Abstract
The influential notion that the hippocampus supports associative memory by interacting with functionally distinct and distributed brain regions has not been directly tested in humans. We therefore used targeted noninvasive electromagnetic stimulation to modulate human cortical-hippocampal networks and tested effects of this manipulation on memory. Multi-session stimulation increased functional connectivity among distributed cortical-hippocampal network regions and concomitantly improved associative memory performance. These alterations involved localized long-term plasticity, because increases were highly selective to the targeted brain regions, and enhancements of connectivity and associative memory persisted for ~24 hours following stimulation. Targeted cortical-hippocampal networks can thus be enhanced noninvasively, demonstrating their role in associative memory.
The hippocampus is necessary for associative (relational/declarative) memory (1, 2). It is a neuroanatomical convergence zone for highly processed sensory information regarding qualities of objects and contexts and therefore could serve as a “hub” to support binding of information from distinct processing modules into associative memories (1–4). However, hippocampal interactivity with distributed brain regions has yet to be demonstrated as necessary for associative memory in humans. Few experiments have used functional magnetic resonance imaging (fMRI) to identify hippocampal interactions with distributed cortical regions that are correlated with associative memory (5). Although brain-lesion studies have shown the necessity of an intact hippocampus for associative memory, they cannot readily demonstrate the necessity of hippocampal interactivity with other regions.
We therefore developed methods to modulate cortical-hippocampal brain networks in healthy adults (N=16) to test their role in associative memory (6). We focused modulatory stimulation on the lateral parietal cortex component of a well-characterized cortical-hippocampal network (4), based on hypothesized interactions between hippocampus and lateral parietal cortex in memory (7) as well as robust functional connectivity between these regions (8), likely mediated by lateral parietal projections to retrosplenial and parahippocampal cortex (9, 10). We defined a target within left hippocampus for each subject and used resting-state fMRI to identify a subject-specific left lateral parietal location that demonstrated high functional connectivity with the hippocampal target (Fig. 1A; Fig. S1)(6). Noninvasive high-frequency repetitive transcranial magnetic stimulation (rTMS; (6)) was delivered to the parietal location for five consecutive days, based on evidence that rTMS can induce changes in connectivity within stimulated networks (11, 12), and that such effects can increase over multi-day stimulation sessions (13).
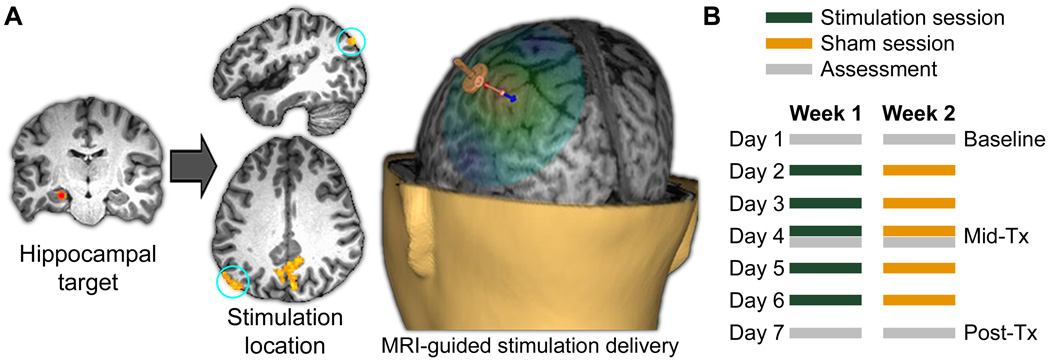
Fig. 1. Targeted cortical-hippocampal network stimulation.
(A) For each subject, a parietal stimulation location was selected based on maximum local fMRI connectivity with a hippocampal target, and stimulation was applied to this location under MRI guidance (6). (B) Timing of assessments and stimulation sessions for the stimulation and sham weeks, with week order counterbalanced (6). Post-Tx assessment was ~24 h after the final stimulation session.
We measured changes in cortical-hippocampal network fMRI connectivity and associative memory using pre-treatment (baseline), mid-treatment (Mid-Tx), and post-treatment (Post-Tx) assessments (Fig. 1B). Stimulation effects were measured relative to a sham-control condition involving the same parameters, but at sub-threshold intensity for neural stimulation (6). Compared to sham, Post-Tx resting-state fMRI connectivity was significantly greater than baseline in four regions, including (1) precuneus/retrosplenial cortex, (2) fusiform/parahippocampal cortex, (3) superior parietal cortex, and (4) left lateral parietal cortex (Fig. 2A; Table S1). These stimulation-responsive regions include elements of well-characterized hippocampal intrinsic connectivity networks (8) hypothesized to interact with the hippocampus to support associative memory (4, 14, 15), including the approximate location of lateral parietal cortex that was stimulated.
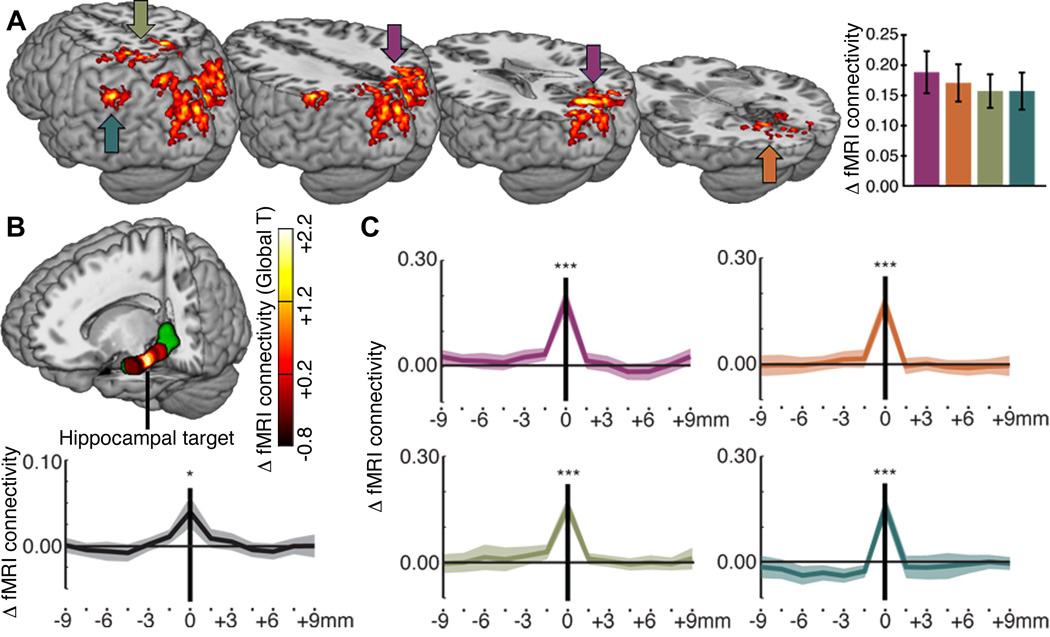
Fig. 2. Stimulation-induced fMRI connectivity increases selective to hippocampal targets.
(A) Regions showing significant change in fMRI connectivity with the hippocampal target (Post-Tx versus baseline for stimulation compared to sham) (6) shown on a template brain viewed from the back left. (B) Stimulation-induced changes in whole-brain fMRI connectivity (T values of differences in global average connectivity) are colorized for the target and other locations along the anterior-posterior axis of the left hippocampus (which is displayed in green on the rendered brain) (6). The plot shows changes in fMRI connectivity values for the subject-specific hippocampal target (0 mm) and for 1.5-mm steps along the anterior-posterior hippocampal axis (negative values indicate anterior to the target). (C) The same change values are plotted for the four stimulation-responsive regions shown in (A). *P<0.05 versus zero; ***P<0.001 versus zero. Error bars and line shading indicate standard error of the mean.
Increased fMRI connectivity was highly specific to the individual hippocampal target selected for each subject. By assessing Post-Tx versus baseline connectivity changes due to stimulation (relative to sham) along the anterior-posterior axis of the targeted hippocampus (6), we noted a rapid decline in stimulation effects on fMRI connectivity with increasing distance from the target (Fig. 2B). At average distances of 1.5 mm and 3.0 mm from the target in either direction, the T values of the change in whole-brain (global) connectivity from baseline were ~48% and ~17% of the connectivity change for the target, respectively, and were not statistically significant (Fig. 2B). Stimulation-induced changes in connectivity of the hippocampal target with the four stimulation-responsive regions were similarly selective (Fig. 2C). We found no reliable changes for right hippocampal locations that mirrored left hippocampal target locations and no reliable changes for the left hippocampus treated as a unit (6).
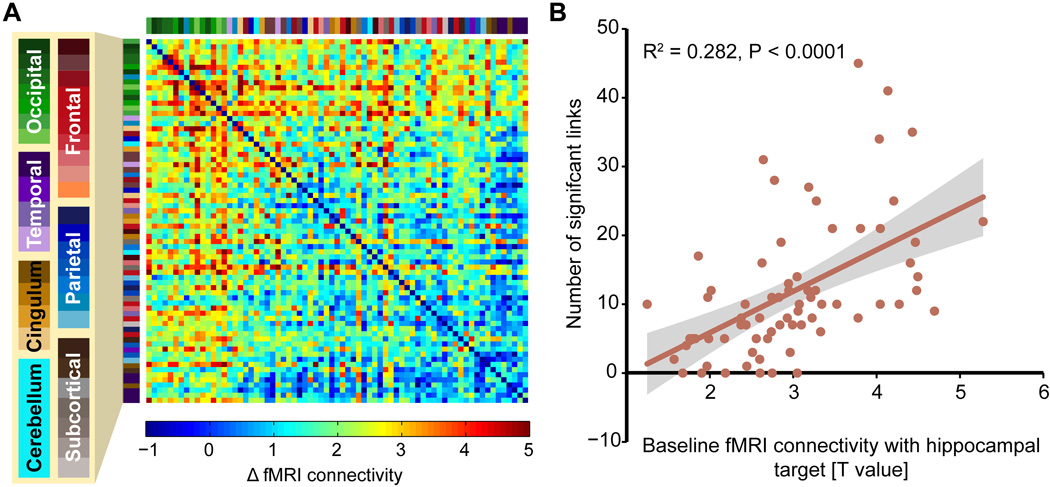
Stimulation also increased interconnectivity among stimulation-responsive regions. A correlation-weighted fMRI connectivity matrix formed from locations with at least minimal stimulation-related change in connectivity with the hippocampal target (6) indicated robust increases in regional interconnectivity (Fig. 3A; Fig. S2). To test whether increases in regional interconnectivity were associated with the degree to which an anatomically defined region was part of the hippocampal resting-state network, the matrix was sorted by each region’s baseline fMRI connectivity with the hippocampal target. Indeed, the number of significant interregional links (P < 0.05, FDR-corrected) was significantly correlated with baseline fMRI connectivity with the hippocampal target (Fig. 3B; R2(adj) = 0.27, df = 69, P < 0.0001). Lateral parietal cortex stimulation thus increased fMRI interconnectivity to a greater extent among regions that were more versus less strongly within baseline cortical-hippocampal networks.
Fig. 3. Stimulation-induced fMRI regional interconnectivity scales with baseline connectivity with hippocampal targets.
(A) Coloration indicates the effect of stimulation (Post-Tx versus baseline for stimulation relative to sham, T value) on fMRI connectivity among stimulation-responsive regions (6). Regions are sorted by baseline fMRI connectivity with hippocampal targets (top rows and left columns are highest). Region labels are colorized and expanded in Fig. S2. (B) The degree of interconnectivity for a region (number of significant links with other regions surviving P < 0.05 FDR correction) correlated with the strength of baseline connectivity with hippocampal targets for the region. Shading indicates 95% CI.
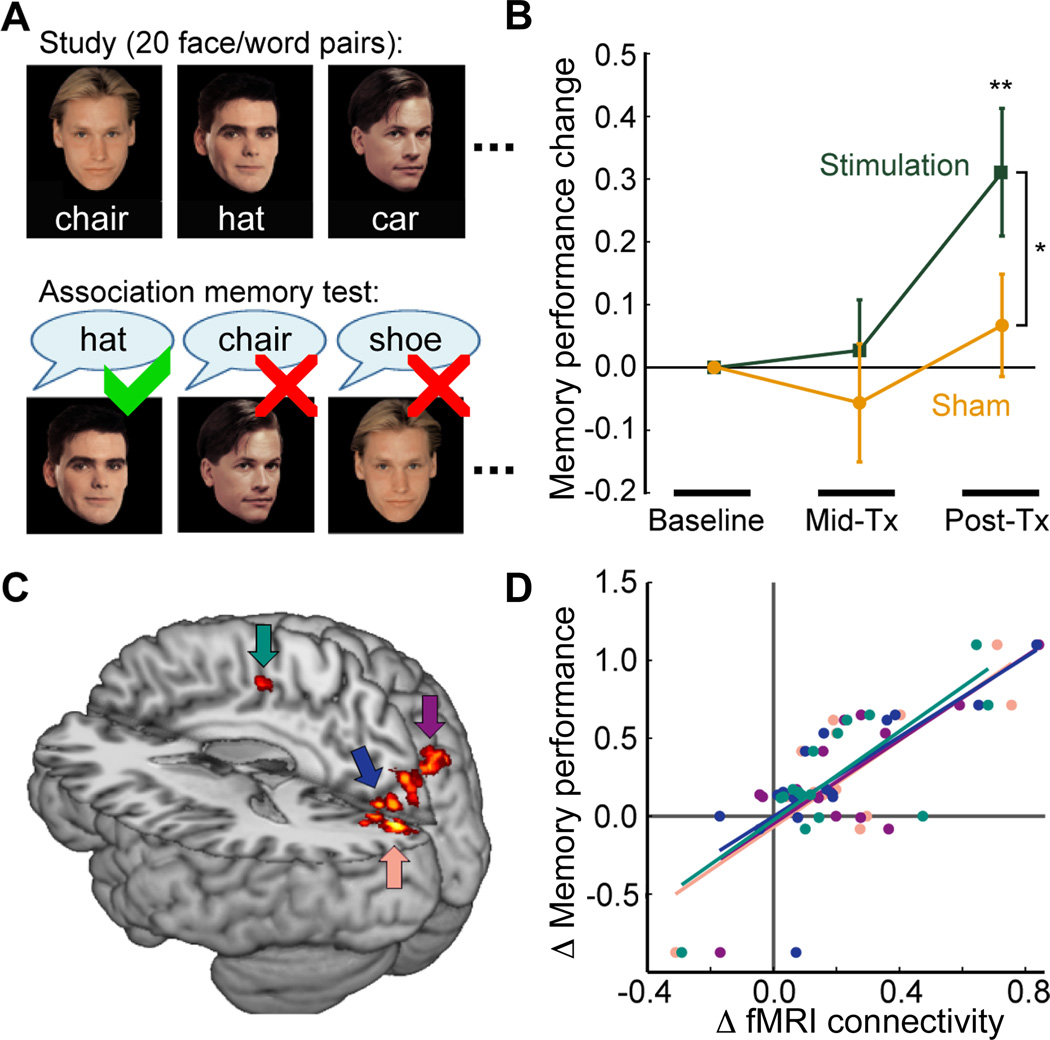
We next tested for corresponding changes in associative memory. Stimulation increased associative memory performance (face-cued word recall, Fig. 4A) from baseline to Post-Tx, (T(15) = 3.05, P = 0.008) whereas sham treatment caused no significant performance change (T(15) = 0.82, P = 0.425) (Fig. 4B). The increase in performance for baseline to Post-Tx was greater for stimulation than for sham (T(15) = 2.21, P = 0.043). Using regionally constrained correlation analysis (6), we found that baseline to Post-Tx changes in performance due to stimulation (relative to sham) correlated significantly with corresponding changes in fMRI connectivity with the hippocampal target for a portion of treatment-responsive brain regions (Fig. 4C–D). Subjects demonstrating larger stimulation-induced connectivity changes for these regions exhibited greater memory improvements. Targeted analysis of left lateral parietal cortex identified the same relationship for a portion of this region (6), but at sub-threshold size for the primary analysis. We administered a battery of additional cognitive tests (6) to assess selectivity of stimulation effects for associative memory. No such changes were observed on any of these tests (P value range = 0.33 to 0.99 for all pairwise Post-Tx versus baseline comparisons performed separately for each test).
Fig. 4. Stimulation-induced associative memory enhancement.
(A) Structure of the face-cued word recall test of associative memory, involving recall during test of words arbitrarily paired with faces at study (6). Different word-face pairs were used for each assessment. (B) Stimulation increased memory whereas sham did not. Mean performance change for each assessment is expressed as a proportion of baseline. (C) Subset of treatment-responsive regions that demonstrate significant correlation between stimulation-induced fMRI connectivity change with hippocampal targets and memory improvement. (D) Plot of memory improvement values (Post-Tx vs. baseline for treatment relative to sham) and corresponding values of fMRI connectivity increase with respect to hippocampal targets from each subject for the four areas indicated in (C). *P < 0.05 Stimulation versus Sham; **P < 0.01 versus zero.
A control experiment tested whether aforementioned stimulation effects could have resulted from nonspecific influences of above-threshold brain stimulation rather than targeted stimulation of cortical-hippocampal networks via lateral parietal cortex. Subjects receiving the same stimulation protocol to a primary motor cortex region that is not reliably included in cortical-hippocampal networks did not exhibit any reliable changes in cortical-hippocampal connectivity or associative memory performance (6) (Fig. S4).
These findings confirm the proposed role of cortical-hippocampal interactions in associative memory (1–4). Enhanced memory via neurosurgical (invasive) stimulation of entorhinal cortex (the primary input to hippocampus) has been reported (16), although effects were specific to the material studied during stimulation and network-level function was not tested. Our findings are thus novel in demonstrating persistent memory changes and in substantiating fMRI correlative evidence for cortical-hippocampal network involvement in associative memory (5).
Although effects of noninvasive stimulation on neurophysiology are not fully characterized, findings that resemble NMDA-receptor-dependent long-term potentiation (LTP) of hippocampal circuits (17, 18) have been observed using rTMS parameters similar to those reported here (13, 19). fMRI connectivity changes due to stimulation could reflect LTP-like effects throughout cortical-hippocampal networks (20). Indeed, changes were evident ~24 hours after stimulation (Post-Tx), indicating long-term plasticity. Alternatively, non-specific physiological effects (e.g., neuromodulatory, neurochemical, or other more global processes (13, 19)) and/or psychological factors (e.g., memory recall during fMRI, effort during memory testing, placebo effects, etc.) could have changed connectivity and memory. However, fMRI connectivity changes were remarkably specific for hippocampal targets (Fig. 2B–C), were significantly correlated with associative memory improvements (Fig. 4C–D), and did not occur when a motor-cortex region distinct from cortical-hippocampal networks was stimulated in the control experiment, providing strong evidence against these possible nonspecific influences. Additional research is required to determine whether other cortical-hippocampal networks can be modulated with similarly high specificity and to identify neurophysiological mechanisms.
Although memory-related processing by cortical-hippocampal networks is relevant for many cognitive domains (e.g., attention, language, executive control; (21, 22)), there were no stimulation effects on standardized measures of those domains. Notably however, the instruments were not designed to provide specificity to cortical-hippocampal network influences (6). Specialized tests could potentially be used to identify broader effects of stimulation on cognition. Stimulation-responsive regions and hippocampus are elements of a network that shows high interconnectivity even during periods of quiescence (i.e., the “default-mode” network (23)), further underscoring the potential broader influences of stimulation-induced changes on cognition.
Following hippocampal damage, residual tissue might retain function (24) and assume functions previously supported by damaged tissue (25). Further, cortical-hippocampal network dysfunction has been implicated in various memory disorders (26). The methods reported here potentially could be modified to treat memory disorders by targeting residual hippocampal tissue to improve impaired cortical-hippocampal networks.
Supplementary Material
Acknowledgments
Research was supported by award numbers P50-MH094263 from the National Institute of Mental Health and F32-NS083340 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Neuroimaging was performed at the Northwestern University Center for Translational Imaging facility supported by the Northwestern University Department of Radiology. All primary behavioral data are archived in paper and electronic format in the Laboratory for Human Neuroscience of the Northwestern University Feinberg School of Medicine. All primary MRI data are archived in the Northwestern University Neuroimaging Data Archive (https://nunda.northwestern.edu).
Footnotes
Supplementary Materials
Materials and Methods
Supplementary Text
Figs. S1 to S4
Tables S1 to S2
References (27–38)
References
- 1.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York City, NY: Oxford University Press; 2001. [Google Scholar]
- 3.Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci. 2011;15:310–318. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 5.Staresina BP, Cooper E, Henson RN. Reversible information flow across the medial temporal lobe: the hippocampus links cortical modules during memory retrieval. J Neurosci. 2013;33:14184–14192. doi: 10.1523/JNEUROSCI.1987-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Materials, methods and supplementary findings are available online as supplementary material on Science Online.
- 7.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 10.Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- 11.Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62:2232–2243. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Ther. 2012;133:98–107. doi: 10.1016/j.pharmthera.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci Biobehav Rev. 2012;36:1579–1596. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suthana N, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown TH, Chapman PF, Kairiss EW, Keenan CL. Long-term synaptic potentiation. Science. 1988;242:724–728. doi: 10.1126/science.2903551. [DOI] [PubMed] [Google Scholar]
- 19.Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013;16:838–844. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-Salvado E, Pallares V, Moreno A, Canals S. Functional MRI of long-term potentiation: imaging network plasticity. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130–20152. doi: 10.1098/rstb.2013.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shohamy D, Turk-Browne NB. Mechanisms for widespread hippocampal involvement in cognition. J Exp Psychol Gen. 2013;142:1159–1170. doi: 10.1037/a0034461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci. 2012;6:146. doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullally SL, Hassabis D, Maguire EA. Scene construction in amnesia: an FMRI study. J Neurosci. 2012;32:5646–5653. doi: 10.1523/JNEUROSCI.5522-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finke C, Bruehl H, Duzel E, Heekeren HR, Ploner CJ. Neural correlates of short-term memory reorganization in humans with hippocampal damage. J Neurosci. 2013;33:11061–11069. doi: 10.1523/JNEUROSCI.0744-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Joie R, et al. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer's and semantic dementia-targeted networks. Neuron. 2014;81:1417–1428. doi: 10.1016/j.neuron.2014.01.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.