Abstract
Purpose
To assess autonomic arousal associated with speech and nonspeech tasks in school-age children and young adults.
Method
Measures of autonomic arousal (electrodermal level, electrodermal response amplitude, blood pulse volume, and heart rate) were recorded prior to, during, and after the performance of speech and nonspeech tasks by twenty 7- to 9-year-old children and twenty 18- to 22-year-old adults.
Results
Across age groups, autonomic arousal was higher for speech tasks compared with nonspeech tasks, based on peak electrodermal response amplitude and blood pulse volume. Children demonstrated greater relative arousal, based on heart rate and blood pulse volume, for nonspeech oral motor tasks than adults but showed similar mean arousal levels for speech tasks as adults. Children demonstrated sex differences in autonomic arousal; specifically, autonomic arousal remained high for school-age boys but not girls in a more complex open-ended narrative task that followed a simple sentence production task.
Conclusions
Speech tasks elicit greater autonomic arousal than nonspeech tasks, and children demonstrate greater autonomic arousal for nonspeech oral motor tasks than adults. Sex differences in autonomic arousal associated with speech tasks in school-age children are discussed relative to speech-language differences between boys and girls.
Keywords: autonomic nervous system, autonomic arousal, speech production, oral motor development
Speaking requires the complex operation and coordination of neural networks underlying cognitive, linguistic, and motor processes. In addition, speakers monitor, evaluate, and correct their speech while anticipating listener evaluations (Levelt, 1989). It is not surprising, given the coordination of these complex processes, that speech production has been found to reliably increase autonomic arousal in adults (e.g., Heponiemi, Ravaja, Elovainio, Näätänen, & Keltikangas-Järvinen, 2006; Het, Rohleder, Schoofs, Kirschbaum, & Wolf, 2009; Kirschbaum, Pirke, & Hellhammer, 1993), with arousal levels for speech exceeding that of high-effort nonspeech tasks such as the Valsalva maneuver (Weber & Smith, 1990), mirror writing, or intelligence test items (Peters & Hulstijn, 1984).
Although they typically elicit less autonomic arousal than speech tasks, high-effort nonspeech tasks are also effective elicitors of autonomic arousal. The Valsalva maneuver, which involves motor subsystems that are shared with speech tasks, reliably elicits autonomic arousal (Delius, Hagbarth, Hongell, & Wallen, 1972; Weber & Smith, 1990). Intelligence test items and other nonspeech tasks that require cognitive–linguistic processing are also effective at eliciting autonomic arousal (e.g., Peters & Hulstijn, 1984; Renaud & Blondin, 1997; Tulen, Moleman, Van Steenis, & Boomsma, 1989). Speech may elicit more autonomic arousal than these nonspeech tasks because it combines these arousal-eliciting motor and cognitive–linguistic elements for successful execution.
When does this speech-related autonomic arousal develop? Because childhood is a time of considerable speech and language growth, as well as maturation of the motor system, it follows that the relationship between speech production and autonomic arousal might differ between children and adults. The evidence as to whether speech is associated with higher levels of autonomic arousal in children than in adults is mixed. Studies that have used speech tasks to elicit autonomic arousal in children (Schmidt, Fox, Schulkin, & Gold, 1999; Stroud et al., 2009) have reported fewer indicators of arousal than similar studies of adults (Gonzolez-Bono et al., 2002; Heponiemi et al., 2006; Het et al., 2009; Parente, Garcia-Leal, Del-Ben, Guimarães, & Graeff, 2005). For example, Het et al. (2009) reported increases in salivary alpha amylase, a metabolic measure of sympathetic arousal, and heart rate (HR) during a speech task for adults. However, Stroud et al. (2009) found, using the same two measures, only HR increases with a similar speech task in 7- to 12-year-old children. It is important to note that none of these studies that showed more consistent autonomic arousal in adults directly compared speech-related autonomic arousal in adults and children.
When Kleinow and Smith (2006) directly compared autonomic arousal in adults and children, school-age children demonstrated elevated sympathetic arousal compared to adults, as evidenced by decreased peripheral blood volume during sentence repetition tasks. These findings suggest that speech-related sympathetic arousal decreases with age as individuals develop greater proficiency with speech tasks. Evidence indicates that language and speech motor proficiency are not fully developed until the late teens (for a review see Nippold, 2000; Walsh & Smith, 2002). Reflecting the continued development of speech motor skills, oral motor coordination continues to be more variable in children than adults through at least age 16 (Walsh & Smith, 2002). With regard to language skills, syntax and semantics also undergo development through the adolescent years (Nippold, 2000). Because of their still-maturing speech-language skills, children may expend more effort in speaking, resulting in higher speech-related autonomic arousal than is seen in adults. However, given the mixed evidence in the literature, further direct comparison between adults and children would help clarify scholars’ understanding of age differences in speech-related arousal.
Because there are notable sex differences in speech-language development, it is important to consider potential sex differences in autonomic arousal related to speech production. A meta-analysis of 165 studies of preschool-age children through adults indicated that speech production abilities are superior in girls compared to boys of the same age (Hyde & Linn, 1988). For example, Smit, Hand, Freilinger, Bernthal, and Bird (1990) found that girls lead boys in speech sound accuracy between the ages of 3;0 (years;months) and 6;0, acquiring correct production of 11 speech sounds earlier than boys. Sex differences in the consistency of oral motor coordination during repeated sentence productions appear in 4- to 5-year-old children, with boys lagging girls, but are not evident in school-age children (Sadagopan & Smith, 2008; Smith & Zelaznik, 2004; Walsh & Smith, 2002). In addition to sex differences in speech production skills, early language acquisition under age 3 in boys lags behind that of girls (Bouchard, Trudeau, Sutton, Boudreault, & Deneault, 2009). Thus, sex differences in speech-language skills are evident during the first 4 to 5 years and appear to become less evident as children develop.
Also relevant to sex differences in speech development is the fact that boys are more prone to speech-language disorders, such as specific language impairment (Shriberg, Tomblin, & McSweeny, 1999) and persistent developmental stuttering (Bloodstein & Bernstein Ratner, 2008). Considering the evidence of sex differences in speech-language development, speech tasks may be more challenging for boys and result in elevated speech-related autonomic arousal compared to girls. However, sex differences in speech-related arousal have not been assessed in children. This is an important area of inquiry, given that increased levels of arousal could contribute to the development and/or persistence of disorders, such as stuttering (Walden et al., 2012).
The nature of speech-related arousal is also important to consider because autonomic arousal has been shown to alter subsequent speech output (Caruso, Chodzko-Zajko, Bidinger, & Sommers, 1994; Kleinow & Smith, 2006). This may be because physiological processes underlying speech—including respiratory, laryngeal, and oral motor processes—are influenced by sympathetic activity (Weber & Smith, 1990; Zimmermann, 1980). If the speech-language productions of children, who are undergoing marked growth of communicative skill, are affected by heightened autonomic arousal, then arousal could contribute to the development of speech-language disorders. In adults who stutter, autonomic arousal prior to speech tasks appears to have an stronger effect on speech unit duration than in typically fluent adults (Caruso et al., 1994). This arousal has also been found to relate to the occurrence of subsequent speech disfluencies in adults who stutter (Caruso et al., 1994; Weber & Smith, 1990). High levels of autonomic arousal could have an important impact on the speech-language systems of children, whose developing skills may be more vulnerable to disruption. Therefore, investigating how speech-related autonomic arousal develops could aid in better understanding the development of speech-language disorders.
To gain insight into the development of autonomic arousal associated with speech and nonspeech tasks, we adapted tasks from Weber and Smith (1990) for use with typically speaking adults and school-age children. Nonspeech tasks were included because they provide a good contrast to the speech conditions, requiring oral motor activity but less cognitive–linguistic involvement. We also varied the difficulty of the speech and nonspeech tasks to better understand how task requirements contribute to autonomic arousal. To obtain a more complete picture of the activity of the autonomic nervous system (ANS), we recorded peripheral blood flow (i.e., finger blood pulse volume [BPV] and heart rate [HR]) and electrodermal activity (EDA; i.e., tonic electrodermal level [EDL] and phasic electrodermal response [EDR] amplitude). All four measures have been used in similar studies that have compared speech- and nonspeech-related autonomic arousal (Peters & Hulstijn, 1984; Weber & Smith, 1990). These measures reflect both rapidly changing (BPV, HR, and peak EDR amplitude) and background, steady-state autonomic activity (EDL). Collectively, these measures also reflect activity from the sympathetic and parasympathetic branches of the ANS, given that HR changes result from coactivation of both branches. In contrast, EDA and peripheral BPV reflect the activity of only the sympathetic branch (Jennings, Tahmoush, & Redmond, 1980; Venables & Christie, 1980). Using these measures and tasks, we designed the present experiment to assess the difference in autonomic arousal between speech and nonspeech tasks and to investigate whether age and sex differences in autonomic arousal are associated with these tasks.
Method
Participants
Twenty adults (10 women) and 20 children (10 girls) participated in this study. Adults were undergraduate students age 18 to 22 years (range: 19;0–22;8, M = 19;11, SD = 1;3). Children were ages 7 to 9 years (range: 7;0–9;11, M = 8;5 years, SD = 0;9). The selected autonomic variables have been shown to be easily measured in children in this age range (Schmidt et al., 1999; Stroud et al., 2009), and this is a period when children are still considerably less mature than adults in speech motor and language skills (Nippold, 2000; Walsh & Smith, 2002). All participants spoke Standard American English as their first and primary language.
Participants exhibited typical speech, language, and hearing abilities and had no reported history of speech, language, or hearing problems. Children scored within normal limits on expressive language subtests of the Clinical Evaluation of Language Fundamentals—Fourth Edition (Semel, Wiig, & Secord, 2003). All participants demonstrated normal oral motor function on the Oral Speech Mechanism Screening Examination, Third Edition (St. Louis & Ruscello, 2000) and passed a bilateral pure-tone hearing screening at 500, 1000, 2000, 4000, and 6000 Hz at 20 dB HL. Handedness was determined using an abbreviated handedness inventory (five tasks adapted from Oldfield, 1971) so that all physiological recordings could be made using the nonpreferred hand, which was less likely to be moved by participants, resulting in potential artifact.
According to self-report for the adults and parent report for the children, no participant had a history of heart disease, hypertension, attention-deficit disorder, or neurological problems, conditions that could potentially affect autonomic recordings. The speech, language, and hearing screening lasted approximately 90 min for the children and 20 min for the adults.
Experimental Tasks
The experimental tasks, modified from those of Weber and Smith (1990), included two nonspeech tasks (jaw open–close and Valsalva maneuver) and two speech tasks (sentence production and narrative production) designed to elicit a range of autonomic activity. Similar tasks used by Weber and Smith elicited varying levels of autonomic arousal, with a jaw open–close task eliciting the smallest autonomic responses and the spontaneous speech task, which best corresponds to our narrative production task, eliciting the largest ANS responses (Heilmann, Nockerts, & Miller, 2010). Participants were seated 5 ft from a computer monitor, which displayed instructions for nonspeech tasks, picture stimuli for speech tasks, and the word REST paired with a visually interesting picture for each intertrial rest period. For all tasks described below, trials were followed by a 20-s rest period. At the completion of the series of trials for each condition, participants rested 30 s before receiving instructions for the next condition.
JAW task
Each participant was instructed to move his or her jaw up and down at a comfortable rate when the phrase “Open and Close Jaw” appeared on the computer screen. When the word REST appeared on the screen, the participant was instructed to stop and sit quietly while looking at the picture on the screen until the instructions appeared again. Jaw movements continued for approximately 5 s per trial, followed by a period of 20 s before the next trial of jaw movements started. After viewing a demonstration by the experimenter during precondition instructions, six trials of jaw movements were completed, and data were recorded continuously. The JAW condition was included to serve as a low-arousal, nonspeech task (Weber & Smith, 1990).
VAL task
In this condition, participants were instructed to take a deep breath and hold it while simultaneously trying to force the air out. This is called the Valsalva maneuver, and it was demonstrated by the experimenter during the precondition instructions. Participants initiated each maneuver after seeing the phrase “Hold Breath” on the computer screen and continued to hold their breath for 5 s, until the word REST appeared on the screen, prompting them to stop. Six trials of Valsalva maneuvers were completed, and data were recorded continuously. The VAL condition was included as a high-arousal, nonspeech task because there is evidence from sympathetic nerve recordings that it consistently produces increased sympathetic activity (e.g., Delius et al., 1972).
SENT task
Participants were instructed to describe three similar pictures in a row, resulting in three simple sentence productions per trial. The experimenter demonstrated an example during precondition instructions. Sentences were elicited by a series of pictures of an object or animal in a box or barn (e.g., “The cow is in the barn”). Participants began the sentence productions when the first picture stimulus was presented and stopped after the third picture stimulus when the word REST appeared on the monitor. Participants completed 12 trials of sequences of three simple sentence productions. This task was believed to be a less challenging speech task because it required a simple, active, declarative sentence structure.
NARR task
Participants were instructed to describe detailed pictures from the text-free storybook Frog Where Are You? (Mayer, 1969). Each picture and the associated narrative served as one trial. The experimenter demonstrated an example during precondition instructions. After participants completed their responses for each trial, the word REST appeared on the computer screen for the 20-s rest period until another picture from the storybook was presented. Participants completed 12 trials of the narrative productions. This was included as a more challenging speech task, because narratives require generative speech and tend to elicit more complex syntax (e.g., past tense and causal inferences; Heilmann et al., 2010).
As noted earlier, each of the four experimental tasks was preceded by a 30-s rest period. A 15-s portion of this rest period was selected to obtain reference autonomic levels, or baselines, for the data collected in the trial-related intervals. These between-condition rest intervals ideally would have been much longer so that the participants’ ANS levels could return to what has been referred to as “rest, baseline” levels in previous work (Weber & Smith, 1990). For example, Weber and Smith (1990) allowed participants to rest for several minutes between conditions so that the ANS signals could resolve (e.g., the experimenter could observe tonic EDA slowly drift back to a much lower rest level compared to that observed in the active trials). This strategy simply was not feasible with our young participants given the already-lengthy experimental protocol. This is an important experimental detail because it means that our task-related ANS measures are expressed relative to higher levels of baseline arousal compared to the much lower, resting baseline levels used by Weber and Smith. For each rest period, participants were asked to sit quietly and look at an interesting still image, consisting of abstract patterns of colors, on the monitor. Experimental conditions were presented in order of expected increase in autonomic arousal, JAW, VAL, SENT, and NARR, based on difficulty of the task and past results indicating that speech tasks are more arousing than nonspeech tasks for adults (Weber & Smith, 1990). The presentation of nonspeech tasks prior to speech tasks is also consistent with Peters and Hulstijn’s (1984) study.
Autonomic Signals
We assessed EDA using a Biopac system (Model MP150WS, GSR 100C) with electrodes attached to the thenar and hypothenar eminences of the palm of the non-dominant hand (Dawson, Schell, & Filion, 2007). EDA signals were recorded with a gain of 10 μS/V and were low-pass-filtered at 1 Hz with a third-order Butterworth IIR filter. EDA and all other autonomic signals were recorded with a sampling rate of 2500 Hz. The hand and arm were stabilized by having the participant rest them on a splint. BPV was obtained using an infrared photoelectric plethysmographic transducer (Biopac Model MP150WS, PPG100C) with a gain of 100. The BPV signal (from which pulse period was measured and HR calculated) was bandpass filtered at 0.5–3 Hz with a 100-order FIR filter. The transducer was placed over the distal phalange of the middle finger and held in place with a Velcro strap. The transducer was left in place throughout the entire experimental session so that any deformation of the vascular bed remained constant.
Kinematic, Audio, and Video Recordings
Lower lip/jaw movements were transduced in the superior-inferior dimension using a resistive-wire strain gauge system (Barlow, Cole, & Abbs, 1983) to determine the onset and offset of task performance. The strain gauge system was mounted on an adjustable arm and affixed to an appropriately sized bicycle helmet fitted to the participant’s comfort and so that the cantilever of the system could be positioned next to and in parallel with the lower lip. The cantilever was threaded through a small bead attached to the lower edge of the vermilion border of the lower lip at midline. Participants’ speech signals were audio recorded, and, along with movement signals, the audio recordings were used offline to determine the intervals corresponding to 5-s periods prior to, during, and after the participants’ task performance. The output signals from the lip/jaw motion tracking system and audio amplifier were recorded along with the autonomic signals by the Biopac system and Acq 3.8.2 software for offline analysis. The audio signal was sampled at 10,000 Hz and the jaw movement signals at 2500 Hz. All signals, including ANS, kinematic, and audio, were digitized with the Biopac analog to digital system. Video recordings of each participant’s face and upper body were made and used later to make judgments of speech fluency and to exclude periods contaminated with movement artifact.
Procedure
Participants were asked to refrain from caffeine or alcohol consumption for at least 3 hr before participation. Experimental sessions were not scheduled within 3 hr of stressful events such as college exams or heavy physical activity (Tulen et al., 1989). Each experimental session lasted approximately 40 min. All recording sessions took place between 1:00 p.m. and 6:00 p.m. to control for potential effects of diurnal rhythms on electrodermal responding (Dawson et al., 2007).
After completing screening procedures, participants washed their hands with soap and water (Dawson et al., 2007). EDA electrodes were applied, and the BPV and lip/jaw motion transducers were attached. The general procedures and experimental tasks were explained to and demonstrated for the participants. The room temperature was maintained at 68–71° F for each experimental session.
Signal Analysis
We imported all physiological signals into MATLAB (Mathworks) and down-sampled them from 2,500 to 250 samples/s. Tonic EDL was measured directly from the EDA signal. Phasic EDR was derived from the EDA signal by filtering (i.e., direct-form II, second-order, Chebyshev high-pass filter, cutoff frequency of 0.07 Hz, pass-band ripple of 0.5 dB) in MATLAB. BPV and HR were measured using an automatic peak detection program in MATLAB. All measures and peaks were verified by the experimenter. The experimenter viewed the data in a graphic user interface in MATLAB and selected a 15-s baseline in the middle of the 30-s recording occurring immediately prior to each experimental condition. As shown in Panel A of Figure 1, precondition measures were taken for three 5-s nonoverlapping samples from the pretrial rest period, and the measures from the three samples were averaged to produce the reference values for each condition for EDL, BPV, and HR.
Figure 1.
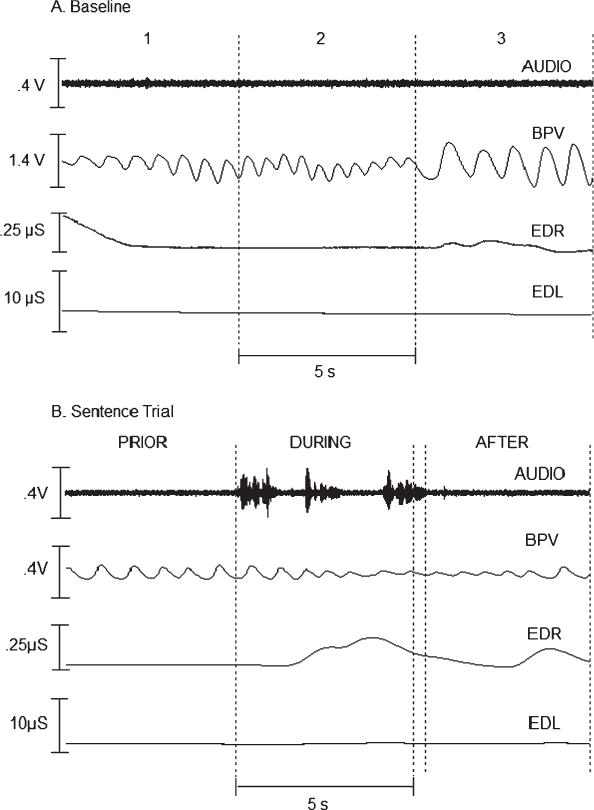
Records of autonomic activity for a 7-year-old boy during (Panel A) baseline measurement and (Panel B) a single trial of the simple sentence task. Traces in each panel, from top to bottom, are the audio (AUDIO), blood pulse volume (BPV), phasic electrodermal response (EDR), and tonic electrodermal level (EDL) signals.
For each autonomic signal within each experimental task, measurements were taken for three intervals: (a) 5 s prior to onset of the task, (b) the first 5 s during the task, and (c) 5 s immediately after the task. These intervals are illustrated for one speech trial in Panel B of Figure 1. We selected three intervals to obtain measures of autonomic activity that were related to task (a) preparation, (b) performance, and (c) postperformance (Weber & Smith, 1990). The onset of task performance for each condition was determined from the jaw motion and/or acoustic signal(s). The end of a trial was taken as the cessation of the jaw movement and/or acoustic signal(s). Speech task performance intervals were often longer than 5 s; therefore, the intervals during and after a task were separated by whatever portion exceeded 5 s. This is illustrated in Panel B of Figure 1. Because they were extemporaneous, NARR responses were in rare cases shorter than 5 s. Following the practice of Weber and Smith (1990), we excluded from the analysis those shorter than 2.5 s. The measures we made are described in the following sections.
BPV was based on peak-to-peak amplitude (in volts) of pulse cycles. We calculated BPV using an automatic peak-picking algorithm. For intervals containing very small pulse cycle changes not identified by the automatic algorithm, pulse maxima and minima were identified by the experimenter. The mean peak-to-peak amplitude was calculated for each prior, during, and after interval. BPV for experimental tasks was expressed as a percentage change from the mean baseline peak-to-peak BPV (hereafter, BPV%bl). A decrease in pulse volume indicates peripheral blood vessel vasoconstriction produced by an increase in sympathetic activity (Jennings et al., 1980).
We computed HR, in beats per minute, from the BPV signal using the automatic peak-picking algorithm. As described above for the BPV measure, records with small peaks were identified by visual inspection. Mean HR was computed for each prior, during, and after interval and was expressed as percentage change from the mean baseline HR (hereafter, HR%bl). Changes in HR reflect activity in both branches of the ANS (Pick, 1970). Increases in HR indicate increased sympathetic activity and/or parasympathetic withdrawal, whereas decreases in HR indicate increased parasympathetic activity and/or decreased sympathetic activity.
Electrodermal response (EDR) amplitude was quantified as the peak-to-peak change in phasic EDA, measured in μS (hereafter, EDRpk). EDRs often stretched across the 5-s intervals, preventing them from being measured discretely for each prior, during, or after interval. Thus, the largest amplitude EDR was measured from the onset to peak of the response for each trial (across prior, during, and after intervals) using an automatic peak- and onset-picking algorithm. A higher amplitude EDRpk signifies increased sweat secretion resulting from increased sympathetic activity (Dawson et al., 2007).
The mean tonic EDL was measured in μS for each prior, during, and after interval, with the mean baseline tonic EDL subtracted (hereafter, EDL-bl). Greater EDL-bl values signify greater tonic levels of sweat secretion resulting from increased sympathetic activity (Dawson et al., 2007).
Statistical Method
We analyzed the data to assess the main research questions related to condition effects and age and sex differences in autonomic measures for the experimental conditions. We used the general linear model procedure in SPSS 18.0 with a mixed-model analysis of variance (ANOVA) with condition (JAW, VAL, SENT, or NARR) and interval (prior, during, or after) as within-subject factors and age (children or adults) and sex serving as between-groups factors for three of the dependent variables (EDL-bl, BPV%bl, and HR%bl). We applied the same ANOVA design to EDRpk, with the interval factor left out given that EDRpk could not be measured separately for each time interval. Main effects of intervals were examined at a preliminary level to verify that the experimental conditions effectively elicited autonomic arousal.
To simplify the interpretation of this complex analysis, we did not consider interval interactions but instead interpreted findings related to the main research questions. Therefore, the results of interest for the main and subsequent analyses were condition and group main effects (age and sex) as well as interactions among condition, age, and sex. We followed up significant condition main effects by creating a combined speech variable and comparing it to a combined nonspeech variable, retaining interval, sex, and age factors in the model.
Finally, we wished to examine the possible contribution of differences within speech and nonspeech categories to significant condition main effects. We did this by repeating the original ANOVA design twice: (a) with only the two speech tasks (SENT and NARR) for the task condition factor and (b) with only the two nonspeech tasks (JAW and VAL) for the task condition factor.
Because time series autonomic measures are known to violate the sphericity assumption, we used Huynh and Feldt’s (1976) estimator of Box’s ε to adjust the degrees of freedom for F tests on all within-subject variables. Alpha levels were set at p < .05. Only the first five trials of the tasks were used to compute the means in these analyses so that comparisons between conditions could be made and because later trials showed evidence of considerable adaptation in the autonomic responses.
Results
Interval Effects
The means and standard errors for the three measures that were taken across the prior, during, and after intervals (EDL-bl, BPV%bl, and HR%bl) are displayed in Figure 2. Note that the task-related arousal levels at times fall below the precondition baseline measures (e.g., for the JAW task, the EDL is lower than the EDL in the baseline, which is the zero reference value indicated by the dotted line). This is likely due to the fact that our intercondition rest periods were very brief and because of autonomic habituation during later trials of each condition. Habituation is clearly visible in the continuous autonomic data reported over long periods of task performance by Peters and Hulstijn (1984). Changes in autonomic activity occurred in each task, but the time course and degree of autonomic arousal differed across conditions.
Figure 2.
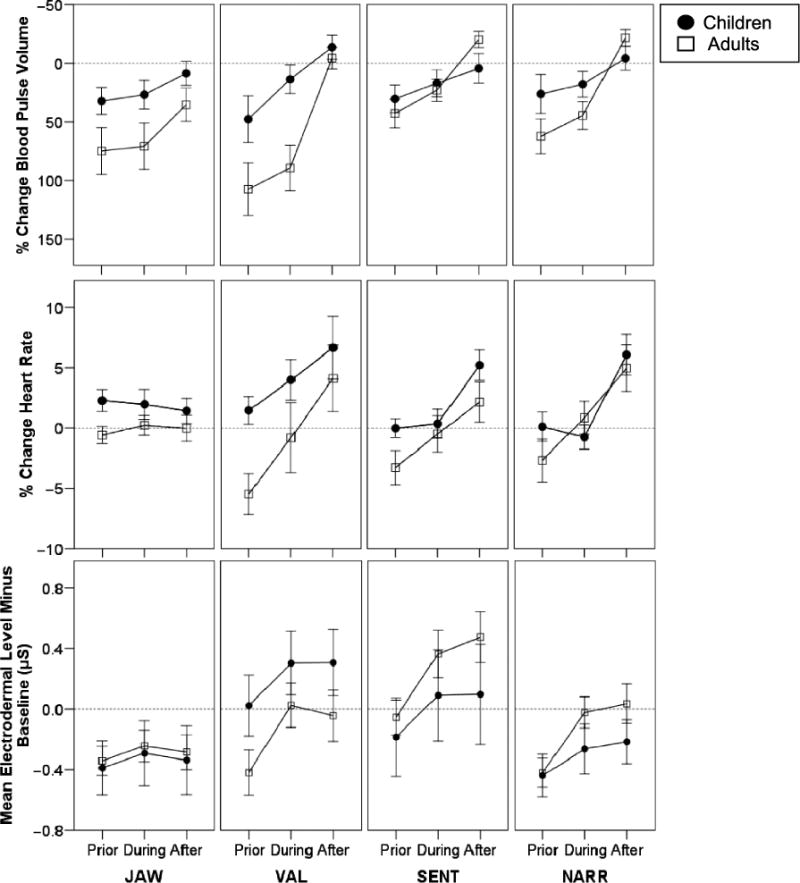
Means and standard error bars for autonomic responses corresponding to intervals prior to, during, and after each of the experimental tasks are plotted for children and adults. Percentage change in BPV is plotted with negative percentages going upward. Thus, for each of the measures, increased arousal is plotted by higher points on the graphs. JAW = jaw movement task; VAL = Valsalva maneuvers; SENT = sentence task; NARR = narrative task.
Repeated-measures ANOVAs revealed large, significant main effects of interval for all three variables with interval data: BPV%bl, F(2, 72) = 65.9, p < .001, ; HR%bl, F(2, 72) = 24.8, p < .001, ; and EDL-bl, F(2, 72) = 67.0, p < .001, . These interval effects indicated that the tasks effectively elicited autonomic arousal. For all three measures, the highest autonomic arousal was observed in the interval after task performance.
Group Comparisons and Condition Main Effects
There were significant main effects of age group for the two autonomic measures related to peripheral blood flow: BPV%bl, F(1, 36) = 4.3, p < .05, , and HR%bl, F(1, 36) = 5.2, p < .05, . Children had lower BPV%bl and higher HR%bl than adults, indications of higher auto-nomic arousal in children compared to adults for both measures. There were no significant main effects of sex for any of the measures.
Relevant to our major aim to assess the differences between speech and nonspeech task arousal, the mixed-model ANOVAs revealed statistically significant main effects of task condition for three of the four autonomic measures: BPV%bl, F(3, 108) = 3.0, p < .05, ; EDL-bl, F(3, 108) = 3.2, p < .05, (see Figure 2 for graphs of these two measures); and EDRpk, F(3, 108) = 24.2, p < .05, (see Figure 3 for a graph of EDRpk).
Figure 3.
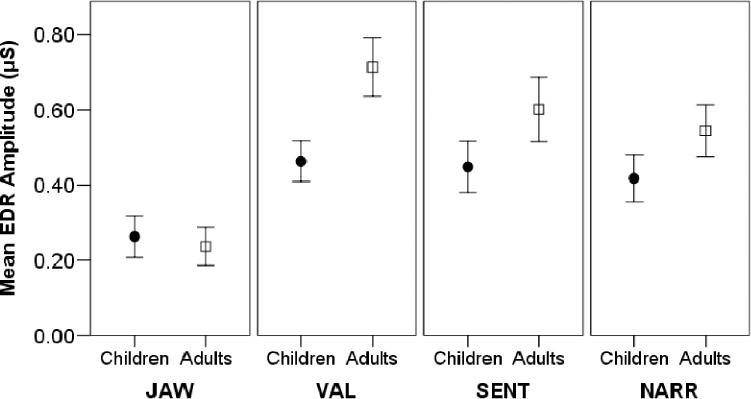
Means and standard error bars for peak electrodermal response amplitude are plotted for children and adults.
Speech- Versus Nonspeech-Related Arousal
To assess whether these condition main effects were due to overall differences between speech and nonspeech tasks, we averaged JAW and VAL data to create a non-speech variable and SENT and NARR to create an equally weighted speech variable. We then used mixed-model ANOVAs to compare speech and nonspeech for BPV%bl, EDL-bl, and EDRpk. Significant differences were found between speech and nonspeech for BPV%bl, F(1, 36) = 7.66, p < .01, , and EDRpk, F(1, 36) = 4.88, p < .05, . For both measures, autonomic arousal was higher for speech compared to nonspeech conditions.
For BPV%bl, there was a Condition × Age interaction, F(1, 36) = 5.30, p < .05, , indicating that adults demonstrated a larger difference between speech and nonspeech conditions than children. ANOVAs examining speech and nonspeech separately for age differences revealed no significant main effect of age for BPV%bl for speech, p > .1, but significantly lower mean nonspeech BPV%bl (greater vasoconstriction) for children than for adults, F(1, 36) = 7.26, p < .05, ; that is, children’s mean BPV%bl indicated greater vasoconstriction than adults’ in the nonspeech conditions but levels similar to adults’ in the speech conditions.
Nonspeech Condition Comparisons
In addition to speech versus nonspeech differences, condition main effects from the initial ANOVAs may have been due to differences between the two nonspeech conditions, JAW and VAL. To investigate this possibility, we repeated the above ANOVAs for BPV%bl, EDL-bl, and EDRpk with only JAW and VAL included. For BPV%bl and EDL-bl, there were no significant main effects of condition for JAW or VAL (p > .05). However, EDRpk was significantly higher for VAL than for JAW, F(1, 36) = 97.2, p < .001, . There was also a Condition × Age interaction for EDRpk, F(1, 36) = 16.4, p < .001, , indicating that adults, compared to children, had a larger difference in peak EDR amplitude between VAL and JAW. Follow-up t tests indicated no age group difference for JAW (p > .05) but did indicate that adults had significantly greater EDRpk than children for VAL, t(38) = −2.7, p < .05. Thus, the adults’ larger difference in EDRpk between JAW and VAL was due to greater sympathetic arousal than children in the VAL condition.
Speech Condition Comparisons
The next step was to assess whether differences between the two speech tasks contributed to the main effect of condition. Similar to the above procedure with the non-speech conditions, we repeated the ANOVAs for BPV%bl, EDL-bl, and EDRpk with only SENT and NARR included. There were no statistically significant main effects of condition for BPV%bl and EDRpk (p >.05), although EDL-bl was marginally higher for SENT than for NARR, F(1, 36) = 4.1, p = .05, .
There was, however, a significant three-way Condition × Age × Sex interaction for BPV%bl, F(1, 36) = 5.8, p = .02, . This interaction was particularly relevant to our major aim of examining age and sex differences in speech-related arousal, so we conducted the mixed-model ANOVAs with each age group separately. These analyses indicated a statistically significant Condition × Sex interaction for children, F(1, 16) = 7.3, p = .015, , but not for adults (p > .10). In Figure 4, the BPV%bl data are plotted for the children, illustrating that although boys maintained similar levels of BPV%bl from the first, more simple sentence production task (SENT), to the second, more open-ended narrative task (NARR), girls demonstrated a marked increase in BPV%bl (i.e., decrease in vasoconstriction and sympathetic arousal) for the NARR task.
Figure 4.
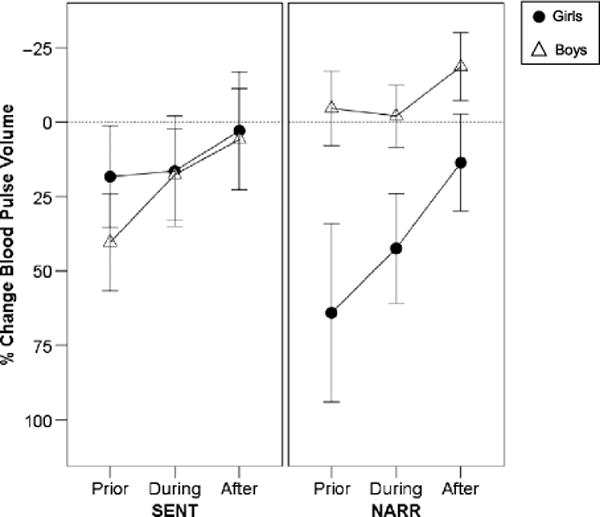
Means and standard error bars for autonomic responses for intervals prior to, during, and after each of the experimental tasks are plotted for girls and boys. Percentage change in BPV (from baseline) is plotted with negative percentages going upward so that increased arousal is plotted by higher points on the graphs.
Discussion
The purpose of the current study was to assess whether autonomic arousal associated with speech and nonspeech task performance differs and whether there are differences in autonomic arousal for these tasks in 7- to 9-year-old children, as compared to young adults. There were three main findings. First, mean levels of autonomic arousal were higher for speech compared to nonspeech tasks, based on peripheral BPV levels and EDR peak amplitude. Second, children demonstrated greater relative arousal, based on BPV, for nonspeech tasks than adults but similar arousal levels for speech tasks as adults. Third, there were sex differences in speech-related arousal for children but not adults. Specifically, autonomic arousal remained high for school-age boys when completing the second, more open-ended speaking task, compared to the first, simple sentence task. In comparison, school-age girls and young adults demonstrated lower autonomic arousal for the second, presumably more demanding open-ended speech task. We discuss these three findings below.
Autonomic Arousal in Speech Versus Nonspeech Tasks
Previous work indicates that adults demonstrate greater autonomic arousal during speech tasks compared to nonspeech tasks, even when the nonspeech tasks included the Valsalva maneuver (Weber & Smith, 1990), mirror writing, or intelligence test items (Peters & Hulstijn, 1984). Consistent with these findings, the current study indicates that adults and children demonstrate greater autonomic arousal, indexed by BPV and EDR amplitude, for the speech tasks compared to the nonspeech tasks. This finding of greater arousal in speech compared to nonspeech tasks was somewhat less consistent across measures for this study than for Weber and Smith’s (1990) findings with adults. Speech tasks in the current study may not have elicited as strong a response because the speech topics were less emotional and personal in nature than those used by Weber and Smith.
The measures used in this study provide information about the phasic and tonic elements of autonomic arousal for these speech and nonspeech tasks. The amplitudes of phasic EDRs (EDRpk) for speech tasks were greater than for nonspeech tasks, but were not significantly different between speech and nonspeech tasks for the tonic responses (EDL-bl). This may reflect the rapidity of cognitive-linguistic and oral motor planning and execution required for speech, resulting in a faster sympathetic response. Furthermore, although the Valsalva maneuver, the high-arousal nonspeech task, requires a high level of physical effort resulting in strong sympathetic outflow (Delius et al., 1972), it does not equal speech’s requirements for oral motor speed and precision.
The measures also provide information about the relative contribution of sympathetic and parasympathetic arousal for these speech and nonspeech tasks. BPV and EDR amplitude, the measures differentiating speech and nonspeech in this study, indicate activation of the sympathetic branch of the ANS (Jennings et al., 1980; Venables & Christie, 1980). In contrast, HR measures, which were not found to be different across conditions, result from coactivation of the sympathetic and parasympathetic branches of the ANS. Thus, mean HR may not have been higher for speech compared to nonspeech tasks because of parasympathetic activation, which reduces HR. These results may indicate that the parasympathetic branch effectively regulated the sympathetic arousal that speech elicited.
Overall, the findings strengthen the evidence that speech is a powerful elicitor of sympathetic arousal, compared to nonspeech oral motor tasks. The processes that support speech—including, but not limited to, cognitive-linguistic planning and oral motor execution—likely contribute to this arousal. In contrast, nonspeech oral motor tasks do not require the cognitive-linguistic element, which is known to elicit autonomic arousal absent overt speech requirements (e.g., Peters & Hulstijn, 1984; Renaud & Blondin, 1997; Tulen et al., 1989).
Age Differences in Speech Versus Nonspeech Autonomic Arousal
We observed some notable age effects when we compared autonomic variables across speech and nonspeech tasks. Children demonstrated higher overall HR and lower BPV relative to baseline than adults for the nonspeech tasks. It is important to note that the use of a baseline reference for these measures allowed us to examine task-related differences as opposed to the expected age-related physiological differences (e.g., higher overall HR in children than adults). This finding of higher baseline-referenced HR and lower BPV for the nonspeech tasks may reflect how these novel oral motor tasks taxed the relatively less mature oral motor systems of children (Smith & Zelaznik, 2004), resulting in greater autonomic arousal. This interpretation is in line with normative data indicating lower speed and accuracy of nonspeech oral motor performance in children compared to adults (St. Louis & Ruscello, 2000).
One exception to this finding was that adults demonstrated higher amplitude peak EDRs than children during one of the nonspeech tasks, the Valsalva maneuver. Taken together, these findings provide clues about sympathetic and parasympathetic activation in this task. EDR amplitude findings, which reflect only sympathetic branch activation, indicate that sympathetic drive was stronger in adults than in children. Adults’ lower mean HR during the Valsalva maneuver suggests that this strong sympathetic response was regulated by a strong parasympathetic response, which acted to rapidly bring HR back down. This combination of findings indicates that both adults and children have strong sympathetic responses to the Valsalva task but that parasympathetic activation is stronger in adults. This supposition is consistent with previous findings of increasing HR variability as children age to young adulthood (Kazuma, Otsuka, Wakamatsu, Shirase, & Matsuoka, 2002; Nunan, Gavin, Sandercock, & Brodie, 2010). Increased HR variability (i.e., beat-to-beat changes in HR) indicates that the parasympathetic branch is active in down-regulating sympathetic drive (Berntson, Quigley, & Lozano, 2007). Thus, the current findings support previous findings that the ANS, in particular the parasympathetic branch, functions differently between adults and children.
For speech tasks, overall levels of autonomic arousal were not significantly different between adults and children. This contrasts with the findings of a study that also directly compared adults and children performing speech tasks (Kleinow & Smith, 2006). This variance in findings may be due to important differences in the speech tasks used. In the current study, the sentences (e.g., “The cow is in the barn”) were shorter, syntactically much simpler, and less variable in structure than many of Kleinow and Smith’s (2006) targets (e.g., “The birds that saw butterflies played by the pond”). Therefore, the speech targets in the current study may have been too short and simple to tax the speech planning and production systems in children more than adults. For the narrative condition of the current study, children formulated speech spontaneously. With the freedom to produce any utterance relevant to the storybook picture, it is possible that children adjusted the linguistic and motoric complexity of their utterances to match their skills. In contrast, the children in Kleinow and Smith’s study produced a high level of linguistic complexity in many of the sentence targets, which changed in structure throughout the experiment. These task requirements might have been a greater challenge than what the children would produce spontaneously. Thus, it may be that speech-related autonomic arousal is greater only in children when the linguistic difficulty of the speech targets reaches a particular threshold.
Sex Differences in Speech-Related Arousal for Children
Our two speech tasks were designed to require different levels of effort. We thought that the narrative task, like Weber and Smith’s (1990) spontaneous speech task (i.e., responding to questions from the experimenter), would elicit greater autonomic arousal than the simple sentence task. Contrary to this prediction, the narrative and sentence tasks we used did not, on the whole, elicit different levels of autonomic arousal.
Again, this finding may be better understood when we consider important distinctions between Weber and Smith’s (1990) tasks and ours. Our sentence task required more linguistic formulation than Weber and Smith’s reading task. Meanwhile, our narrative task may have been less arousing because it required none of the personal disclosure of Weber and Smith’s spontaneous speech task (e.g., “If there was one thing you could change in your life, what would it be?”). The combination of these factors may help explain why the sentence and narrative tasks in the current study were, on the whole, not differentiated according to the strength of autonomic responses.
One subgroup of participants demonstrated a different pattern of arousal than the others. Although adults of both sexes and girls demonstrated lower levels of autonomic arousal during the second, presumably most demanding, speech condition, boys maintained their high level of autonomic arousal, as measured by BPV. It may be that boys, who tend to lag behind girls in linguistic development, found the open-ended narrative task more challenging or were more sensitive to the change in speaking requirements, resulting in maintenance of the autonomic arousal elicited by the first speech task. As a result, boys may not have habituated as quickly to the successive speech tasks as did girls. Due to lack of investigation, little is known about sex differences in autonomic habituation, particularly in children. However, a study of adults repeatedly listening to pure tone indicates that BPV habituated more quickly in men than women (McGuiness, 1973). In contrast, our results suggest that BPV did not habituate in boys as it did in girls or adults, resulting in lower mean BPV measures (indicating higher arousal) across successive speech tasks. This finding may be because of the particular challenges associated with speech at the children’s developmental level.
One of the challenges for the boys might have been the greater number of semantic target choices available in the narrative task (e.g., frog, dog, boy, etc.) compared to the sentence stimuli. In addition, the narrative task was not associated with a particular syntactic frame as the sentence task was. This meant that participants had to generate the syntactic frame spontaneously. Perhaps these cognitive-linguistic challenges were greater for boys than girls, and this sex difference is diminished with continued development of speech and language skills during later childhood and adolescence, explaining why this difference was not found in adults.
Although by school age there are fewer differences in speech and language skills between boys and girls, boys use these skills less frequently than girls. Studies of play styles indicate that girls at age 5 demonstrate more interactive play with peers than boys, who are more likely to engage in disconnected or disruptive play (Coolahan, Fantuzzo, Mendez, & McDermott, 2000). A study of children age 8–12 years indicated that girls prefer social activities more than boys (Brown, O’Keefe, Brown, & Stagnitti, 2011). Evidence also indicates that mothers talk more to daughters than to sons (Leaper, Anderson, & Sanders, 1998). Thus, girls appear to have or seek out more opportunities to talk with others. Girls, tending to have more experience with speech-language tasks, may have demonstrated lower arousal to the successive speech tasks presented in this study due to this increased exposure in daily life. Boys, however, who may have or seek out fewer opportunities to use their speech-language skills in daily life, may have less familiarity and comfort with a range of speech-language tasks.
Considering evidence that heightened autonomic arousal is associated with decreased speech motor coordination in children (Kleinow & Smith, 2006), boys’ increased autonomic arousal with speech may relate to their increased likelihood of developing speech-language disorders. In particular, boys are more prone to specific language impairment (Shriberg et al., 1999) and persistent developmental stuttering (Bloodstein & Bernstein Ratner, 2008; Yairi & Ambrose, 2005). Thus, it may be that boys’ heightened autonomic arousal across successive speech tasks relates to sex differences in the development of speech-language disorders.
Conclusion
We investigated whether there were differences in autonomic arousal between adults and children when performing speech and nonspeech tasks. In a confirmation of past findings, speech tasks elicited greater sympathetic arousal than nonspeech tasks. In addition, children demonstrated greater autonomic arousal for the nonspeech tasks than adults, potentially reflecting the less mature oral-motor systems of children. Finally, sex differences in how children respond to changes in speech task requirements, in particular given boys’ heightened susceptibility to speech-language disorders, should receive further investigation.
Acknowledgments
This research was supported by National Institute on Deafness and Other Communication Disorders Grant R01 DC00559, awarded to Purdue University. We thank Chris Weber-Fox and Lisa Goffman for their consultation regarding the methods for this study.
Footnotes
Disclosure: The authors have declared that no competing interests existed at the time of publication.
Associate Editor: Megha Sundara
References
- Barlow SM, Cole KJ, Abbs JH. A new head-mounted lip–jaw movement transduction system for the study of motor speech disorders. Journal of Speech and Hearing Research. 1983;26:283–288. doi: 10.1044/jshr.2602.283. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Lozano D. Cardiovascular psychophysiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. New York, NY: Cambridge University Press; 2007. pp. 182–210. [Google Scholar]
- Bloodstein O, Bernstein Ratner N. A handbook on stuttering. 6. Clifton Park, NY: Delmar Cengage Learning; 2008. [Google Scholar]
- Bouchard C, Trudeau N, Sutton A, Boudreault A, Deneault J. Gender differences in language development in French Canadian children between 8 and 30 months of age. Applied Psycholinguistics. 2009;30:685–707. doi: 10.1017/S0142716409990075. [DOI] [Google Scholar]
- Brown T, O’Keefe S, Brown T, Stagnitti K. Activity preferences and participation of school-age children living in urban and rural environments. Occupational Therapy in Health Care. 2011;25:225–239. doi: 10.3109/07380577.2011.589889. [DOI] [PubMed] [Google Scholar]
- Caruso AJ, Chodzko-Zajko WJ, Bidinger DA, Sommers RK. Adults who stutter: Responses to cognitive stress. Journal of Speech and Hearing Research. 1994;37:746–754. doi: 10.1044/jshr.3704.746. [DOI] [PubMed] [Google Scholar]
- Coolahan K, Fantuzzo JW, Mendez J, McDermott P. Preschool peer interactions and readiness to learn: Relationships between classroom peer play and learning behaviors and conduct. Journal of Educational Psychology. 2000;92:458–465. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. New York, NY: Cambridge University Press; 2007. pp. 159–181. [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallen BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiologica Scandinavica. 1972;84:82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- Gonzolez-Bono E, Moya-Albiol L, Salvador A, Carrillo E, Ricarte J, Gomez-Amor J. Anticipatory autonomic response to a public speaking task in women: The role of trait anxiety. Biological Psychology. 2002;60:37–49. doi: 10.1016/s0301-0511(02)00008-x. [DOI] [PubMed] [Google Scholar]
- Heilmann J, Nockerts A, Miller JF. Language sampling: Does the length of the transcript matter? Language, Speech, and Hearing Services in Schools. 2010;41:393–404. doi: 10.1044/0161-1461(2009/09-0023). [DOI] [PubMed] [Google Scholar]
- Heponiemi T, Ravaja N, Elovainio M, Näätänen P, Keltikangas-Järvinen L. Experiencing positive affect and negative affect during stress: Relationships to cardiac reactivity and to facial expressions. Scandinavian Journal of Psychology. 2006;47:327–337. doi: 10.1111/j.1467-9450.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the “Trier Social Stress Test”. Psychoneuroendocrinology. 2009;34:1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in the randomized block and split-block designs. Journal of Educational Statistics. 1976;1:69–82. [Google Scholar]
- Hyde JS, Linn MC. Gender differences in verbal ability: A meta-analysis. Psychological Bulletin. 1988;104:53–69. [Google Scholar]
- Jennings JR, Tahmoush AJ, Redmond DP. Non-invasive measurement of peripheral vascular activity. In: Martin I, Venables PH, editors. Techniques in psychophysiology. London, United Kingdom: Wiley; 1980. pp. 69–137. [Google Scholar]
- Kazuma N, Otsuka K, Wakamatsu K, Shirase E, Matsuoka I. Heart rate variability in normotensive healthy children with aging. Clinical and Experimental Hypertension. 2002;24:83–89. doi: 10.1081/ceh-100108718. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. “The Trier Social Stress Test”—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kleinow J, Smith A. Potential interactions among linguistic, autonomic, and motor factors in speech. Developmental Psychobiology. 2006;48:275–287. doi: 10.1002/dev.20141. [DOI] [PubMed] [Google Scholar]
- Leaper C, Anderson KJ, Sanders P. Moderators of gender effects on parents’ talk to their children: A metaanalysis. Developmental Psychology. 1998;34:3–27. doi: 10.1037/0012-1649.34.1.3. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. Speaking: From intention to articulation. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- MathWorks. MATLAB: The language of technical computing [Computer program] Natick, MA: Author; 2005. [Google Scholar]
- Mayer M. Frog where are you? New York, NY: Dial Books for Young Readers; 1969. [Google Scholar]
- McGuiness D. Cardiovascular responses during habituation and mental activity in anxious men and women. Biological Psychology. 1973;1:115–123. doi: 10.1016/0301-0511(73)90003-3. [DOI] [PubMed] [Google Scholar]
- Nippold M. Language development during adolescent years: Aspects of pragmatics, syntax, and semantics. Topics in Language Disorders. 2000;20:15–28. [Google Scholar]
- Nunan D, Gavin RH, Sandercock GRH, Brodie D. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology. 2010;33:1407–1417. doi: 10.1111/j.1540-8159.2010.02841.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parente A, Garcia-Leal C, Del-Ben CM, Guimarães FS, Graeff FG. Subjective and neurovegetative changes in healthy volunteers and panic patients performing simulated public speaking. European Neuropsychopharmacology. 2005;15:663–671. doi: 10.1016/j.euroneuro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Peters HFM, Hulstijn W. Stuttering and anxiety: The difference between stutterers and nonstutterers in verbal apprehension and physiologic arousal during the anticipation of speech and non-speech tasks. Journal of Fluency Disorders. 1984;9:67–84. [Google Scholar]
- Pick J. The autonomic nervous system: Morphological, comparative, clinical and surgical aspects. Philadelphia, PA: Lippincott; 1970. [Google Scholar]
- Renaud P, Blondin J. The stress of Stroop performance: Physiological and emotional responses to color–word interference, task pacing, and pacing speed. International Journal of Psychophysiology. 1997;27:87–97. doi: 10.1016/s0167-8760(97)00049-4. [DOI] [PubMed] [Google Scholar]
- Sadagopan N, Smith A. Developmental changes in the effects of utterance length and complexity on speech movement variability. Journal of Speech, Language, and Hearing Research. 2008;51:1138–1151. doi: 10.1044/1092-4388(2008/06-0222). [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology. 1999;35:199–135. [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals. Fourth. San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- Shriberg LD, Tomblin JB, McSweeny JL. Prevalence of speech delay in 6-year-old children and comorbidity with language impairment. Journal of Speech, Language, and Hearing Research. 1999;42:1461–1481. doi: 10.1044/jslhr.4206.1461. [DOI] [PubMed] [Google Scholar]
- Smit A, Hand L, Freilinger J, Bernthal J, Bird A. The Iowa Articulation Norms Project and its Nebraska replication. Journal of Speech and Hearing Disorders. 1990;55:779–798. doi: 10.1044/jshd.5504.779. [DOI] [PubMed] [Google Scholar]
- Smith A, Zelaznik H. Development of functional synergies for speech motor coordination in childhood and adolescence. Developmental Psychobiology. 2004;45:22–33. doi: 10.1002/dev.20009. [DOI] [PubMed] [Google Scholar]
- St Louis KO, Ruscello DM. Oral Speech Mechanism Screening. Third. Austin, TX: Pro-Ed; 2000. [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulen JHM, Moleman P, Van Steenis HG, Boomsma F. Characterization of stress reactions to the Stroop Color Word Test. Pharmacology Biochemistry and Behavior. 1989;32:9–14. doi: 10.1016/0091-3057(89)90204-9. [DOI] [PubMed] [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Psychophysiological techniques. London, United Kingdom: Wiley; 1980. pp. 4–67. [Google Scholar]
- Walden T, Frankel C, Buhr A, Johnson K, Conture E, Karrass J. Dual diathesis–stressor model of emotional and linguistic contributions to developmental stuttering. Journal of Abnormal Child Psychology. 2012;40:633–644. doi: 10.1007/s10802-011-9581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Smith A. Articulatory movements in adolescents: Evidence for protracted development of speech motor control processes. Journal of Speech, Language, and Hearing Research. 2002;45:1119–1133. doi: 10.1044/1092-4388(2002/090). [DOI] [PubMed] [Google Scholar]
- Weber CM, Smith A. Autonomic correlates of stuttering and speech assessed in a range of experimental tasks. Journal of Speech and Hearing Research. 1990;33:690–706. doi: 10.1044/jshr.3304.690. [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering: For clinicians by clinicians. Austin, TX: Pro-Ed; 2005. [Google Scholar]
- Zimmermann G. Stuttering: A disorder of movement. Journal of Speech and Hearing Research. 1980;23:122–136. [PubMed] [Google Scholar]


