Abstract
Polybrominated diphenyl ethers (PBDEs) were widely used as flame retardants in the past three decades. These compounds are lipophilic and easily cross the placenta from pregnant woman to fetus. It is not clear whether hydroxylated PBDEs (OH-PBDEs), with greater hydrophilicity, have different concentrations in maternal and cord serum samples. We analyzed PBDEs (BDE-28, -47, -99, -100, -153, -154, -209) and OH-PBDEs (6-OH-BDE-47, 5-OH-BDE-47, 4′-OH-BDE-49, 5′-OH-BDE-99) in 20 pairs of maternal and cord serum samples collected in Cincinnati, OH in 2011. The geometric mean concentration of ΣOH-BDEs (the sum of four OH-PBDEs) was 49.76 pg/ml in cord sera, higher than 32.84 pg/ml in maternal sera. Similarly, cord serum total BDEs had a higher geometric mean than maternal serum (45.51 vs. 32.07 ng/g lipid). Equal or higher levels of total OH-BDEs and total BDEs in cord serum were observed in 85% and 80% of the mother-neonate pairs, respectively. The study suggests fetuses might receive higher OH-PBDE and PBDE exposure than their mothers.
Keywords: Birth weight, Delivery, Fetus, Hydroxylated metabolites, Mother, Polybrominated diphenyl ethers (PBDEs)
Introduction
Polybrominated diphenyl ethers (PBDEs) are a group of brominated flame retardants widely used in polyurethane foams, carpet pads, furniture, and electronic devices. Because of extensive use of PBDEs in the U.S., the body burden of PBDEs is about an order of magnitude higher in the U.S. than in European or Asian countries.1 PBDEs are lipophilic chemical compounds and can cross placenta and distribute to fetus.2–5 The presence of PBDEs in developing fetuses has raised concerns about potential health and developmental risks. Prior epidemiologic research in the U.S. has suggested that prenatal exposure to PBDEs is related to cognitive deficits in young children.6 A study in the Netherlands reported deficits in fine motor function and attention in school age children related to prenatal exposure to PBDEs, even though other measures of coordination, visual perception, and behavior were positively related to exposure.7 A Spanish study indicated a non-significant cognitive and motor function deficits related to prenatal exposure to BDE-47, the dominant congener of PBDE compounds.8 Another U.S. study reported lower birth weight in neonates in relation to maternal PBDEs.9
One working hypothesis with regard to the potential toxicity of PBDEs in developing humans is the disruption of hypothalamus-pituitary-thyroid (HPT) axis, a key neuroendocrine pathway related to brain maturation, body growth, and metabolism.10, 11 The observation of inverse associations of PBDEs and thyroid hormones, especially thyroxine (T4) and triiodothyronine (T3): in various animal studies lends strong support to the hypothesis.12 The underlying rationale is the similarity of PBDE compounds and thyroxine in chemical structure. Human studies in pregnant women and newborns, however, have been inconclusive regarding the association between PBDEs and maternal or fetal thyroid hormones.13–17
The two recent studies14, 15 on PBDEs and thyroid hormones also investigated hydroxylated PBDEs (OH-PBDEs) because the OH-PBDEs are structurally more similar to thyroid hormones, with stronger binding capability to human transthyretin (TTR).18–22 OH-PBDEs are possible metabolites of PBDEs in humans, however, recent research also suggests OH-PBDEs can be derived from naturally occurring methoxylated PBDEs (MeO-PBDEs) in marine environment and thus be found in fish.23–26 OH-PBDEs also bind to thyroxine binding globulin (TBG), the major T4 transport protein in human plasma.21, 22 In addition to thyroid hormone transporters, OH-PBDEs might bind to thyroid hormone receptors α and β (TRα and TRβ) and affect thyroid hormone signaling through nuclear receptor antagonism.27 Thyroid hormone disruption is not the only pathway for the toxicity of OH-PBDEs; recent studies have also suggested gamma-aminobutyric acid (GABAA) and α4β2 nicotinic acetylcholine (nACh) receptor binding, estrogen receptor α and β (ERα and ERβ) binding, and aromatase activity inhibition.27–30
Despite potential toxicity of OH-PBDEs in human fetuses, very few studies have been able to quantify OH- PBDEs in neonates, probably due to the complexity of the analytical assays.31–33 Although PBDE concentrations appear to be similar in maternal circulation and cord blood, suggesting cross-placenta transfer of lipophilic original compounds,2, 4 it is not clear whether more polar hydroxylated PBDEs cross the placenta in a fashion similar to PBDEs. A research study of 6 pairs of mothers and neonates in Japan found lower median 6-OH-BDE-47 in neonate serum samples (0.6 pg/g wet weight [ww]) than mothers (2.1 pg/g ww),33 but a study of 26 pairs in South Korea disputed this finding (<4 pg/g ww in mothers vs. 26 pg/g ww in neonates).31 The only study of maternal and cord serum OH-PBDEs in the U.S. also suggests higher concentrations in neonates than mothers, but the study offered little for paired comparison because only one mother-neonate pair was among the 4 maternal and 16 cord sera tested.32 To examine the relationship between maternal and fetal exposure to OH-PBDEs as well as PBDEs, we conducted a study in pregnant women and measured the hydroxylated and original compounds in the U.S.
Materials and Methods
Study participants
We enrolled 20 pregnant women with singleton pregnancy before delivery in the clinic at the University of Cincinnati Department of Obstetrics and Gynecology, Cincinnati, OH, from January to June 2011. The inclusion criteria were: 1) pregnant women with singleton pregnancy after 28 weeks of gestation; 2) maternal age 18–45 years; 3) intending to continue prenatal care and deliver in the recruiting clinic; 4) willing to give informed consent to participate in a questionnaire interview and donation of maternal and cord blood samples. The exclusion criteria were: 1) twin or other multiple pregnancy; 2) having been diagnosed with thyroid diseases, e.g., clinical hyperthyroidism or hypothyrodism, thyroiditis, goiters, thyroid neoplasms, or taking medications to treat thyroid diseases during pregnancy; 3) severe diseases of the heart, lung, liver, kidneys or cancers that would affect pregnancy outcomes; 4) severe maternal and fetal complications at the time of enrollment, for example pre-existing diabetes or hypertension before pregnancy, preeclampsia, placenta abruption, placenta previa, diagnosed fetal malformation in utero. The study protocol was approved by the University of Cincinnati Institutional Review Board.
Data and biospecimen collection
Pregnant women attending prenatal care were contacted by a research nurse regarding the possibility of participation in the study. After informed consent, a short structured questionnaire was given to pregnant women to complete. The questions included demographic and socioeconomic characteristics, life style, exposure to PBDEs (occupation in flame retardant-related business, foam and carpet padding recycling, electronics recycling, computer repairing, house vacuuming frequency, vacuum with HEPA filter, car upholstery type (and driving hours), dietary intake (iodine, fish consumption, fatty food consumption, vitamin supplements), reproductive history (parity, prior pregnancy outcomes, lactation history), and medical history (endocrine diseases). Information on birth outcomes were extracted from medical records after delivery. The cord blood samples were collected immediately after delivery. Study participants donated venous blood at the time of the 24-hour postpartum regular blood draw. Serum samples were separated from the BD Vacutainer® red top tubes after at least 30 min of clotting and stored in Nalgene® cryovials at −80°C until shipping on dry ice to the analytical laboratory.
Chemical Assays
The maternal and cord serum samples were tested in the Environmental Chemistry Laboratory at California Department of Toxic Substances Control for PBDEs and OH-PBDEs with methods adopted from the lab’s previous publications.14, 34, 35 We used standard liquid-liquid serum extraction and phase separation techniques. Analysis of PBDE was performed by gas chromatography/high resolution mass spectrometry (GC-HRMS, Thermo-Finnegan) coupled with isotope dilution. OH-PBDEs were determined as methyl derivatives (MeO-PBDEs) by using Varian 1200 GC/MS with negative chemical ionization mode. The levels of total cholesterol and triglycerides were determined and used to calculate the lipid content based on Phillips’ formula.36 The levels of lipophilic PBDEs (BDE-28, -47, -99, -100, -153, -154, -209) were expressed on a lipid basis (ng/g lipid). The levels of more hydrophilic OH-PBDEs (6-OH-BDE-47, 5-OH-BDE-47, 4′-OH-BDE-49, 5′-OH-BDE-99) were expressed on wet weight basis (pg/ml).
13C12 labeled PBDEs (13C12-BDE-28, 47, 99, 153, 154, 183, 197, and 207) (purchased from Wellington Laboratory, Guelph, Ontario, Canada) were used as internal standards. 13C12-PCB-209 (purchased from Cambridge Isotope Laboratory, Andover, MA, USA) was used as a recovery standard. Native PBDE standards were purchased from Wellington Laboratories (Guelph, Ontario, Canada). MeO-BDE standards (2′-MeO-BDE28 (internal standard), 2′-MeO-BDE68, 6-MeO-BDE47, 5-MeO-BDE47, 4′-MeO-BDE49, 5′-MeO-BDE100, 4′-MeO-BDE103, 5′-MeO-BDE99 and 4′-MeO-BDE101) were purchased from Wellington Laboratories or donated by Dr. Robert Letcher. OH-PBDEs were methylated by using diazomethane. Diazomethane was synthesized in hexane by using N-nitroso-N-methylurea (Sigma-Aldrich, USA) as described elsewhere.37
All glassware was washed, rinsed with acetone and hexane, and baked (500 °C for 3 hours). Samples were analyzed using standard laboratory QA/QC protocol; each batch of nine samples were accompanied with a matrix blank (bovine serum; HyClone), a matrix spike control (permatrix-spiked bovine serum), and a standard reference material (NIST SRM 1589a). For the OH-PBDE quantification, we used five point external calibration curve of MeO-PBDEs and corrected the final concentrations by the surrogate recoveries. Precision and accuracy of PBDEs from surrogate spikes, reference material, and control samples were within reasonable analytical error ranges. The mean recovery (± standard deviation [SD]) of surrogate standards for 2′-OH-BDE28 was 85±24%.
The limit of detection (LOD) was calculated as three times the SD of the blank concentrations. For PBDE or OH-PBDE concentrations below the wet weight based LOD (BDE-28 2.2 pg/ml, BDE-47 38.5 pg/ml, BDE-99 11.9 pg/ml, BDE-100 3 pg/ml, BDE-153 3.1 pg/ml, BDE-154 4 pg/ml, BDE-209 32 pg/ml, 6-OH-BDE-47 5 pg/ml, 5-OH-BDE-47 6.8 pg/ml, 4′-OH-BDE-49 5 pg/ml, 5′-OH-BDE-99 16.4 pg/ml), the values were replaced with LOD/2.
Data Analysis
We first calculated summary statistics on maternal and cord serum concentrations of both PBDEs and OH-PBDEs in this set of mother and neonate pairs. Because the concentrations of PBDEs and OH-PBDEs did not follow a normal distribution, we used a natural logarithm transformation to perform statistical analysis and present data. We calculated total BDEs as the sum of BDE-28, -47, -99, -100, -153, -154, -209, and total OH-BDEs as the sum of 6-OH-BDE-47, 5-OH-BDE-47, 4′-OH-BDE-49, 5′-OH-BDE-99. Then, we compared the maternal and cord PBDE concentrations as well as OH-PBDE concentrations using paired t-tests after natural log transformation. The comparison was performed for both congener-specific and total PBDEs and OH-PBDEs. We then examined data in each of the 20 pairs of mothers and neonates graphically and calculated the cord to maternal (C:M) ratio of PBDE and OH-PBDE congeners. We analyzed the pattern of PBDE and OH-PBDE congeners using Principal Component Analysis (PCA) in mothers and neonates. We further examined, in an exploratory way, whether maternal or cord total BDEs and total OH-BDEs were related to birth weight and gestational age in the study participants. SAS 9.2 (SAS Institute Inc., Cary, NC) was used for statistical analysis with two sided tests at a significance level of 0.05.
Results
The study participants had a mean (±SD) age of 26±6 years, with 50% Non-Hispanic White and 50% African American. In the study sample, 80% had annual household income below $40,000. None of the mothers was involved in the following occupation related to PBDE exposure: electronic device recycling, computer repair and maintenance, foam and carpet padding recycling, carpet installation, rubber manufacturing, or electronic cable manufacturing. Of 20 study participants, 70% had mostly carpet floor at home, 25% mostly hardwood, and 5% had even residential carpet and hardwood. Analogously, for car upholstery type, 60% had fabric, 25% had leather, 5% had both fabric and leather, and 10% had no cars. The mean time per week in a car is 6.5±5.5 hours.
In the study sample, 40% women had no prior live birth, 35% smoked during this pregnancy, and 5% reported drinking alcohol during this pregnancy. The neonates were born from 35 to 41 gestational weeks, with mean of 39.0±1.3 weeks; the mean birth weight was 3361± 491 grams. Only 1 neonate was preterm (35 weeks) and low birth weight (1901 grams) in the study sample, with the remaining all term birth with normal birth weight. Vaginal delivery accounted for 75% of the study sample, with another 25% cesarean section.
The geometric mean (GM) and other summary statistics of PBDEs and OH-PBDEs in paired maternal and cord serum samples are shown in Table 1. The GM of total BDEs was 45.51 ng/g lipid in cord serum, higher than maternal serum (GM 32.07 ng/g lipid, p<0.01). Of the PBDE congeners, BDE-47 was still the major congener detected, accounting for about one third of total BDEs. Interestingly, BDE-153 concentrations were higher in maternal serum than cord serum, while all other congeners were higher in cord serum. The percentage of OH-PBDEs above LOD appeared lower than that of PBDEs. Nevertheless, the GM of total OH-BDEs was 49.76 pg/ml in cord sera and higher than in maternal sera (GM 32.84 pg/ml, p<0.01). There were no differences of 4′-OH-BDE-49 and 5′-OH-BDE-99 between maternal and cord serum sample (p>0.05).
Table 1.
Summary statistics of PBDE and OH-PBDE congeners in paired maternal and cord serum samples (n=20)
| Congeners | Maternal | Cord | p* | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| %≥LOD | Geometric mean | Median | Range | %≥LOD | Geometric mean | Median | Range | ||
| PBDEs (ng/g lipid) | |||||||||
| BDE-28 | 90 | 1.02 | 1.43 | 0.14–5.85 | 65 | 1.66 | 3.11 | 0.41–6.76 | 0.0011 |
| BDE-47 | 90 | 12.52 | 10.96 | 2.53–90.61 | 65 | 16.80 | 18.01 | 7.29–95.65 | 0.0091 |
| BDE-99 | 95 | 3.89 | 3.17 | 0.79–26.55 | 80 | 6.24 | 6.19 | 2.26–25.07 | 0.0034 |
| BDE-100 | 85 | 1.50 | 1.80 | 0.20–20.48 | 90 | 2.44 | 2.30 | 0.57–20.14 | 0.0343 |
| BDE-153 | 100 | 5.46 | 5.73 | 0.79–22.45 | 85 | 2.75 | 2.95 | 0.58–15.92 | <0.0001 |
| BDE-154 | 25 | 0.36 | 0.27 | 0.27–2.41 | 5 | 0.79 | 0.76 | 0.76–1.83 | <0.0001 |
| BDE-209 | 55 | 4.28 | 5.07 | 2.11–13.97 | 40 | 10.51 | 6.06 | 6.06–60.93 | 0.0004 |
| Total BDEs | 32.07 | 28.55 | 7.46–175.66 | 45.51 | 41.51 | 17.92–171.43 | 0.0028 | ||
| OH-PBDEs (pg/ml) | |||||||||
| 6-OH-BDE-47 | 40 | 4.61 | 2.50 | 2.50–82.43 | 55 | 6.60 | 5.62 | 2.50–168.89 | 0.0013 |
| 5-OH-BDE-47 | 55 | 8.82 | 8.29 | 3.40–84.12 | 85 | 19.78 | 19.08 | 3.40–167.80 | 0.0003 |
| 4′-OH-BDE-49 | 10 | 2.84 | 2.50 | 2.50–12.18 | 15 | 3.26 | 2.50 | 2.50–21.05 | 0.3902 |
| 5′-OH-BDE-99 | 25 | 12.88 | 8.20 | 8.20–140.20 | 35 | 15.57 | 8.20 | 8.20–225.85 | 0.2086 |
| Total OH-BDEs | 32.84 | 25.52 | 16.60–230.58 | 49.76 | 39.74 | 16.60–445.63 | 0.0011 | ||
p value in paired t-test of maternal and cord PBDE or OH-PBDE concentrations after natural log transformation
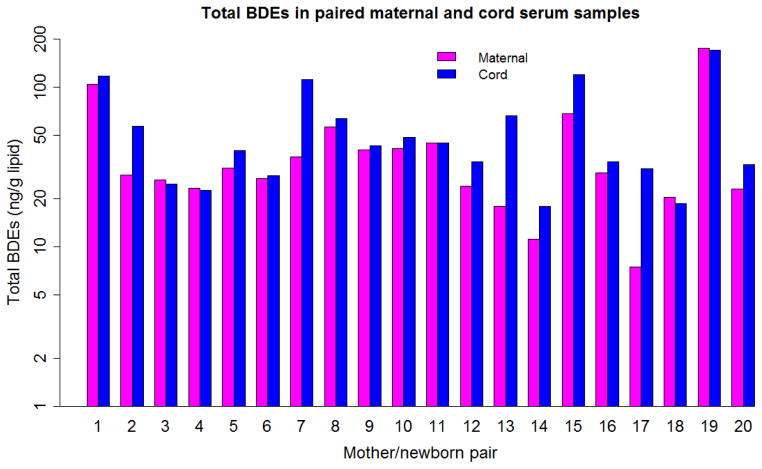
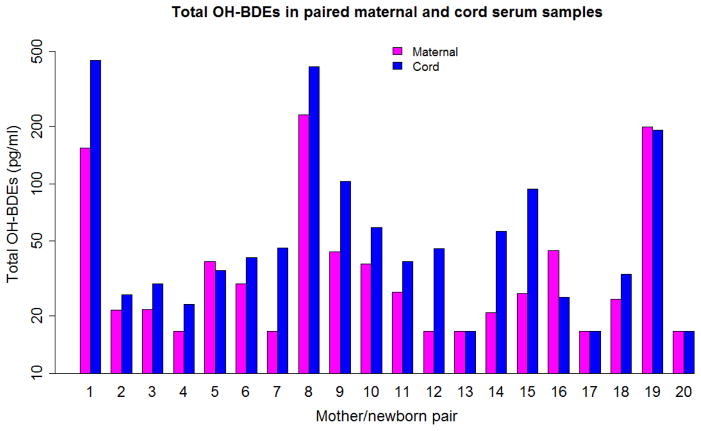
Comparing each mother and neonate pair for total BDEs yielded Figure 1, and, for total OH-BDEs, Figure 2. In both figures, the y axes used a log scale. Most cord serum samples had equal or higher total BDEs and total OH-BDEs than the corresponding maternal serum sample. The ratios of C:M concentration of PBDE and OH-PBDE congeners in the paired samples are shown in Table 2. The mean C:M ratio was higher than 1 for all PBDE and OH-PBDE congeners except BDE-153. The highest C:M ratio was observed for BDE-209.
Figure 1.
Total BDEs in paired maternal and cord serum samples in Cincinnati, OH 2011 (n=20, ng/g lipid)
Figure 2.
Total OH-BDEs in paired maternal and cord serum samples in Cincinnati, OH 2011 (n=20, pg/ml)
Table 2.
The ratios of cord to maternal (C:M) concentrations of PBDEs and OH-PBDEs in paired samples (n=20)
| Congeners | Mean±SD | Median | Range | % ≥ 1 |
|---|---|---|---|---|
| PBDEs (ng/g lipid) | ||||
| BDE-28 | 1.85±0.85 | 1.87 | 0.44–3.52 | 85 |
| BDE-47 | 1.49±0.78 | 1.29 | 0.66–3.59 | 80 |
| BDE-99 | 2.07±2.07 | 1.48 | 0.82–9.41 | 80 |
| BDE-100 | 2.96±4.41 | 0.99 | 0.73–18.30 | 50 |
| BDE-153 | 0.52±0.13 | 0.50 | 0.31–0.73 | 0 |
| BDE-154 | 2.41±0.83 | 2.87 | 0.63–2.87 | 90 |
| BDE-209 | 4.17±6.39 | 2.88 | 0.60–28.94 | 80 |
| Total BDE | 1.59±0.94 | 1.17 | 0.91–4.12 | 80 |
| OH-PBDEs (pg/ml) | ||||
| 6-OH-BDE-47 | 1.57±0.77 | 1.10 | 1.00–3.79 | 100 |
| 5-OH-BDE-47 | 3.21±3.19 | 1.78 | 0.85–12.79 | 95 |
| 4′-OH-BDE-49 | 1.57±1.85 | 1.00 | 0.21–8.42 | 95 |
| 5′-OH-BDE-99 | 1.52±1.25 | 1.00 | 0.40–5.65 | 85 |
| Total OH-BDEs | 1.69±0.83 | 1.38 | 0.57–3.55 | 85 |
In both maternal and cord serum samples, the patterns of PBDE and OH-PBDE congeners were similar in the PCA. Two principal components explained about 80% of variance of the PBDEs or OH-PBDEs. For PBDEs, BDE-28, -47, -99, -100, -153, -154 had similar loading on the first component (~60% variance), while BDE-209 mostly explained the second component (~20% variance). For OH-PBDEs, 6-OH-BDE-47, 5-OH-BDE-47, 5′-OH-BDE-99 had similar loading on the first component (~60% variance), and 4′-OH-BDE-49 mostly explained the second component (~20% variance). The correlation between PBDEs and OH-PBDEs were high, for example, the Pearson correlation coefficient of total BDEs and total OH-BDEs after natural log transformation was 0.76 in mothers and 0.57 in neonates (both p<0.01).
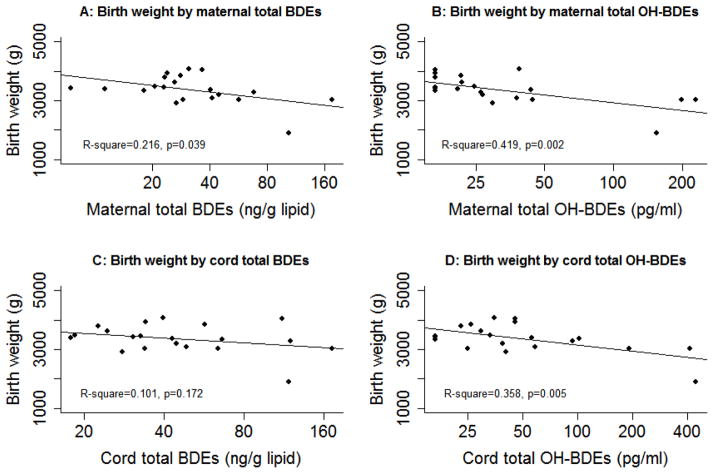
In the sample, higher maternal or cord total BDE concentrations were observed in younger mothers, and African Americans (data not shown). Floor type, car upholstery type, hours traveling by car, live birth order, breastfeeding history, and smoking during pregnancy were not associated with total BDE concentrations in mothers. Maternal total BDEs or total OH-BDEs was not related to gestational age in the linear regression model. For birth weight, there is an inverse trend for maternal or cord total BDEs or total OH-BDEs in scatter plots and unadjusted regression models (Figure 3). The results of adjusted association are shown in Table 3, with maternal age (continuous), race (Non-Hispanic White or African American), smoking during pregnancy (yes or no), neonate’s sex (male or female), and gestational week at birth (continuous) as covariates. Maternal total OH-BDEs were significantly associated with lower birth weight, with −319 (95% confidence interval [CI] −579, −60) grams related to each unit natural log total OH-BDEs. This estimate for cord total OH-BDEs was −262 (95% CI: −533, 10) grams, with p=0.058. A further sub-analysis by excluding the only preterm and low birth weight neonate reduced the estimate for maternal total OH-BDEs to marginal significance (p=0.07). However, the association between maternal or cord total BDEs and birth weight did not reach statistical significance in either full or restricted sample.
Figure 3.
Scatter plot and simple regression line of birth weight by concentrations of total BDEs and total OH-BDEs in maternal and cord serum samples, with x-axis on natural log scale. A: Birth weight by maternal total BDEs; B: Birth weight by maternal total OH-BDEs; C: Birth weight by cord total BDEs; D: Birth weight by cord total OH-BDEs.
Table 3.
Adjusted* regression coefficients and 95% confidence intervals (CIs) of maternal and cord BDEs and OH-BDEs on birth weight
| Exposure measurement | All subjects (n=20) | Excluding 1 preterm and low birth weight neonate (n=19) | ||
|---|---|---|---|---|
|
| ||||
| β (95% CI) | p | β (95% CI) | p | |
| ln Maternal total BDEs | −274 (−789, 242) | 0.27 | −183 (−662, 296) | 0.42 |
| ln Maternal total OH-BDEs | −319 (−579, −60) | 0.02 | −248 (−515, 20) | 0.07 |
| ln Cord total BDEs | −137 (−668, 394) | 0.59 | 6 (−495, 508) | 0.98 |
| ln Cord total OH-BDEs | −262 (−533, 10) | 0.06 | −174 (−465, 117) | 0.22 |
Adjusted for maternal age (continuous), race (Non-Hispanic White or African American), smoking during pregnancy (yes or no), neonate’s sex (male or female), and gestational week at birth (continuous)
Discussion
In this urban clinical unit in the Midwest U.S., comparison of paired mothers and neonates revealed slightly higher total BDEs and OH-BDEs in cord sera than maternal sera collected immediately after delivery. This suggests fetuses and neonates might have higher circulating concentration than mothers to this group of potential endocrine disruptors and developmental neurotoxicants. The increased levels of PBDEs and OH-PBDEs in cord sera were evident in vast majority of the mother-neonate pairs, indicating involvement of possible mechanisms of placental partition and variations in metabolic conversion, tissue deposit, and excretion between mothers and fetuses.
The results from this study sample are similar to the study in 26 pairs of mother and neonate from South Korea for 6-OH-BDE-47, although that study was only able to detect 6-OH-BDE-47 but not other OH-PBDE congeners.31 In that study, the mean±SD C:M ratio was 1.4±1.1 for 10 pairs with 6-OH-BDE-47 above LOD, while this ratio in the current study was 1.90±0.85 in 8 pairs with 6-OH-BDE-47 above LOD, largely supporting higher levels of 6-OH-BDE-47 in fetal circulation than in maternal circulation. The median cord serum level of 6-OH-BDE-47 in the study from South Korea (26 pg/g ww) was much higher than the maternal serum level (<4 pg/g ww), although the mean level in cord serum samples was about 1.7 times higher than maternal level. The cord serum levels of 6-OH-BDE-47 were lower in the current study than that study. It might be that fish consumption, which contributes to high dietary intake of 6-MeO-BDE-47 and 6-OH-BDE-47,26 could explain the difference between this study (lower fish consumption) and the study from South Korea. Recent investigations of the source of OH-PBDEs in mammals have suggested a significant role of naturally occurring MeO-PBDEs (based on radiocarbon measurement) because these organobromine compounds can be transformed to OH-PBDEs in marine environment.25, 26 Therefore, fish consumption might be another exposure route to OH-PBDE in addition to the exposure from the metabolites of PBDEs. More research is warranted in humans to differentiate the source of exposure to OH-PBDEs. The study in Japan reported much lower 6-OH-BDE-47 levels (median 2.1 pg/g ww in maternal serum vs. 0.6 pg/g ww in cord serum) and total BDEs levels (median 3 ng/g lipid in maternal serum vs. 0.65 ng/g lipid in cord serum),33 which is in accordance to the lower PBDEs in Asian countries compared with the U.S.38, 39 The notion of higher maternal total BDE levels than cord serum levels in the Japanese study, however, is not supported by majority of paired mother-neonate studies.2–5
The only other study of maternal and fetal OH-PBDEs in the U.S. suggested higher total OH-BDEs in cord samples (median 69 pg/g plasma) than in maternal samples (median 45 pg/g plasma), despite the fact that only 1 mother-neonate pair was included among 4 mothers and 16 neonates.32 It also suggested similar median total BDEs between the mothers and the neonates (31 vs. 34 ng/g lipid respectively). In that study, the congener profile of OH-PBDEs was similar to the current study. For example, in cord plasma, the median levels of 6-OH-BDE-47, 5-OH-BDE-47, 4′-OH-BDE-49, and 5′-OH-BDE-99 were 2.58, 17.53, below LOD, and 14.01 pg/g ww, with 5-OH-BDE-47 and 5′-OH-BDE-99 as dominant congeners.32 In that study, blood samples were collected in Indianapolis, IN during 2003–2004, also representing a Midwest U.S. population but from an earlier time period. Data from this study and the current study suggest fetus might encounter equivalent or high exposure to PBDEs and OH-PBDEs. Different from Asian reports, 5-OH-BDE-47 was the major OH-PBDE congener with the highest proportion above LOD in both maternal and cord sera in the U.S.31–33
The congener profiles of the PBDEs are similar between mothers and neonates, with BDE-47 explained about one third of the total PBDEs. It is plausible that in the principal component analysis BDE-209 accounted for most of the second component while the other congeners explained the first component given that BDE-209 is still in use. The reason we observed unanimously lower BDE-153 levels among all major congeners in cord than maternal serum is not clear. Similar studies have found lower4, 5, 32 or higher2, 3, 38 BDE-153 levels in cord serum samples compared with maternal samples. Nevertheless, this is not unprecedented and might be related to chance findings rather than specific chemical transportation or excretion mechanisms. For OH-PBDEs, 5-OH-BDE-47 and 5′-OH-BDE-99 are major congeners with highest concentration in both mothers and neonates followed by 6-OH-BDE-47. It is important to determine their exposure sources and biotransformation in humans.
The inverse association between birth weight and maternal PBDEs has been shown in a California study of a predominantly Mexican farming community (n=286) in Salinas Valley,9 and a smaller Taiwanese study (n=20):40 but neither measured OH-PBDEs. The current exploratory study suggests an inverse association between total OH-BDEs and birth weight while total BDEs did not shown statistical significance. The levels of PBDEs in this study were similar to the Harley et al. study and lower than the U.S. national average exposure levels measured in 2003–2004.1, 9 This study has a small sample size and very limited power; thus caution should be exercised when interpreting the findings on birth weight. The potential role of OH-PBDEs on fetal development needs attention in future larger prospective studies.
This study has limitations for its assessment on maternal-fetal PBDE and OH-PBDE concentrations. We did not have early or mid-pregnancy OH-PBDE concentrations, although presumably PBDEs maintain relatively constant concentrations throughout pregnancy because of their long half-lives. It will be technically difficult to assess fetal PBDEs or OH-PBDEs before birth, but it would be ideal to include both preterm and term birth newborns to evaluate maternal to fetal transfer of PBDEs and OH-PBDEs at different gestational ages. The study also lacks the possibility to investigate the association between OH-PBDEs and thyroid hormones due to the limited serum volume collected and the need to measure both PBDEs and OH-PBDEs in the same individual. Two studies have examined maternal OH-PBDEs and maternal thyroid hormones in pregnant women.14, 15 One suggested an inverse association with total T315 and another indicated a positive association with thyroid stimulating hormone (TSH).14 Nevertheless, there is a need to examine both maternal and fetal exposure to OH-PBDEs and fetal thyroid profiles because fetal exposure to OH-PBDEs might be higher than maternal levels, and after first trimester fetal thyroid hormones increase dramatically while maternal levels remain constant.41
Despite small sample size, this study is among the first to systematically measure PBDEs and OH-PDBEs in paired mother and neonate serum sample in the U.S. In this exploratory study, neonates had slightly higher OH-PBDEs and PBDEs than their corresponding mothers in the U.S. The major OH-PBDE congener in cord serum is 5-OH-BDE-47 with 85% detection. Further studies need to examine the exposure sources of OH-PBDEs, the role of OH-PBDEs on the fetal hormonal profile, perinatal health, and neurodevelopment with adequate sample size.
Acknowledgments
We thank Dr. Rose Maxwell, Ms. Christine DeArmond, and Ms. Stephani Kim for their contribution to the field work, data and specimen collection, and sample handling and storage. This work is supported by the Center for Environmental Genetics grant P30ES006096 from the National Institute of Environmental Health Sciences.
Footnotes
Part of the work has been presented at the 21st Annual Meeting of The International Society of Exposure Science, October 23–27, 2011, Baltimore, MD.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
References
- 1.Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG., Jr Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ Sci Technol. 2008;42(4):1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- 2.Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111(9):1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster WG, Gregorovich S, Morrison KM, Atkinson SA, Kubwabo C, Stewart B, Teo K. Human maternal and umbilical cord blood concentrations of polybrominated diphenyl ethers. Chemosphere. 2011;84(10):1301–1309. doi: 10.1016/j.chemosphere.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Frederiksen M, Thomsen C, Froshaug M, Vorkamp K, Thomsen M, Becher G, Knudsen LE. Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort. Int J Hyg Environ Health. 2010;213(4):233–242. doi: 10.1016/j.ijheh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Vizcaino E, Grimalt JO, Lopez-Espinosa MJ, Llop S, Rebagliato M, Ballester F. Polybromodiphenyl ethers in mothers and their newborns from a non-occupationally exposed population (Valencia, Spain) Environ Int. 2011;37(1):152–157. doi: 10.1016/j.envint.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal Exposure to PBDEs and Neurodevelopment. Environ Health Perspect. 2010;118(5):712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117(12):1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M, Sunyer J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4years of age. Environ Int. 2011;37(3):605–611. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Harley KG, Chevrier J, Schall RA, Sjodin A, Bradman A, Eskenazi B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am J Epidemiol. 2011;174(8):885–892. doi: 10.1093/aje/kwr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 11.Shields BM, Knight BA, Hill A, Hattersley AT, Vaidya B. Fetal thyroid hormone level at birth is associated with fetal growth. J Clin Endocrinol Metab. 2011;96(6):E934–938. doi: 10.1210/jc.2010-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28(6):1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated Diphenylether (PBDE) Flame Retardants and Thyroid Hormone during Pregnancy. Environ Health Perspect. 2010;118(10):1444–1449. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45(18):7896–7905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119(10):1454–1459. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chevrier J, Harley KG, Bradman A, Sjodin A, Eskenazi B. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol. 2011;174(10):1166–1174. doi: 10.1093/aje/kwr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggesbo M, Thomsen C, Jorgensen JV, Becher G, Odland JO, Longnecker MP. Associations between brominated flame retardants in human milk and thyroid-stimulating hormone (TSH) in neonates. Environ Res. 2011;111(6):737–743. doi: 10.1016/j.envres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92(1):157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 19.Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56(1):95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- 20.Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119(7):900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao J, Lin Y, Guo LH, Zhang AQ, Wei Y, Yang Y. Structure-based investigation on the binding interaction of hydroxylated polybrominated diphenyl ethers with thyroxine transport proteins. Toxicology. 2010;277(1–3):20–28. doi: 10.1016/j.tox.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, Takeuchi M, Irth H, Murk AJ. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol. 2008;232(1):150–160. doi: 10.1016/j.taap.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Wan Y, Jones PD, Wiseman S, Chang H, Chorney D, Kannan K, Zhang K, Hu JY, Khim JS, Tanabe S, Lam MH, Giesy JP. Contribution of synthetic and naturally occurring organobromine compounds to bromine mass in marine organisms. Environ Sci Technol. 2010;44(16):6068–6073. doi: 10.1021/es100914r. [DOI] [PubMed] [Google Scholar]
- 24.Wan Y, Liu F, Wiseman S, Zhang X, Chang H, Hecker M, Jones PD, Lam MH, Giesy JP. Interconversion of hydroxylated and methoxylated polybrominated diphenyl ethers in Japanese medaka. Environ Sci Technol. 2010;44(22):8729–8735. doi: 10.1021/es102287q. [DOI] [PubMed] [Google Scholar]
- 25.Wan Y, Wiseman S, Chang H, Zhang X, Jones PD, Hecker M, Kannan K, Tanabe S, Hu J, Lam MH, Giesy JP. Origin of hydroxylated brominated diphenyl ethers: natural compounds or man-made flame retardants? Environ Sci Technol. 2009;43(19):7536–7542. doi: 10.1021/es901357u. [DOI] [PubMed] [Google Scholar]
- 26.Wiseman SB, Wan Y, Chang H, Zhang X, Hecker M, Jones PD, Giesy JP. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: environmental sources, metabolic relationships, and relative toxicities. Mar Pollut Bull. 2011;63(5–12):179–188. doi: 10.1016/j.marpolbul.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009;117(8):1210–1218. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canton RF, Scholten DE, Marsh G, de Jong PC, van den Berg M. Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs) Toxicol Appl Pharmacol. 2008;227(1):68–75. doi: 10.1016/j.taap.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109(4):399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendriks HS, Antunes Fernandes EC, Bergman A, van den Berg M, Westerink RH. PCB-47, PBDE-47, and 6-OH-PBDE-47 differentially modulate human GABAA and alpha4beta2 nicotinic acetylcholine receptors. Toxicol Sci. 2010;118(2):635–642. doi: 10.1093/toxsci/kfq284. [DOI] [PubMed] [Google Scholar]
- 31.Wan Y, Choi K, Kim S, Ji K, Chang H, Wiseman S, Jones PD, Khim JS, Park S, Park J, Lam MH, Giesy JP. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: placental transfer and potential risks. Environ Sci Technol. 2010;44(13):5233–5239. doi: 10.1021/es1002764. [DOI] [PubMed] [Google Scholar]
- 32.Qiu X, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ Health Perspect. 2009;117(1):93–98. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawashiro Y, Fukata H, Omori-Inoue M, Kubonoya K, Jotaki T, Takigami H, Sakai S, Mori C. Perinatal exposure to brominated flame retardants and polychlorinated biphenyls in Japan. Endocr J. 2008;55(6):1071–1084. doi: 10.1507/endocrj.k08e-155. [DOI] [PubMed] [Google Scholar]
- 34.Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, Drobna B, Trnovec T, Bergman A, Hertz-Picciotto I. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBS) in pregnant women from eastern Slovakia. Environ Health Perspect. 2007;115(1):20–27. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers E, Petreas M, Park JS, Zhao G, Charles MJ. Evaluation of four capillary columns for the analysis of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human serum for epidemiologic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;813(1–2):269–285. doi: 10.1016/j.jchromb.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 36.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 37.Sandau CD. Analytical chemistry of hydroxylated metabolites of pcbs and other halogenated phenolic compounds in blood and their relationship to thyroid hormone and retinol homeostasis in humans and polar bears. Carleton University; Ottawa: 2000. [Google Scholar]
- 38.Kim TH, Bang du Y, Lim HJ, Won AJ, Ahn MY, Patra N, Chung KK, Kwack SJ, Park KL, Han SY, Choi WS, Han JY, Lee BM, Oh JE, Yoon JH, Lee J, Kim HS. Comparisons of polybrominated diphenyl ethers levels in paired South Korean cord blood, maternal blood, and breast milk samples. Chemosphere. 2012;87(1):97–104. doi: 10.1016/j.chemosphere.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 39.Kim UJ, Lee IS, Kim HS, Oh JE. Monitoring of PBDEs concentration in umbilical cord blood and breast milk from Korean population and estimating the effects of various parameters on accumulation in humans. Chemosphere. 2011;85(3):487–493. doi: 10.1016/j.chemosphere.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Chao HR, Wang SL, Lee WJ, Wang YF, Papke O. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Int. 2007;33(2):239–245. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Hume R, Simpson J, Delahunty C, van Toor H, Wu SY, Williams FL, Visser TJ. Human fetal and cord serum thyroid hormones: developmental trends and interrelationships. J Clin Endocrinol Metab. 2004;89(8):4097–4103. doi: 10.1210/jc.2004-0573. [DOI] [PubMed] [Google Scholar]