risk for lung cancer were randomly assigned to three annual screenings with either low-dose CT or radiography. The study protocol was approved by the institutional review board at each of 33 screening centers, and written informed consent was obtained from each participant before randomization.
SCREENING
Acquisition factors and measures to control image quality for both low-dose CT and radiographic screenings were standardized throughout the trial.5 Images were interpreted by individual radiologists on soft-copy display stations without computer-assisted image analysis. A positive low-dose CT screening test was defined as the finding of one or more indeterminate (noncalcified) nodules measuring at least 4 mm in the longest diameter or, less commonly, mediastinal masses, pleural disease, or atelectasis of more than one segment. A positive radiographic screening test was defined as the finding of a noncalcified nodule of any size or another abnormality potentially related to lung cancer. At the discretion of the radiologist, nodules at incidence screenings that showed no change in growth or consistency from those detected at the previous screening could be classified as “positive, stable”; nodules that were stable across all three annual screenings could be classified as “positive, stable” or “negative.”
OUTCOMES AND FOLLOW-UP
The primary end point of the NLST was lung-cancer–specific mortality. Data on participants were obtained by means of medical-record abstraction and included the types and results of downstream diagnostic tests; histologic features, grade, and stage of lung cancers diagnosed; histologic features of other cancers; details of first-line treatments for lung cancer; and deaths from all causes. The methods for the histopathological classification of lung cancer, lung-cancer staging, and determination of vital status and cause of death have been described previously.1,6-9
STATISTICAL ANALYSIS
We compared the two screening groups with respect to adherence of the participants to the testing protocol, screening results, types of diagnostic procedures and results, and initial treatment information. Measures of diagnostic and predictive accuracy at the T1 and T2 rounds were derived with the use of data only from participants who had adequate screening examinations in the respective rounds and for whom lung-cancer status was known. For each dichotomous (positive vs. negative) screening result, lung cancer was classified as present or absent at the time of the screening on the basis of specific rules related to the timing of lung-cancer diagnosis relative to screening intervals and diagnostic testing (see the Supplementary Appendix, available with the full text of this article at NEJM.org). The sensitivity, specificity, positive predictive value, and negative predictive value were calculated according to accepted definitions, and confidence intervals were calculated with the use of bootstrapping.10 Screenings of participants with unknown lung-cancer status were excluded from calculations of screening test performance. We calculated the confidence intervals for incidence ratios assuming a Poisson distribution for the number of events and a normal distribution of the logarithm of the ratio, using asymptotic methods. All tabulations were performed with the use of SAS/STAT software, version 9.1 of the SAS System for Unix or version 9.2 for PC (SAS Institute).
RESULTS
SCREENING
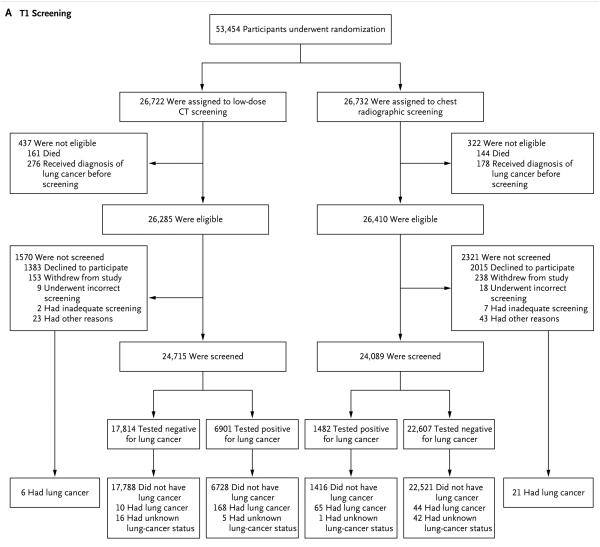
At the T1 screening, 94.0% of eligible participants in the low-dose CT group (24,715 of 26,285) and 91.2% of eligible participants in the radiography group (24,089 of 26,410) underwent screening (Table 1). T1 screening results were positive in 27.9% of the participants who were screened in the low-dose CT group (6901 of 24,715) and in 6.2% of the participants who were screened in the radiography group (1482 of 24,089). A total of 186 participants received a diagnosis of lung cancer in the low-dose CT group at T1: 168 of 6901 participants with positive screening results, 10 of 17,814 participants with negative screening results, 6 of 1570 participants who were not screened at T1, and 2 of 437 ineligible participants with lung cancers that were first diagnosed during the T1 screening year (Fig. 1A). In the radiography group, 133 participants received a diagnosis of lung cancer at T1: 65 of 1482 participants with positive screening results, 44 of 22,607 participants with negative screening results, 21 of 2321 participants who were not screened, and 3 of 322 ineligible participants with lung cancers that were diagnosed during the T1 screening year. The sensitivity of low-dose CT was 94.4% (95% confidence interval [CI], 90.8 to 97.6), the specificity was 72.6% (95% CI, 72.0 to 73.1), the positive predictive value was 2.4% (95% CI, 2.1 to 2.8), and the negative predictive value was 99.9% (95% CI, 99.9 to 100.0) (Table S1 in the Supplementary Appendix). The sensitivity of radiography was 59.6% (95% CI, 50.0 to 69.0), the specificity was 94.1% (95% CI, 93.8 to 94.4), the positive predictive value was 4.4% (95% CI, 3.3 to 5.5), and the negative predictive value was 99.8% (95% CI, 99.7 to 99.9).
Table 1.
Screening Eligibility, Compliance, and Rates of Positive Screening Tests According to Screening Round and Study Group.
| Screening Round |
Low-Dose CT | Chest Radiography | ||||
|---|---|---|---|---|---|---|
| Eligible for Screening |
Screened | Positive Result |
Eligible for Screening |
Screened | Positive Result |
|
| no. |
no. (% of eligible) |
no. (% of screened) |
no. |
no. (% of eligible) |
no. (% of screened) |
|
| T1 | 26,285 | 24,715 (94.0) | 6901 (27.9) | 26,410 | 24,089 (91.2) | 1482 (6.2) |
| T2 | 25,942 | 24,102 (92.9) | 4054 (16.8) | 26,110 | 23,346 (89.4) | 1174 (5.0) |
Figure 1. Randomization and Follow-up of the Study Participants.
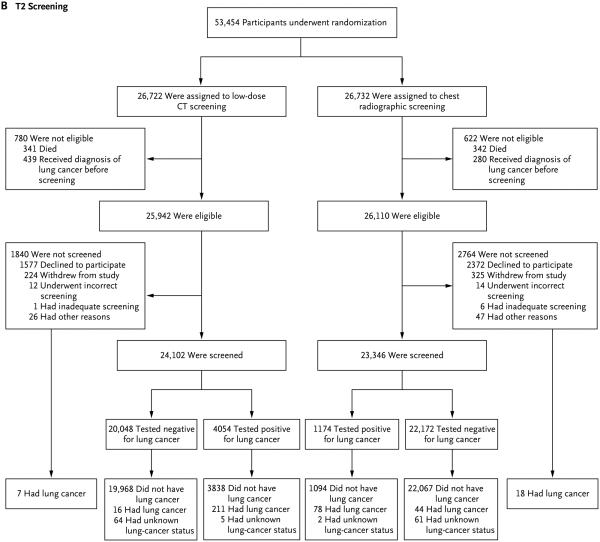
Panel A shows randomization and follow-up for the T1 screening. During the T1 study year, 2 of 437 ineligible participants in the low-dose computed tomography (CT) group and 3 of 322 ineligible participants in the radiography group received a diagnosis of lung cancer (data not shown). Panel B shows randomization and follow-up for the T2 screening. During the T2 study year, 3 of 780 ineligible participants in the low-dose CT group and 4 of 622 ineligible participants in the radiography group received a diagnosis of lung cancer (data not shown).
At the T2 screening, 92.9% of eligible participants in the low-dose CT group (24,102 of 25,942) and 89.4% of eligible participants in the radiography group (23,346 of 26,110) underwent screening (Fig. 1B and Table 1). T2 screening results were positive in 16.8% of persons who were screened in the low-dose CT group (4054 of 24,102) and in 5.0% of the persons who were screened in the radiography group (1174 of 23,346). A total of 237 participants received a diagnosis of lung cancer at T2 in the low-dose CT group: 211 of 4054 participants with positive screening results, 16 of 20,048 participants with negative screening results, 7 of 1840 participants who were not screened at T2, and 3 of 780 ineligible participants with lung cancers that were diagnosed during the T2 screening year (Fig. 1B). In the radiography group, 144 participants received a diagnosis of lung cancer at T2: 78 of 1174 participants with positive screening results, 44 of 22,172 participants with negative screening results, 18 of 2764 participants who were not screened, and 4 of 622 participants who were ineligible for the T2 screening but received a diagnosis of lung cancer during the T2 screening year (Fig. 1B). The sensitivity of low-dose CT was 93.0% (95% CI, 89.7 to 96.3), the specificity was 83.9% (95% CI, 83.4 to 84.3), the positive predictive value was 5.2% (95% CI, 4.6 to 5.9), and the negative predictive value was 99.9% (95% CI, 99.9 to 100.0) (Table S1 in the Supplementary Appendix). The sensitivity of radiography was 63.9% (95% CI, 55.2 to 72.1), the specificity was 95.3% (95% CI, 95.0 to 95.6), the positive predictive value was 6.7% (95% CI, 5.2 to 8.2), and the negative predictive value was 99.8% (95% CI, 99.7 to 99.9).
The rates of positive screening tests in both groups decreased at the T2 screening, primarily because abnormalities that were stable across all three rounds could be categorized as negative screening results (Table 1). Data on lung-cancer status according to the participants’ age, sex, and race or ethnic group are provided in Tables S2a and S2b in the Supplementary Appendix. In both the low-dose CT and radiography groups, 43% of participants who underwent incidence screening were 55 to 59 years of age at study entry, but the rates of lung cancer among participants in this age group were the lowest (in the low-dose CT group, 0.4% at the T1 screening and 0.7% at the T2 screening, and in the radiography group, 0.3% at both the T1 and T2 screenings).
FOLLOW-UP OF POSITIVE RESULTS
Information on diagnostic follow-up of positive screening results was available for the majority of participants in both groups at both screening rounds (Tables S3a and S3b in the Supplementary Appendix). Beyond office visits and physical examinations, imaging examinations, including diagnostic chest CT and 18F-fluorodeoxyglucose–positron-emission tomography (FDG-PET), were the most commonly performed procedures for follow-up of positive tests in both groups and at both screening rounds. FDG-PET was performed much more frequently in participants with a lung-cancer diagnosis than in those with no lung cancer. In the low-dose CT group, both surgical and bronchoscopic interventional procedures were performed more commonly than percutaneous biopsies in both participants with and those without lung-cancer diagnoses; the frequencies of these interventional procedures were more evenly distributed in the radiography group. Among participants in the low-dose CT group who underwent thoracotomy because of positive screening results, the proportion in whom lung cancer was not diagnosed was 18.9% at the T1 screening and 15.9% at the T2 screening. The respective proportions in the radiography group were 11.4% and 13.6%.
RELATIONSHIP OF NODULE SIZE TO LUNG CANCER
At the T1 screening, 161 of 168 CT-detected lung cancers (95.8%) and 60 of 65 radiography-detected lung cancers (92.3%) were diagnosed on the basis of an observed lung nodule or mass (Table 2). Among patients with lung cancers that were diagnosed with the use of low-dose CT screening at T1, 58 (34.5%) had nodules that were 4 to 10 mm in diameter, 74 (44.0%) had nodules that were 11 to 20 mm, 20 (11.9%) had nodules that were 21 to 30 mm, and 8 (4.8%) had masses larger than 30 mm. The positive predictive value for the detection of a nodule of any size with the use of low-dose CT at T1 was 2.4%, but it increased to 58.2% for positive screening results with subsequent biopsy. For nodules that were 4 to 6 mm diameter, the positive predictive value at T1 was 0.3%. In the radiography group, the largest nodule or mass observed among 65 lung cancers detected at the T1 screening was 4 to 10 mm in diameter in 9 participants (13.8%), 11 to 20 mm in 22 participants (33.8%), 21 to 30 mm in 16 participants (24.6%), and more than 30 mm in 10 participants (15.4%). The positive predictive value for the detection of a nodule of any size at the T1 screening in the radiography group was 4.4%, but it increased to 67.4% for positive screening results with subsequent biopsy. In both groups, the positive predictive value for detection of a nodule increased as the nodule size increased from 4 to 30 mm. In the radiography group, the positive predictive value for nodules smaller than 4 mm was relatively high; it is unclear whether these nodules corresponded to a lung cancer or prompted follow-up assessments that led to an ultimate diagnosis of lung cancer, although the latter explanation is more likely. In both groups, detection of masses larger than 30 mm had a slightly decreased positive predictive value relative to the detection of nodules that were 21 to 30 mm, probably because pneumonia was interpreted as a positive screening result.
Table 2.
Frequency of Positive Screening Results and Positive Predictive Value (PPV) at T1 Screening, According to Study Group.*
| Variable | Low-Dose CT | Chest Radiography | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confirmed Lung Cancer | Total | PPV† | PPV Range |
Confirmed Lung Cancer | Total | PPV† | PPV Range |
|||||
| yes | no | unknown | yes | no | unknown | |||||||
| number (percent) | percent | number (percent) | percent | |||||||||
| Positive screening result | 168 | 6728 | 5 | 6901 | 2.4 | 2.1–2.8 | 65 | 1416 | 1 | 1482 | 4.4 | 3.4–5.5 |
| With subsequent biopsy |
167 (99.4) | 120 (1.8) | 0 | 287 (4.2) | 58.2 | 52.7–64.0 | 64 (98.5) | 31 (2.2) | 0 | 95 (6.4) | 67.4 | 56.8–76.7 |
| With noncalcified nodule or mass |
161 (95.8) | 6620 (98.4) | 5 (100.0) | 6786 (98.3) | 2.4 | 2.0–2.7 | 60 (92.3) | 1296 (91.5) | 1 (100.0) | 1357 (91.6) | 4.4 | 3.4–5.6 |
| Size of nodule or mass‡ | ||||||||||||
| <4 mm | 0 | 1 (0) | 0 | 1 (0) | 0.0 | 0.0–0.0 | 1 (1.5) | 28 (2.0) | 0 | 29 (2.0) | 3.4 | 0.0–11.7 |
| 4–6 mm | 12 (7.1) | 3809 (56.6) | 1 (20.0) | 3822 (55.4) | 0.3 | 0.2–0.5 | 1 (1.5) | 304 (21.5) | 0 | 305 (20.6) | 0.3 | 0.0–1.1 |
| 7–10 mm | 46 (27.4) | 1909 (28.4) | 4 (80.0) | 1959 (28.4) | 2.4 | 1.7–3.0 | 8 (12.3) | 433 (30.6) | 0 | 441 (29.8) | 1.8 | 0.7–3.2 |
| 11–20 mm | 74 (44.0) | 741 (11.0) | 0 | 815 (11.8) | 9.1 | 7.0–11.1 | 22 (33.8) | 321 (22.7) | 0 | 343 (23.1) | 6.4 | 4.0–9.3 |
| 21–30 mm | 20 (11.9) | 94 (1.4) | 0 | 114 (1.7) | 17.5 | 10.6–24.6 | 16 (24.6) | 46 (3.2) | 1 (100.0) | 63 (4.3) | 25.8 | 15.2–36.8 |
| >30 mm | 8 (4.8) | 47 (0.7) | 0 | 55 (0.8) | 14.5 | 6.2–24.6 | 10 (15.4) | 43 (3.0) | 0 | 53 (3.6) | 18.9 | 9.0–30.6 |
| Unknown | 1 (0.6) | 19 (0.3) | 0 | 20 (0.3) | 5.0 | 0.0–16.7 | 2 (3.1) | 121 (8.5) | 0 | 123 (8.3) | 1.6 | 0.0–4.4 |
| Other findings | ||||||||||||
| Atelectasis, segmental or more extensive |
3 (1.8) | 77 (1.1) | 0 | 80 (1.2) | 3.8 | 0.0–8.5 | 1 (1.5) | 12 (0.8) | 0 | 13 (0.9) | 7.7 | 0.0–26.4 |
| Noncalcified hilar or mediastinal adenopathy or mass |
23 (13.7) | 151 (2.2) | 1 (20.0) | 175 (2.5) | 13.2 | 8.5–18.4 | 8 (12.3) | 25 (1.8) | 0 | 33 (2.2) | 24.2 | 10.1–41.3 |
| Consolidation | 1 (0.6) | 50 (0.7) | 0 | 51 (0.7) | 2.0 | 0.0–6.4 | 2 (3.1) | 30 (2.1) | 0 | 32 (2.2) | 6.3 | 0.0–15.0 |
| Pleural thickening or effusion |
11 (6.5) | 427 (6.3) | 0 | 438 (6.3) | 2.5 | 1.2–4.0 | 2 (3.1) | 98 (6.9) | 1 (100.0) | 101 (6.8) | 2.0 | 0.0–5.4 |
The cases of confirmed lung cancer do not include the following cancers diagnosed during the T1 study year: 32 in participants who did not undergo screening at T1, 10 in participants with negative results of low-dose CT screening at T1, and 44 in participants with negative results of radiographic screening at T1.
PPV is defined as the proportion of confirmed lung cancers among the participants with positive screening tests who had a known lung-cancer status.
Nodule size refers to the diameter of the largest nodule recorded on the screening examination.
In both groups, the proportions of cancers associated with a lung nodule or mass at the T2 screening were similar to those seen at the T1 screening (Table 3). At T2, the positive predictive value for detection of a nodule was 5.2% on low-dose CT and 6.4% on radiographic screening; the corresponding positive predictive values for detection of nodules that were 4 to 6 mm in diameter were 0.7% and 2.5%. With low-dose CT, the positive predictive value increased with increasing nodule size at T2; this was not the case with radiography because of a decrease in the positive predictive value for masses larger than 30 mm and two cases in which nodules smaller than 4 mm were associated with lung cancer. As expected, in both groups, detection of stable nodules at T2 was less predictive of lung cancer than detection of new nodules. For positive screening tests with subsequent biopsy, the positive predictive value increased to 65.8% with low-dose CT and to 67.9% with radiography. At both the T1 and T2 screenings, there was no clear relationship between nodule size and stage of non–small-cell lung cancer in either group (Table S4 in the Supplementary Appendix).
Table 3.
Frequency of Positive Screening Results and Positive Predictive Value at T2 Screening, According to Study Group.*
| Variable | Low-Dose Computed Tomography | Chest Radiography | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confirmed Lung Cancer | Total | PPV† | PPV Range |
Confirmed Lung Cancer | Total | PPV† | PPV Range |
|||||
| yes | no | unknown | yes | no | unknown | |||||||
| number (percent) | percent | number (percent) | percent | |||||||||
| Positive screening result | 211 | 3838 | 5 | 4054 | 5.2 | 4.5–6.0 | 78 | 1094 | 2 | 1174 | 6.7 | 5.4–8.0 |
| New finding | 196 (92.9) | 1622 (42.3) | 4 (80.0) | 1822 (44.9) | 10.8 | 9.4–12.2 | 74 (94.9) | 865 (79.1) | 1 (50.0) | 940 (80.1) | 7.9 | 6.3–9.5 |
| Stable finding | 15 (7.1) | 2216 (57.7) | 1 (20.0) | 2232 (55.1) | 0.7 | 0.4–1.0 | 4 (5.1) | 229 (20.9) | 1 (50.0) | 234 (19.9) | 1.7 | 0.4–3.6 |
| With subsequent biopsy | 206 (97.6) | 107 (2.8) | 0 | 313 (7.7) | 65.8 | 60.2–71.1 | 74 (94.9) | 35 (3.2) | 0 | 109 (9.3) | 67.9 | 59.0–76.3 |
| With noncalcified nodule or mass |
203 (96.2) | 3715 (96.8) | 3 (60.0) | 3921 (96.7) | 5.2 | 4.5–5.9 | 67 (85.9) | 975 (89.1) | 2 (100.0) | 1044 (88.9) | 6.4 | 5.0–8.0 |
| Size of nodule or mass‡ | ||||||||||||
| <4 mm | 0 | 7 (0.2) | 0 | 7 (0.2) | 0 | 0.0–0.0 | 2 (2.6) | 11 (1.0) | 0 | 13 (1.1) | 15.4 | 0.0–37.5 |
| 4–6 mm | 15 (7.1) | 2007 (52.3) | 1 (20.0) | 2023 (49.9) | 0.7 | 0.4–1.1 | 5 (6.4) | 197 (18.0) | 0 | 202 (17.2) | 2.5 | 0.5–4.7 |
| 7–10 mm | 58 (27.5) | 1072 (27.9) | 1 (20.0) | 1131 (27.9) | 5.1 | 4.0–6.4 | 9 (11.5) | 335 (30.6) | 0 | 344 (29.3) | 2.6 | 1.1–4.4 |
| 11–20 mm | 86 (40.8) | 502 (13.1) | 0 | 588 (14.5) | 14.6 | 11.7–17.5 | 22 (28.2) | 263 (24.0) | 0 | 285 (24.3) | 7.7 | 4.9–10.6 |
| 21–30 mm | 23 (10.9) | 77 (2.0) | 0 | 100 (2.5) | 23.0 | 14.1–31.7 | 19 (24.4) | 47 (4.3) | 0 | 66 (5.6) | 28.8 | 18.2–40.0 |
| >30 mm | 20 (9.5) | 41 (1.1) | 1 (20.0) | 62 (1.5) | 32.8 | 21.0–45.1 | 2 (2.6) | 22 (2.0) | 1 (50.0) | 25 (2.1) | 8.3 | 0.0–20.9 |
| Unknown | 1 (0.5) | 9 (0.2) | 0 | 10 (0.2) | 10.0 | 0.0–33.3 | 8 (10.3) | 100 (9.1) | 1 (50.0) | 109 (9.3) | 7.4 | 3.0–12.5 |
| Other findings | ||||||||||||
| Atelectasis, segmental or more extensive |
4 (1.9) | 90 (2.3) | 1 (20.0) | 95 (2.3) | 4.3 | 0.9–8.8 | 3 (3.8) | 21 (1.9) | 0 | 24 (2.0) | 12.5 | 0.0–28.6 |
| Noncalcified hilar or me diastinal adenopathy or mass |
19 (9.0) | 78 (2.0) | 0 | 97 (2.4) | 19.6 | 12.1–28.1 | 7 (9.0) | 33 (3.0) | 0 | 40 (3.4) | 17.5 | 5.8–30.4 |
| Consolidation | 4 (1.9) | 35 (0.9) | 0 | 39 (1.0) | 10.3 | 2.2–20.6 | 2 (2.6) | 34 (3.1) | 0 | 36 (3.1) | 5.6 | 0.0–14.3 |
| Pleural thickening or ef fusion |
15 (7.1) | 278 (7.2) | 1 (20.0) | 294 (7.3) | 5.1 | 2.9–7.9 | 6 (7.7) | 80 (7.3) | 0 | 86 (7.3) | 7.0 | 2.1–12.6 |
The cases of confirmed lung cancer do not include the following cancers diagnosed during the T2 study year: 32 in participants who did not undergo screening at T2, 16 in participants with negative results of low-dose CT screening at T2, and 44 in participants with negative results of radiographic screening at T2.
PPV is defined as the proportion of confirmed lung cancers among the participants with positive screening tests who had a known lung-cancer status.
Nodule size refers to the diameter of the largest nodule recorded on the screening examination.
STAGE DISTRIBUTION AND TREATMENT OF LUNG CANCER
At the T1 screening, the clinical or pathological stage was known in 183 of 186 participants in the low-dose CT group (Table 4). Among participants with lung cancer of a known stage, 87 had stage IA cancer (47.5%); 39 had stage IB, IIA, or IIB cancer (21.3%); and 57 had stage III or IV cancer (31.1%). Among participants in the radiography group who received a lung-cancer diagnosis at T1, the stage was known in 132 of 133 participants; 31 had stage IA cancer (23.5%); 23 had stage IB, IIA, or IIB cancer (17.4%); and 78 had stage III or IV cancer (59.1%). Stage distributions at T2 were similar to those at T1 in each group.
Table 4.
Stage of Lung Cancers, According to Screening Round, Study Group, and Screening Result.*
| Screening Round and Stage |
Low-Dose CT | Chest Radiography | ||||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | No Screening |
Total | Positive | Negative | No Screening |
Total | |
| number/total number (percent) | ||||||||
| T1 screening round | ||||||||
| Stage IA | 86/165 (52.1) | 1/10 (10.0) | 0 | 87/183 (47.5) | 23/65 (35.4) | 6/43 (14.0) | 2/24 (8.3) | 31/132 (23.5) |
| Stage IB | 18/165 (10.9) | 1/10 (10.0) | 2/8 (25.0) | 21/183 (11.5) | 9/65 (13.8) | 1/43 (2.3) | 1/24 (4.2) | 11/132 (8.3) |
| Stage IIA | 11/165 (6.7) | 0 | 0 | 11/183 (6.0) | 4/65 (6.2) | 2/43 (4.7) | 1/24 (4.2) | 7/132 (5.3) |
| Stage IIB | 6/165 (3.6) | 0 | 1/8 (12.5) | 7/183 (3.8) | 3/65 (4.6) | 1/43 (2.3) | 1/24 (4.2) | 5/132 (3.8) |
| Stage IIIA | 13/165 (7.9) | 1/10 (10.0) | 0 | 14/183 (7.7) | 6/65 (9.2) | 6/43 (14.0) | 2/24 (8.3) | 14/132 (10.6) |
| Stage IIIB | 13/165 (7.9) | 4/10 (40.0) | 2/8 (25.0) | 19/183 (10.4) | 2/65 (3.1) | 6/43 (14.0) | 4/24 (16.7) | 12/132 (9.2) |
| Stage IV | 18/165 (10.9) | 3/10 (30.0) | 3/8 (37.5) | 24/183 (13.1) | 18/65 (27.7) | 21/43 (48.8) | 13/24 (54.2) | 52/132 (39.4) |
| Unknown† | 3/168 (1.8) | 0 | 0 | 3/186 (1.6) | 0 | 1/44 (2.3) | 0 | 1/133 (0.8) |
| T2 screening round | ||||||||
| Stage IA | 113/204 (55.4) | 2/16 (12.5) | 1/10 (10.0) | 116/230 (50.4) | 27/77 (35.1) | 4/44 (9.1) | 4/22 (18.2) | 35/143 (24.5) |
| Stage IB | 28/204 (13.7) | 0 | 3/10 (30.0) | 31/230 (13.5) | 10/77 (13.0) | 3/44 (6.8) | 0 | 13/143 (9.1) |
| Stage IIA | 8/204 (3.9) | 0 | 0 | 8/230 (3.5) | 7/77 (9.1) | 0 | 2/22 (9.1) | 9/143 (6.3) |
| Stage IIB | 3/204 (1.5) | 2/16 (12.5) | 0 | 5/230 (2.2) | 0 | 3/44 (6.8) | 0 | 3/143 (2.1) |
| Stage IIIA | 15/204 (7.4) | 0 | 0 | 15/230 (6.5) | 10/77 (13.0) | 7/44 (15.9) | 1/22 (4.5) | 18/143 (12.6) |
| Stage IIIB | 15/204 (7.4) | 4/16 (25.0) | 1/10 (10.0) | 20/230 (8.7) | 10/77 (13.0) | 6/44 (13.6) | 5/22 (22.7) | 21/143 (14.7) |
| Stage IV | 22/204 (10.8) | 8/16 (50.0) | 5/10 (50.0) | 35/230 (15.2) | 13/77 (16.9) | 21/44 (47.7) | 10/22 (45.5) | 44/143 (30.8) |
| Unknown‡ | 7/211 (3.3) | 0 | 0 | 7/237 (3.0) | 1/78 (1.3) | 0 | 0 | 1/144 (0.7) |
Cancer-stage classification was based on the sixth edition of the Cancer Staging Manual of the American Joint Committee on Cancer.7 The denominators for cancer stage were the total number of lung cancers of known stage in each screening-result category.
The lung cancers of unknown stage at the T1 screening round included 4 cancers with stage information that could not be classified.
The lung cancers of unknown stage at the T2 screening round included 2 carcinoids, 1 occult carcinoma, 1 with no evidence of malignancy, and 4 with stage information that could not be classified.
In the low-dose CT group, the increase in early-stage lung cancers was associated with a decrease in late-stage lung cancers. Over the course of the trial, the incidence of stage IV lung cancer was 138 cases per 100,000 person-years in the low-dose CT group, as compared with 204 cases per 100,000 person-years in the radiography group (rate ratio, 0.68; 95% CI, 0.57 to 0.80).
The distribution of stage according to screening result was fairly consistent between the T1 and T2 screenings in the low-dose CT group. At both T1 and T2, the majority of stage IA lung cancers were detected in participants with screening results that were positive on low-dose CT; lung cancers diagnosed in participants with negative results (interval cancers) were predominantly advanced-stage cancers. In the radiography group, as compared with the low-dose CT group, lung cancers detected in participants with positive results were more equally distributed among early, intermediate, and advanced stages. Of small-cell lung cancers detected by means of low-dose CT, 92.3% of those detected at T1 and 66.7% of those detected at T2 were late-stage cancers; corresponding percentages in the radiography group were 42.9% and 66.7% (data not shown). For lung cancers at each stage, participants in the low-dose CT and radiography groups received similar treatment (Tables S5a and S5b in the Supplementary Appendix).
HISTOLOGIC CHARACTERISTICS OF DIAGNOSED LUNG CANCERS
The most common histologic types of lung cancer in both screening groups and at both screenings were adenocarcinoma (at T1, 36.6% of cancers diagnosed in the low-dose CT group and 32.8% of those diagnosed in the radiography group; at T2, 34.9% in the low-dose CT group and 33.1% in the radiography group) and squamous-cell carcinoma (at T1, 21.0% of cancers diagnosed in the low-dose CT group and 22.9% of those diagnosed in the radiography group; at T2, 26.0% in the low-dose CT group and 23.9% in the radiography group) (Table S6 in the Supplementary Appendix).
In the low-dose CT group, lung cancers characterized as bronchioloalveolar-cell carcinoma were predominantly diagnosed after a positive screening and accounted for 17.3% and 13.3% of all lung cancers detected on positive screening at T1 and T2, respectively. Bronchioloalveolar-cell carcinoma included pure adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive adenocarcinoma, lepidic predominant (i.e., neoplastic cell growth restricted to preexisting alveolar structures); these histologic subtypes are now classified separately.11 Few bronchioloalveolar-cell carcinomas were diagnosed in the radiography group, probably because they are difficult to discern on planar imaging. The frequency of small-cell carcinoma was similar in the low-dose CT and radiography groups at both screenings, but in the low-dose CT group, small-cell carcinoma was more commonly detected in participants with positive results than in those with negative results, whereas in the radiography group, it was more commonly diagnosed in participants with negative screening tests than in those with positive results.
DISCUSSION
At the T1 and T2 incidence screenings in the NLST, the percentage of positive screening results in the low-dose CT group was more than three times as high as that in the radiography group. With low-dose CT, the number of lung cancers that were detected by screening was increased by a factor of 2.7, and the number of interval lung cancers (diagnosed after a negative screening) was decreased by 70%. The reduction in lung-cancer mortality observed with low-dose CT screening was coupled with a shift to detection of earlier-stage non–small-cell lung cancers at incidence screenings.
In the low-dose CT group, 27.9% of all results at the T1 screening and 16.8% of all results at the T2 screening were positive; 2.4% and 5.2% of positive screening tests were associated with a lung-cancer diagnosis, respectively. The higher positive predictive value at T2 is partly due to the fact that a nodule observed to be stable over three consecutive screenings could be interpreted as “negative” at the last screening.
Although this analysis does not address the unique features of nodules that distinguish lung cancers from noncancers, some preliminary observations can be made. With low-dose CT, the positive predictive value increased as nodule size increased up to 30 mm; this pattern was less pronounced with radiography. For lesions larger than 30 mm, the positive predictive value did not reliably increase in either group, possibly because of misinterpretation of pneumonia as a positive screening result. Moreover, nodules in some size categories in the radiography group were few in number, resulting in large uncertainties (confidence intervals) in the estimates of positive predictive value.
In both groups, more lung cancers were diagnosed at T2 than at T1. It is likely that many positive screening results at T0 were ultimately diagnosed as lung cancer at T2, after observation of growth over time. Also, because T2 was the final screening round in the trial, abnormalities detected in that round may have been followed more aggressively than those detected at earlier rounds.
The performance characteristics of low-dose CT are influenced by the risk (pretest probability) of lung cancer among persons who undergo screening. Given the low proportion of lung cancers in participants 55 to 59 years of age who underwent screening, increasing the minimum age for screening may have merit; however, other risk factors, in addition to age, need to be considered in order to obtain the greatest possible benefit of screening.12 As our knowledge evolves, screening guidelines will be informed by integrating multiple demographic and clinical risk factors, measures of field injury such as airflow obstruction, and validated biomarkers of lung-cancer predisposition, measured in blood or other readily accessible specimens.13-15
The performance of low-dose CT is also influenced by the definition of positive screening results, which will be refined in light of the experience of the NLST as well as other randomized trials and single-group studies. In the NLST, screening interpretations were dichotomous. Other ongoing randomized trials, including the Danish Lung Cancer Screening Trial (ClinicalTrials.gov number, NCT00496977) and the Dutch–Belgian Randomised Controlled Trial for Lung Cancer Screening in High-Risk Subjects (NELSON; Current Controlled Trials number, ISRCTN63545820), have added an “indeterminate” interpretation category for nodules between the lower (negative) and upper (positive) size thresholds, with CT repeated at 3 months to classify the findings as negative or positive.16,17 This two-step approach has little effect on overall medical resource utilization, since patients with nodules within this indeterminate size range typically undergo early repeat low-dose CT; however, the change in classification significantly improves the positive predictive value of low-dose CT screening relative to that observed in the NLST. More important, the designation of “indeterminate” provides a more realistic representation of the risk of lung cancer for both patient and provider, given that the majority of such nodules are ultimately benign.
Increasing the minimum size threshold for positive screening tests can also reduce the frequency of the diagnostic workup.18 In the NLST, nodules that were 4 to 6 mm in diameter accounted for roughly half the positive screening results with low-dose CT at both time points, but such nodules were associated with lung cancer in less than 1% of participants. Future efforts to develop diagnostic prediction models in this rapidly moving field should be informed by aggregating data on nodule size from all screening trials; these efforts should balance the effects of test performance at a given nodule size with potential delays in diagnosis and effects on a reduction in mortality. The size threshold of a nodule that indicates a positive screening, whether based on diameter or computer-assisted volumetric analysis,19,20 will vary depending on the level of risk of lung cancer and must be assessed in the context of the specific cohort undergoing screening.
The NLST had a number of limitations. First, the results may have been influenced by the healthy-volunteer effect, although this effect would be similar in the two screening groups.1 Second, in this analysis, data on medical resource utilization was restricted to participants with positive screening results. Our data underestimate the numbers of additional procedures and attendant risks that could result from reported findings other than those related to potential lung cancer. Data on these findings have been collected in a subgroup of NLST participants but are not reported here. Finally, the NLST included three annual screenings.1 The results of ongoing randomized trials in Europe and mathematical modeling will help to inform the effects of additional years of screening or different screening intervals on stage shift and mortality reduction.
The two incidence screenings in the NLST provide evidence that in a high-risk cohort, annual screening with low-dose CT detects more lung cancers than radiography and results in a stage shift toward early-stage, non–small-cell lung cancers, which are potentially curable. The performance characteristics of low-dose CT may be enhanced by determining the most appropriate risk cohort, refining both algorithms for interpreting the results of screening and definitions of positive findings, and determining the appropriate duration and timing of screening.
Supplementary Material
Acknowledgments
The American College of Radiology Imaging Network (ACRIN)–National Lung Screening Trial (NLST) was supported by grants (U01-CA-80098 and U01-CA-79778) under a cooperative agreement with the Cancer Imaging Program, Division of Cancer Treatment and Diagnosis. The Lung Screening Study (LSS) of the NLST was supported by contracts with the Early Detection Research Group and Biometry Research Group, Division of Cancer Prevention, University of Colorado Denver (N01-CN-25514), Georgetown University (N01-CN-25522), the Pacific Health Research and Education Institute (N01-CN-25515), the Henry Ford Health System (N01-CN-25512), the University of Minnesota (N01-CN-25513), Washington University in St. Louis (N01-CN-25516), the University of Pittsburgh (N01-CN-25511), the University of Utah (N01-CN-25524), the Marshfield Clinic Research Foundation (N01-CN-25518), the University of Alabama at Birmingham (N01-CN-75022), Westat (N01-CN-25476), and Information Management Services (N02-CN-63300).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the study participants for their contributions in making this study possible and Barbara Galen, M.S.N., C.R.N.P., C.N.M.T., ACRIN program director, National Cancer Institute, for her invaluable insights and support of the NLST.
REFERENCES
- 1.National Lung Screening Trial Research Team Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Team Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–91. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Idem The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–53. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idem Baseline characteristics of participants in the randomized National Lung Screening Trial. J Natl Cancer Inst. 2010;102:1771–9. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cagnon CH, Cody DD, McNitt-Gray MF, Seibert JA, Judy PF, Aberle DR. Description and implementation of a quality control program in an imaging-based clinical trial. Acad Radiol. 2006;13:1431–41. doi: 10.1016/j.acra.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Fritz A, Percy C, Jack A, et al., editors. International classification of diseases for oncology. 3rd World Health Organization; Geneva: 2000. [Google Scholar]
- 7.Travis WD, Brambilla E, Muller-Hemer-link HK, Harris CC, editors. Lyons. IARC Press; France: 2004. Pathology and genetics of tumours of the lung, pleura, thymus and heart — World Health Organization classification of tumours. [Google Scholar]
- 8.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th Springer; New York: 2002. [Google Scholar]
- 9.Marcus PM, Gareen IF, Biller AB, et al. The National Lung Screening Trial’s End-point Verification Process: determining the cause of death. Contemp Clin Trials. 2011;32:834–40. doi: 10.1016/j.cct.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davison AC, Hinkley DV. Cambridge University Press; Cambridge, United Kingdom: 1997. Bootstrap methods and their application. [Google Scholar]
- 11.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–36. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risch A, Plass C. Lung cancer genetics and epigenetics. Int J Cancer. 2008;123:1–7. doi: 10.1002/ijc.23605. [DOI] [PubMed] [Google Scholar]
- 14.Young RP, Hopkins RJ, Gamble GD, Etzel C, El-Zein R, Crapo JD. Genetic evidence linking lung cancer and COPD: a new perspective. J Appl Clin Genet. 2011;4:99–111. doi: 10.2147/TACG.S20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barba M, Felsani A, Rinaldi M, Giunta S, Malorni W, Paggi MG. Reducing the risk of overdiagnosis in lung cancer: a support from molecular biology. J Cell Physiol. 2011;226:2213–4. doi: 10.1002/jcp.22558. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial — overall design and results of the prevalence round. J Thorac Oncol. 2009;4:608–14. doi: 10.1097/JTO.0b013e3181a0d98f. [DOI] [PubMed] [Google Scholar]
- 17.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–9. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 18.Henschke CI, Yip R, Yankelevitz DF, Smith JP. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158:246–52. doi: 10.7326/0003-4819-158-4-201302190-00004. [DOI] [PubMed] [Google Scholar]
- 19.Lam S, McWilliams A, Majo J, Tammemagi M. Computed tomography screening for lung cancer: what is a positive screen? Ann Intern Med. 2013;158:289–90. doi: 10.7326/0003-4819-158-4-201302190-00011. [DOI] [PubMed] [Google Scholar]
- 20.El-Baz A, Beache GM, Gimel’farb G, et al. Computer-aided diagnosis systems for lung cancer: challenges and methodologies. Int J Biomed Imaging. 2013 Jan 29; doi: 10.1155/2013/942353. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.