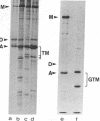
Abstract
A two-dimensional gel electrophoresis system is used to investigate some of the properties of desmin, the major subunit of the 100-A filaments from chick muscle cells, and to compare these properties to those of the other major contractile and regulatory proteins of muscle. Desmin from embryonic and adult smooth, skeletal, and cardiac muscle cells is resolved into two isoelectric variants, alpha and beta, which possess slightly different electrophoretic mobilities in sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Both the alpha and the beta variants from all six preparations appear to be identical in isoelectric point and apparent molecular weight. The alpha and beta desmin are present in approximately equal amounts in all three types of muscle, suggesting that both isoelectric variants of desmin serve as the structural subunits of the 100-A filaments in chick muscle cells. Tropomyosin also can be resolved into two subunits, alpha and beta, in all three types of muscle. However, in each type of muscle both subunits differ from their counterparts in the other types of muscle, either by molecular weight or by isoelectric point. These results indicate that, with regard to apparent isoelectric point and molecular weight, desmin, a major muscle structural protein, is invariant, while tropomyosin, a major muscle regulatory protein, exhibits heterogeneity in the three types of muscle.
Full text
PDF

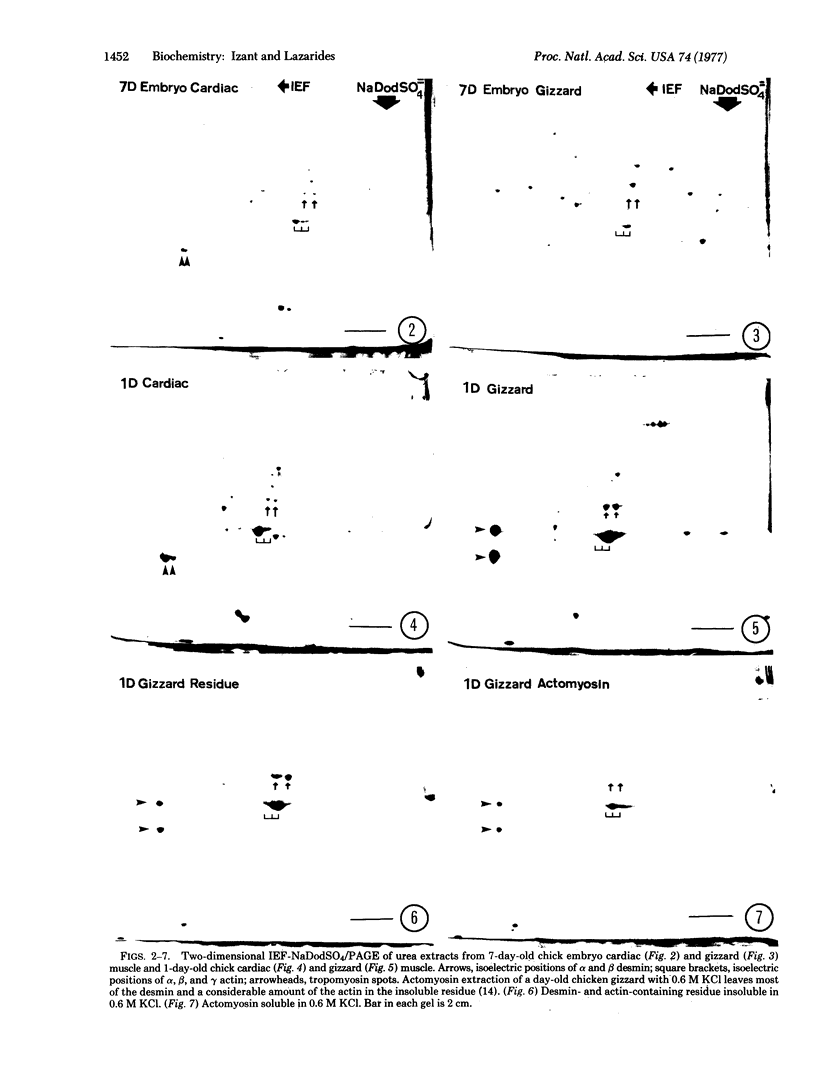

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt I., Pepe F. A. Antigenic specificity of red and white muscle myosin. J Histochem Cytochem. 1975 Mar;23(3):159–168. doi: 10.1177/23.3.47867. [DOI] [PubMed] [Google Scholar]
- Bignami A. Our present knowledge of the pathology of dementias. Mod Trends Neurol. 1975;6:1–16. [PubMed] [Google Scholar]
- Brecher S. The occurrence and possible role of 80-100 A filaments in PtKl cells. Exp Cell Res. 1975 Dec;96(2):303–310. doi: 10.1016/0014-4827(75)90261-x. [DOI] [PubMed] [Google Scholar]
- Burridge K. A comparison of fibroblast and smooth muscle myosins. FEBS Lett. 1974 Sep 1;45(1):14–17. doi: 10.1016/0014-5793(74)80799-4. [DOI] [PubMed] [Google Scholar]
- Burridge K., Bray D. Purification and structural analysis of myosins from brain and other non-muscle tissues. J Mol Biol. 1975 Nov 25;99(1):1–14. doi: 10.1016/s0022-2836(75)80154-9. [DOI] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J. 1974 Jul;141(1):43–49. doi: 10.1042/bj1410043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Glial fibrillary acidic protein from normal and gliosed human brain. Demonstration of multiple related polypeptides. Biochim Biophys Acta. 1975 Mar 28;386(1):41–51. doi: 10.1016/0005-2795(75)90244-5. [DOI] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Heterogeneity of the glial fibrillary acidic protein in gliosed human brains. J Neurol Sci. 1974 Dec;23(4):551–563. doi: 10.1016/0022-510x(74)90027-6. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Maron B. J., Adelstein R. S. Human heart and platelet actins are products of different genes. Science. 1976 Jan 9;191(4222):94–95. doi: 10.1126/science.1246600. [DOI] [PubMed] [Google Scholar]
- Gruenstein E., Rich A. Non-identity of muscle and non-muscle actins. Biochem Biophys Res Commun. 1975 May 19;64(2):472–477. doi: 10.1016/0006-291x(75)90345-9. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. Cyanogen bromide fragments of rabbit skeletal tropomyosin. Can J Biochem. 1973 Jan;51(1):56–70. doi: 10.1139/o73-008. [DOI] [PubMed] [Google Scholar]
- Hood L., Campbell J. H., Elgin S. C. The organization, expression, and evolution of antibody genes and other multigene families. Annu Rev Genet. 1975;9:305–353. doi: 10.1146/annurev.ge.09.120175.001513. [DOI] [PubMed] [Google Scholar]
- Huszar G. Developmental changes of the primary structure and histidine methylation in rabbit skeletal muscle myosin. Nat New Biol. 1972 Dec 27;240(104):260–264. doi: 10.1038/newbio240260a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A. O., Subrahmanyan L., Turnbull C., Kalnins V. I. Localization of the neurofilament protein in neuroblastoma cells by immunofluorescent staining. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3192–3196. doi: 10.1073/pnas.73.9.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Hubbard B. D. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rash J. E., Biesele J. J., Gey G. O. Three classes of filaments in cardiac differentiation. J Ultrastruct Res. 1970 Dec;33(5):408–435. doi: 10.1016/s0022-5320(70)90171-1. [DOI] [PubMed] [Google Scholar]
- Roy R. K., Potter J. D., Sarkar S. Characterization of the Ca2+-regulatory complex of chick embryonic muscles: polymorphism of tropomyosin in adult and embryonic fibers. Biochem Biophys Res Commun. 1976 May 3;70(1):28–36. doi: 10.1016/0006-291x(76)91104-9. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Yamada K. M., Wessells N. K. Microfilaments and cell locomotion. J Cell Biol. 1971 Jun;49(3):595–613. doi: 10.1083/jcb.49.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Sréter F. A., Bálint M., Gergely J. Structural and functional changes of myosin during development: comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975 Oct;46(2):317–325. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Starr R., Offer G. Polarity of the myosin molecule. J Mol Biol. 1973 Nov 25;81(1):17–31. doi: 10.1016/0022-2836(73)90244-1. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Coen D. M., Rich A. Tissue-specific forms of actin in the developing chick. Cell. 1976 Aug;8(4):521–527. doi: 10.1016/0092-8674(76)90220-8. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Rich A. Chick cytoplasmic actin and muscle actin have different structural genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2346–2350. doi: 10.1073/pnas.73.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Campbell G. R., Burnstock G. Cytoplasmic filaments in developing and adult vertebrate smooth muscle. J Cell Biol. 1971 Aug;50(2):484–497. doi: 10.1083/jcb.50.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Greaser M. L., Cassens R. G. Interactions of troponin subunits with different forms of tropomyosin. J Ultrastruct Res. 1974 Jul;48(1):33–58. doi: 10.1016/s0022-5320(74)80043-2. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Dahl D., Schachner M., Shelanski M. L. Biochemistry of the filaments of brain. Proc Natl Acad Sci U S A. 1976 Feb;73(2):529–533. doi: 10.1073/pnas.73.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]