Abstract
Rationale
Alcohol effects on behavioral and cognitive mechanisms influence impaired driving performance and decisions to drive after drinking (Barry 1973; Moskowitz and Robinson 1987). To date, research has focused on the ascending limb of the blood alcohol curve, and there is little understanding of how acute tolerance to impairment of these mechanisms might influence driving behavior on the descending limb.
Objectives
To provide an integrated examination of the degree to which alcohol impairment of motor coordination and inhibitory control contributes to driving impairment and decisions to drive on the ascending and descending limbs of the blood alcohol curve.
Methods
Social-drinking adults (N=20) performed a testing battery that measured simulated driving performance and willingness to drive, as well as mechanisms related to driving: motor coordination (grooved pegboard), inhibitory control (cued go/no-go task), and subjective intoxication. Performance was tested in response to placebo and a moderate dose of alcohol (0.65 g/kg) twice at comparable blood alcohol concentrations: once on the ascending limb and again on the descending limb.
Results
Impaired motor coordination and subjective intoxication showed acute tolerance, whereas driving performance and inhibitory control showed no recovery from impairment. Greater motor impairment was associated with poorer driving performance under alcohol, and poorer inhibitory control was associated with more willingness to drive.
Conclusions
Findings suggest that acute tolerance to impairment of motor coordination is insufficient to promote recovery of driving performance and that the persistence of alcohol-induced disinhibition might contribute to risky decisions to drive on the descending limb.
Keywords: Acute tolerance, Driving, Inhibition, Motor control, Subjective intoxication
Introduction
The impairing effect of alcohol on driving performance is well established. However, driving is a complex skill that involves specific abilities ranging from simple reaction time to higher-order decision making and cognitive control (Groeger 2000; Meda et al. 2009; Wickens et al. 2008), and there is much interest in the specific mechanisms through which alcohol disrupts driving ability. Considerable laboratory research indicates that moderate doses of alcohol impair a broad range of behavioral and cognitive functions, and any of these could contribute to overall driving performance (Holloway 1995; Mitchell 1985; Moskowitz and Robinson 1987; Stapleton et al. 1986). Decades ago, Barry (1973) recognized two distinct behavioral and cognitive mechanisms as critical contributors to driving performance: motor coordination and inhibitory control. Motor impairing effects of alcohol can reduce driver precision, resulting in greater within-lane swerving and line crossings (Holloway 1995). The disinhibiting effects of alcohol can compromise driving performance by increasing reckless behaviors, such as speeding, excessive lane changing, and disregard of traffic signals (Fillmore et al. 2008).
To date, the majority of research examining cognitive and behavioral mechanisms of impaired driving performance has focused on the effects early after the dose as blood alcohol concentration (BAC) is rising. However, for driving-related behavior, the later phase of the dose (when BAC is declining) is of particular importance as decisions to drive are often made after a drinking episode has been terminated. As such, there is a need for this research to examine impairment of mechanisms critical to driving behavior on both the ascending and descending limbs of the blood alcohol curve, particularly in regard to the potential development of acute tolerance. Acute tolerance refers to the diminished intensity of impairment at a given BAC during the descending compared to the ascending limb (Mellanby 1919), and there is reason to believe that motor coordination and inhibitory control might differ in this regard. Acute tolerance to the effects of alcohol has been observed in several behaviors, including motor coordination, reaction time, and subjective intoxication (Beirness and Vogel-Sprott 1984; Fillmore and Vogel-Sprott 1996; Haubenreisser and Vogel-Sprott 1987; Schweizer et al. 2004). However, for more complex cognitive functions, such as inhibitory control, acute tolerance has not been observed (Cromer et al. 2010; Fillmore et al. 2005; Ostling and Fillmore 2010). As such, the degree to which these factors contribute to impaired driving under alcohol might depend on the limb of the blood alcohol curve. Particularly, motor coordination might be a more important predictor on the ascending limb before it begins to tolerate on the descending limb, whereas inhibitory control might influence driving across the blood alcohol curve.
Another factor that has received little attention in this research is the degree to which impairment of motor coordination and inhibitory control, as well as subjective levels of intoxication, might also contribute to the drinker's decision to drive. Alcohol-induced disinhibition is associated with risky, impulsive decision-making, and as such, it could lead to unsafe decisions to drive while intoxicated. Additionally, self-perception of motor impairment and intoxication could affect judgments of one's ability to drive (Beirness 1987; Russ et al. 1986). Acute tolerance to impairment of these mechanisms is also relevant here. The decision to drive is often made after a drinking episode has been terminated, when BAC is likely to be in decline. Thus, the driver is likely to observe some recovery of motor coordination and to feel less intoxicated (Marczinski and Fillmore 2009), yet remain disinhibited by the drug. Together, these mechanisms could serve to increase decisions to drive, particularly on the descending limb.
The purpose of the current study was to provide an integrated examination of the degree to which alcohol-induced impairment of these measures contributes to disruption of driving performance on the ascending limb and again on the descending limb when acute tolerance might be evident in some measures (i.e., motor coordination and subjective intoxication). The study also examined how these three factors contribute to the drinker's decision to drive during the ascending limb and again during the descending limb. It was predicted that alcohol-induced impairment of motor coordination would predict driving disruption more so during the ascending limb than during the descending limb, when acute tolerance may develop and the impairment of motor coordination attenuates. It was also predicted that the three factors would contribute to the drinker's decision to drive, with those reporting a greater willingness to drive displaying greater disinhibition, with less motor impairment and subjective intoxication.
Methods
Participants
Twenty adult social drinkers (ten women and ten men) between the ages of 21 and 31 (mean age=23.2, SD=2.6) were recruited to participate in this study. Screening measures were conducted to determine medical history and current and past drug and alcohol use. Any volunteers who self-reported head trauma, psychiatric disorder, or substance abuse disorder were excluded from participation. Volunteers who reported a potential risk for alcohol dependence, as determined by a score of 5 or higher on the Short-Michigan Alcoholism Screening Test (Selzer et al. 1975), were also excluded. Volunteers were recruited via notices placed on community bulletin boards and by university newspaper advertisements. The University of Kentucky Medical Institutional Review Board approved the study, and the participants received $80 for their participation.
Apparatus and materials
Simulated driving task
A computerized driving simulation task was used to measure driving performance (STISIM Drive, Systems Technology Inc., Hawthorne, CA). Participants sat in front of the 19-in. computer display that presented the driving simulation. The simulation placed the driver within the cab of the vehicle and provided a view of the roadway and dashboard instruments. Drivers controlled the vehicle by moving a steering wheel and manipulating the accelerator and brake pedals. The drive test was a daylight driving scenario that required participants to drive 31,100 ft (5.9 miles) on a busy street in a metropolitan setting while obeying all traffic laws. Other vehicles were presented on the roadway at random intervals but required no passing or braking on the part of the driver. The drive test required between 5 and 10 min to complete, depending on the speed of the driver. In order to encourage attentiveness and optimal performance, participants received small monetary rewards (up to $5) for completion of the drive in a timely manner and for obeying traffic signals. This incentive was intended to model real-world conflicts in driving behavior (i.e., motivation to reach a destination quickly versus need to follow traffic laws).
Willingness to drive
Participants' willingness to drive was measured on a visual analogue scale that has been used in previous research (e.g., Marczinski and Fillmore 2009). Participants placed a vertical line at the point representing their willingness to drive on a 100-mm horizontal line ranging from 0 mm “not at all” to 100 mm “very much.”
Inhibitory control
Inhibitory control was measured by a cued go/no-go reaction time task used in other research to measure the disinhibiting effects of alcohol (e.g., Fillmore et al. 2005; Marczinski and Fillmore 2003). E-Prime experiment generation software (Schneider et al. 2002) was used to operate the task, which was performed on a PC. The task requires finger presses on a keyboard and measures the ability to inhibit the pre-potent behavioral response of executing the key press. Cues provide preliminary information regarding the type of imperative target stimulus (i.e., go or no-go) that is likely to follow, and the cues have a high probability of signaling the correct target. Participants were instructed to press the forward slash (/) key on the keyboard as soon as a go (green) target appeared and to suppress the response when a no-go (blue) target was presented. Key presses were made with the right index finger. To encourage quick and accurate responding, feedback was presented to the participant during the inter-trial interval by displaying the words correct or incorrect along with the reaction time (RT) in milliseconds. A test required approximately 15 min to complete.
Motor coordination
A grooved pegboard task (Lafayette Instruments, Lafayette, IN) was used to measure motor coordination. The grooved pegboard is rectangular in shape and consists of a 5 by 5 in. metal surface that contains 25 “keyhole-shaped” holes arranged in five rows of five holes each. Pegs fit into the holes of the board as a key would fit into a lock. Participants were required to pick up the pegs one at a time and place them in the holes, filling in one row at a time (from left to right) before moving to the next row. A trial was completed once all 25 holes had been filled. Time to complete a trial (in seconds) was the measure of interest. A test consisted of four trials and required approximately 5 min to complete.
Subjective intoxication
Degree of subjective intoxication was measured on a visual analogue scale used in previous research (e.g., Fillmore and Blackburn 2002). Participants rated their degree of subjective intoxication by placing a vertical line at the point representing the extent to which they “feel intoxicated” on a 100-mm horizontal line ranging from 0 mm “not at all” to 100 mm “very much.”
Personal drinking habits questionnaire (Vogel-Sprott 1992)
This questionnaire yielded three measures of a drinker's current, typical drinking habits: (a) frequency (the number of drinking occasions per week), (b) quantity (the number of standard alcoholic drinks (e.g., 1.5 oz of liquor) typically consumed per occasion), and (c) duration (time span in hours of a typical drinking occasion). The questionnaire also provided a measure of history of alcohol use (number of months of regular drinking).
Driving history and experience questionnaire
This questionnaire assessed driving experience in terms of length of time that participants held a driver's license or permit and typical frequency of driving days per week.
Procedure
Interested volunteers responded to advertisements by calling the laboratory to participate in an intake-screening interview conducted by a research assistant. They were informed that the purpose of the study was to examine the effects of alcohol on driving performance and other cognitive and behavioral tasks. All sessions were conducted in the Behavioral Pharmacology Laboratory of the Department of Psychology, and testing began between 10 a.m. and 6 p.m. Participants were tested individually and completed an intake session to become acquainted with laboratory procedures. During this session, informed consent was provided, heights and weights were measured, and the questionnaire measures were completed. They also performed practice tests to become familiar with the laboratory tasks.
Test sessions
Task performance was tested under two doses of alcohol: 0.0 (placebo) and 0.65 g/kg. Each dose was administered on a separate test session, and dose order was randomized. This resulted in nine participants receiving placebo first and alcohol second, and 11 participants receiving the reverse order. Sessions were separated by a minimum of 1 day and a maximum of 1 week, and mean inter-session interval (ISI) did not differ between those receiving placebo first (ISI= 5.6) and those receiving alcohol first (ISI=4.8) (p=ns). Participants were instructed to fast for 4 h prior to each test session, as well as to refrain from consuming alcohol or any psychoactive drugs or medications for 24 h before all sessions. Urine samples were tested for drug metabolites, including amphetamine, barbiturates, benzodiazepines, cocaine, opiates, and tetrahydrocannabinol (ON trak TesTstiks, Roche Diagnostics Corporation, Indianapolis, IN, USA) and, in women, HCG in order to verify that they were not pregnant (Mainline Confirms HGL, Mainline Technology, Ann Arbor, MI, USA). Breath samples were measured by an Intoxilyzer, Model 400 (CMI, Inc., Owensboro, KY) to verify a zero BAC.
The alcohol dose was calculatedon the basis of body weight and administered as absolute alcohol mixed with three parts carbonated soda. Participants consumed the dose in 6 min. The dose produces an average peak BAC of 90 mg/100 ml at approximately 65 min and begins to decline at about 75 min (Fillmore et al. 2005; Ostling and Fillmore 2010). The placebo dose (0.0 g/kg) consisted of a volume of carbonated mix that matched the total volume of the 0.65-g/kg alcohol drink. A small amount (3 ml) of alcohol was floated on the surface of the beverage. It was sprayed with an alcohol mist that resembled condensation and provided a strong alcoholic scent as the beverage was consumed.
Following dose administration, participants performed a 30-min test battery in a fixed order: simulated driving task, cued go/no-go task, subjective measures of intoxication and willingness to drive, and grooved pegboard task. The battery was performed twice: 35-min post-administration and 95-min post-administration. Following alcohol administration, these testing times were expected to occur at comparable BACs on each limb of the blood alcohol curve (approximately 70 mg/100 ml) to allow for testing of acute tolerance (Fillmore et al. 2005; Ostling and Fillmore 2010). Participants' BACs were measured at 35, 45, 65, 95, 105, and 125 min after drinking began. Breath samples were also obtained at these times during the placebo session, ostensibly to measure BACs. Once testing was finished, participants remained at leisure in the lounge until their BACs reached 20 mg/100 ml or below.
Criterion measures and data analyses
Driving performance measures
The dependent measures of driving performance included deviation of lane position, line crossings, and steering rate. Deviation of lane position is an indicator of the degree of adjustment that a driver implements to maintain a desired position within the lane, and greater within-lane deviation indicates poorer driving precision. Within-lane position was sampled at each foot of the drive test, and the standard deviation of the driver's average within-lane position was measured in feet. A single lane position standard deviation (LPSD) score for a test was obtained by averaging the deviation measures sampled at each foot of the driving test. The total number of line crossings (i.e., when the vehicle moved outside the lane) was recorded for each drive. Steering rate is a measure of the average speed with which the driver turns the steering wheel to maintain position on the road. Increase in steering rate indicates more abrupt, quick movements of the steering wheel, reflecting less controlled driving performance (Weafer et al. 2008). Rate of steering movement was measured in terms of degree change in the steering wheel per second. This measure was sampled at every foot of the drive to provide an average rate of steering score for a participant.
Cued go/no-go measures
Failure of response inhibition was measured as the proportion (p) of no-go targets in the go cue condition in which a participant failed to inhibit a response (i.e., p-inhibition failures). Response execution was measured as the mean RT to go targets in the no-go cue condition. Shorter RTs indicated greater facilitation of response execution.
Grooved pegboard
Time in seconds required to insert all of the pegs into the board averaged across the four trials served as the dependent measure. Faster mean completion times indicated greater motor coordination.
All dependent measures were analyzed by 2 (dose 0.0 vs. 0.65 g/kg)×2 (test: ascending limb vs. descending limb) within-subject analyses of variance (ANOVAs). Significant dose X test interactions provided evidence of acute tolerance. Initially, all analyses were conducted with gender (male vs. female) and dose order (placebo first vs. alcohol first) as between-participant factors. There were no effects of gender or dose order on acute tolerance for any measure. Therefore, all subsequent analyses presented are collapsed across gender and dose order. The a priori alpha level of significance was set at .05, and effect sizes (partial η2) are given for all significant effects.
Results
Self-reported drinking habits and driving experience
The sample reported a mean frequency of 1.7 (SD=0.9) drinking occasions per week. On average, participants consumed 4.6 (SD=2.3) standard drinks per occasion, over a mean duration of 3.7 h (SD=1.2). The sample reported a mean history of 76.1 (SD=38.4) months of regular drinking. All participants were regular drivers, reporting a mean history of 93.8 (SD=55.5) months of driving with a valid driver's license or learner's permit. Participants drove an average of 6.3 (SD=1.3) days per week.
BACs
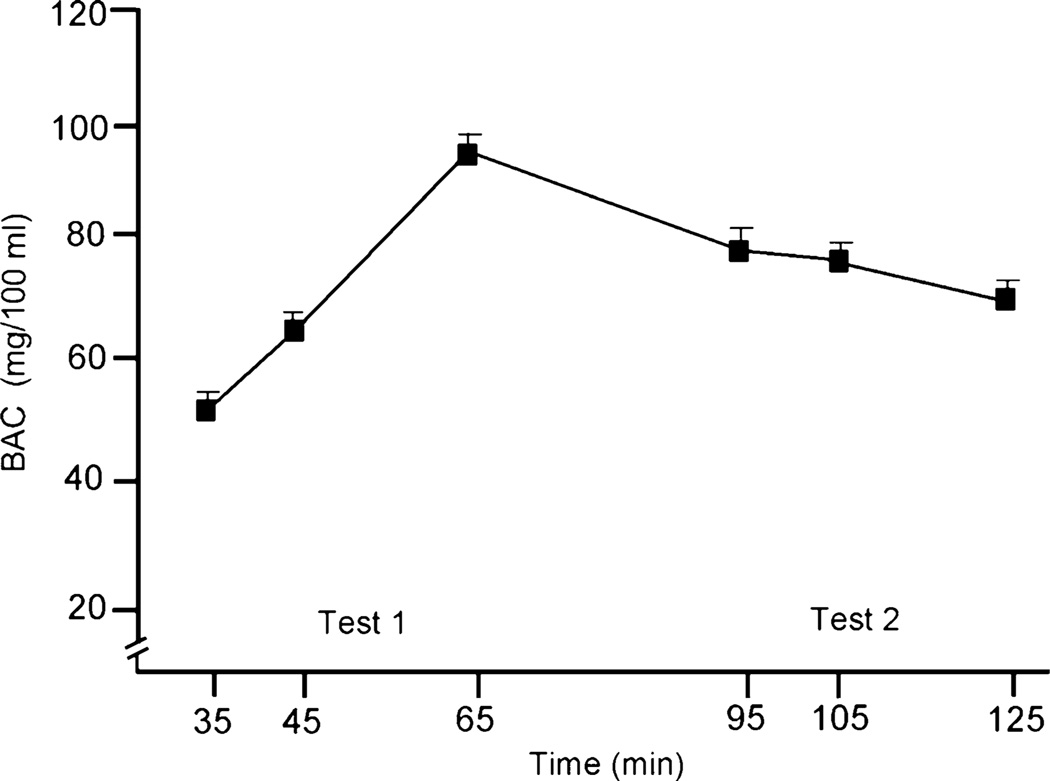
No detectable BACs were observed during the placebo session. BACs during the alcohol session were analyzed by a 2 (gender)×6 (time) mixed-design ANOVA. No main effect or interaction was observed involving gender (ps=ns). There was a main effect of time owing to the rise and fall of BAC over the course of the treatment session F(5, 90)=159.9, p<.01; partial η2=.90. The mean BAC curve for the sample is plotted in Fig. 1. The figure shows that BAC rose to a peak of 94.1 mg/100 ml at 65 min after drinking and then began to decline. To confirm that average BACs during the testing battery for Test 1 (ascending limb) and Test 2 (descending limb) were comparable, we calculated a mean BAC for each participant during each test by averaging the BAC measurement pre- and post-test (35- and 65-min post-drinking for Test 1 and 95- and 125-min post-drinking for Test 2). The mean BAC during Test 1 was 72.4 mg/100 ml (SD=10.7), and the mean BAC during Test 2 was 73.7 mg/100 ml (SD=12.0). A paired-sample t-test obtained no significant difference between mean BAC during Test 1 and Test 2 (t(19)=0.82, p=ns).
Fig. 1.
Mean blood alcohol concentrations (BACs) under alcohol at each interval when breath samples were obtained. The ascending limb test began at 35 min, and the descending limb test began at 95 min. The capped vertical lines show the standard errors of the mean
Simulated driving performance measures
Significant main effects of dose were found for within-lane deviation F(1, 19)=21.8, p<.01; partial η2=.53 and steering rate F(1, 19)=7.5, p<.01; partial η2=.28. A trend toward a significant dose effect was found for line crossings F(1, 19)=3.9, p=.06; partial η2=.17. No main effects or interactions involving test were found for any of the measures (ps=ns). Table 1 presents the mean driving performance values. The table shows that the main effects of dose were due to an increase in scores for each measure following alcohol compared to placebo, indicating poorer driving precision in response to the drug. Furthermore, the degree of alcohol impairment was comparable on both Test 1 (ascending limb) and Test 2 (descending limb), indicating no acute tolerance to alcohol impairment of driving performance.
Table 1.
Mean (SD) simulated driving performance, willingness to drive, and cued go/no-go measures for Test 1 and Test 2 under the alcohol and placebo conditions. Under alcohol (0.65 g/kg), Test 1 coincides with the ascending limb, and Test 2 coincides with the descending limb of the blood alcohol curve
| Alcohol (0.65 g/kg) |
Placebo |
Significance tests |
|||||
|---|---|---|---|---|---|---|---|
| Test 1 M (SD) |
Test 2 M (SD) |
Test 1 M (SD) |
Test 2 M (SD) |
Dose | Test | Dose X test | |
| Driving measures | |||||||
| LPSD (ft) | 1.29 (0.39) | 1.22 (0.31) | 0.96 (0.24) | 1.05 (0.25) | Sig*** | ns | ns |
| Line crossings | 3.95 (7.06) | 3.60 (3.65) | 1.85 (2.13) | 2.05 (3.40) | Sig# | ns | ns |
| Steering rate | 8.91 (3.33) | 8.01 (2.36) | 7.53 (1.76) | 7.47 (2.35) | Sig* | ns | ns |
| Willingness to drive | |||||||
| VAS ratings | 17.1 (27.2) | 38.9 (28.8) | 63.6 (35.1) | 84.0 (30.6) | Sig*** | Sig*** | ns |
| Cued go/no-go measures | |||||||
| P-inhibition failures | 0.22 (0.24) | 0.25 (0.24) | 0.12 (0.16) | 0.15 (0.21) | Sig** | Sig* | ns |
| Reaction time (ms) | 343.3 (23.6) | 346.8 (41.7) | 327.5 (26.2) | 335.4 (40.4) | Sig** | ns | ns |
All contrasts were tested by 2 (dose)×2 (test) within-subjects ANOVAs.
Sig indicates a significance value of p<.05
Sig indicates a significance value of p<.01
Sig indicates a significance value of p<.001.
Sig indicates a significance value of p<.10.
ns indicates a significance value of p>.10
Willingness to drive ratings
Self-reported ratings of willingness to drive revealed a significant main effect of dose F(1, 19)=38.7, p<.01; partial η2=.67 and a significant effect of test F(1, 19)=48.1, p<.01; partial η2=.72. No interaction between dose and test was observed (F(1, 19)=.03, p=ns). Mean willingness to drive ratings are presented in Table 1. Participants were significantly less willing to drive following alcohol compared to placebo. Furthermore, willingness to drive ratings were higher on Test 2 compared to Test 1 for both dose conditions.
Cued go/no-go performance
P-inhibition failures revealed a significant main effect of dose F(1, 19)=9.9, p<.01; partial η2=.34 and a significant main effect of test F(1, 19)=6.0, p<.05; partial η2=.24. No interaction between dose and test was observed (F(1, 19)=.00, p=ns). Mean p-inhibition failures are presented in Table 1. Alcohol impaired inhibitory control as evident by increased failures to inhibit responses under the dose compared with placebo. Moreover, the degree of impairment under alcohol did not subside over the two tests. In fact, the main effect of the test is due to a general increase in p-failures on Test 2 compared to Test 1 following alcohol and placebo. Reaction time analyses revealed a significant main effect of dose F(1, 19)=12.2, p<.01; partial η2=.39 owing to an overall slowing of reaction time under alcohol compared to placebo. No main effect or interaction involving test was observed (ps=ns).
Grooved pegboard task performance
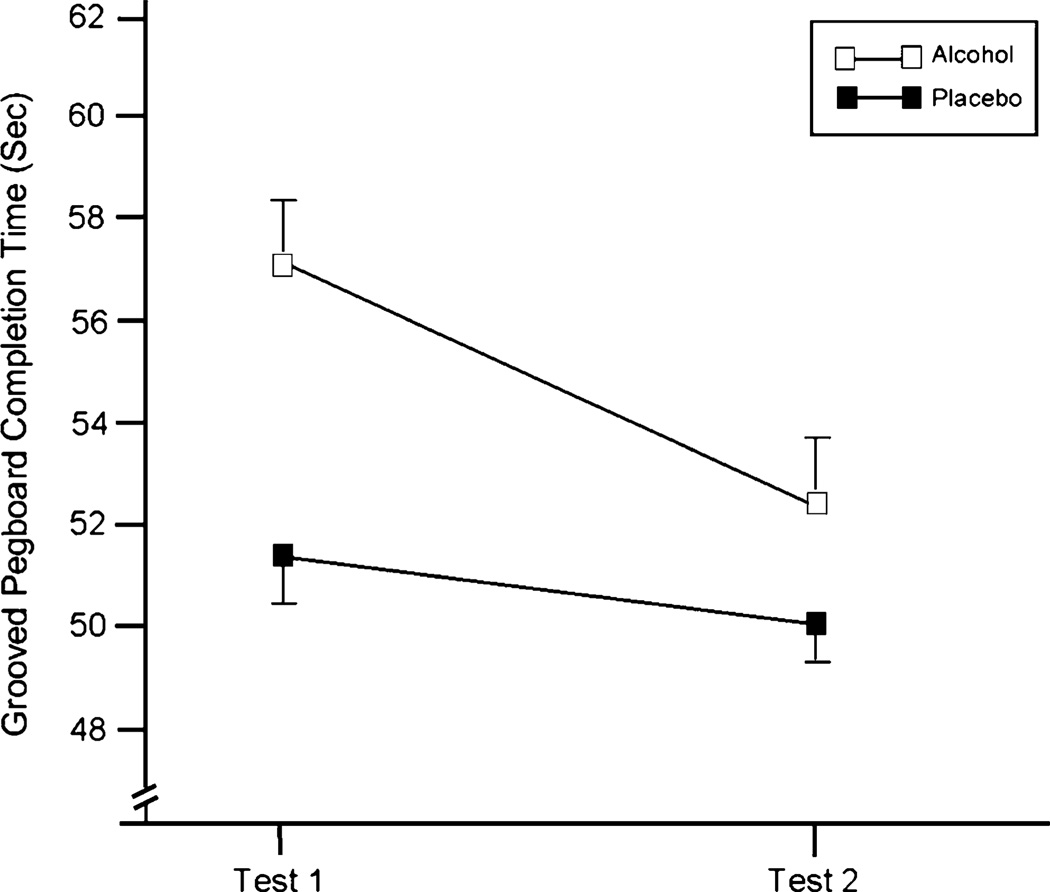
Pegboard task performance revealed significant main effects of dose F(1, 19)=12.4, p<.01; partial η2=.39 and test F(1, 19)=52.0, p<.01; partial η2=.73, and a significant dose X test interaction F(1, 19)=8.1, p<.05; partial η2=.30. The interaction is illustrated in Fig. 2. The figure shows that alcohol impaired (i.e., slowed) performance on this task and that completion time was faster overall on Test 2 compared to Test 1. Moreover, the figure shows that the interaction was due to a decrease in the degree to which alcohol slowed completion time relative to placebo on Test 2 versus Test 1. The magnitude of alcohol impairment at each test was calculated as a difference score by subtracting the completion time under placebo from the completion time under alcohol. For Test 1, alcohol slowed completion time by a mean of 5.7 s. At Test 2, the slowing effect of alcohol was reduced to 2.6 s. This represents a 54% reduction in the magnitude of alcohol impairment from the ascending to the descending limb of the blood alcohol curve, indicating marked acute tolerance to impairing effects of alcohol on motor coordination over this time period.
Fig. 2.
Mean grooved pegboard completion time (s) for Tests 1 and 2 under the 0.0- (placebo) and 0.65-g/kg alcohol dose conditions. The capped vertical lines show the standard errors of the mean
Subjective intoxication ratings
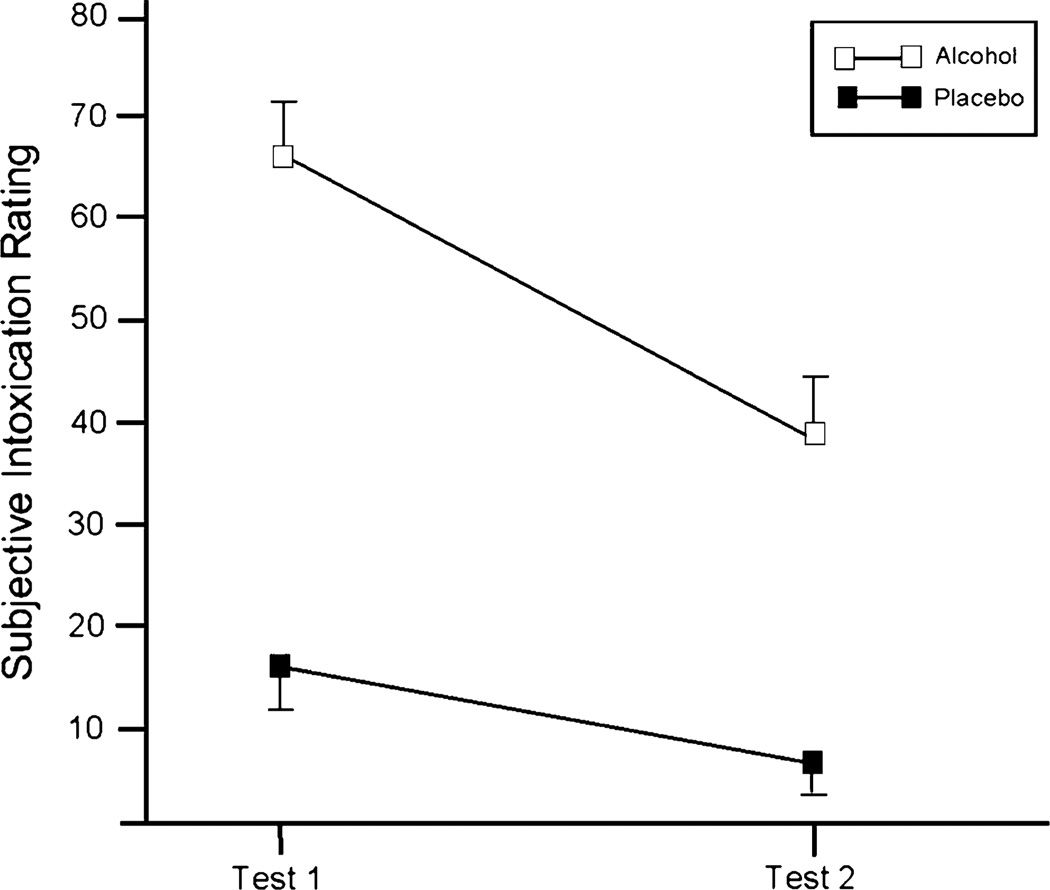
Analysis of subjective intoxication ratings revealed significant main effects of dose F(1, 19)=54.1, p<.01; partial η2=.74 and test F(1, 19)=43.3, p<.01; partial η2=.70, and a significant dose X test interaction F(1, 19)=12.9, p<.01; partial η2=.40. Figure 3 shows that intoxication ratings were greater in response to alcohol compared to placebo and that intoxication ratings decreased overall on Test 2 compared to Test 1. The figure shows that the interaction was due to a pronounced decrease in subjective intoxication following alcohol relative to placebo from Test 1 to Test 2. The magnitude of alcohol effect scores showed that alcohol increased intoxication ratings by a score of 50.0 at Test 1 and only by 32.1 at Test 2. This represents a 36% decrease from the ascending to descending limb, indicating marked acute tolerance to the perceived intoxicating effects of alcohol over this time period.
Fig. 3.
Mean self-reported subjective intoxication for Tests 1 and 2 under the 0.0- (placebo) and 0.65-g/kg alcohol dose conditions. The capped vertical lines show the standard errors of the mean
Predictors of impaired driving performance
To examine the degree to which alcohol impairment of motor coordination and inhibitory control were each associated with impaired driving performance, bi-variate correlational analyses were conducted using the magnitude of impairment scores (i.e., difference score between the alcohol and placebo conditions). These results are presented in Table 2. On the ascending limb, impaired motor coordination significantly predicted alcohol-induced impairment of all three measures of driving performance: LPSD (r=.52, p<.05), line crossings (r=.74, p<.01), and steering rate (r=.55, p<.05). Individuals who exhibited greater alcohol-induced impairment of motor control also exhibited greater alcohol-induced impairment of driving performance. By contrast, on the descending limb, alcohol impairment of motor coordination no longer predicted the degree of impairment on any of the measures of driving performance (ps=ns). Impairment of inhibitory control showed no association to impairment of driving measures on either the ascending or descending limb (ps=ns). Associations between subjective intoxication following alcohol and driving impairment were also examined. Table 2 shows that subjective intoxication did not predict driving impairment on either the ascending or descending limb (ps=ns).
Table 2.
Predictors of impaired driving performance and willingness to drive
| Grooved pegboard |
P-inhibition failures |
Subjective intoxication |
||||
|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | |
| LPSD | 0.52* | 0.29 | −0.25 | −0.16 | 0.29 | 0.03 |
| Line crossings | 0.74*** | 0.08 | 0.12 | 0.14 | 0.37 | 0.06 |
| Steering rate | 0.55* | 0.20 | 0.05 | −0.02 | 0.29 | −0.13 |
| Willingness to drive | −0.28 | −0.16 | 0.10 | 0.52* | −0.45* | −0.44* |
Sig indicates a significance value of p<.05
Sig indicates a significance value of p<.001
Predictors of willingness to drive
Bi-variate correlational analyses were conducted to examine the degree to which alcohol impairment of motor skill and inhibitory control might influence self-reported willingness to drive under alcohol, as well as the degree to which subjective intoxication might influence willingness to drive, and these analyses are presented in Table 2. Alcohol effects on motor coordination did not predict willingness to drive during either the ascending or descending limb (ps=ns). By contrast, alcohol impairment of inhibitory control did predict willingness to drive on the descending limb, with the most disinhibited drivers self-reporting the greatest willingness to drive (r=.52, p<.05). Subjective intoxication ratings were significantly related to willingness to drive on both the ascending (r=−.45, p=.05) and descending limbs (r=−.44, p=.05), with those reporting less intoxication reporting greater willingness to drive.
Discussion
This study examined acute tolerance to alcohol impairment of simulated driving performance and behavioral and cognitive mechanisms considered important to driving. Alcohol impaired driving performance on the ascending limb of the blood alcohol curve. Specifically, intoxicated drivers exhibited greater within-lane swerving, faster and jerkier manipulation of the steering wheel, and more crossings into the opposite lane and onto the road shoulder. Moreover, marked alcohol impairment of each driving measure continued to be observed on the descending limb of the blood alcohol curve, indicating a lack of acute tolerance to impairment of driving ability. Both motor coordination and inhibitory control were also impaired by alcohol. However, acute tolerance was observed specifically to alcohol effects on motor coordination. Indeed, the magnitude of alcohol impairment of motor coordination relative to placebo was reduced by half when measured on the descending limb, despite equivalent BACs between the limbs. By contrast, no acute tolerance was observed to the impairing effects of alcohol on inhibitory control as individuals remained just as disinhibited by alcohol when BAC was declining as when it was rising.
To our knowledge, this is the first study to concurrently examine acute tolerance to alcohol impairment of simulated driving performance and individual skills that are considered important to driving ability. As such, these results provide some important new information as to which behaviors might contribute to the persistence of driving impairment across the blood alcohol curve. It is well established that basic motor skill is impaired by alcohol on the ascending limb and that this affects the ability to operate a motor vehicle (e.g., Holloway 1995; Moskowitz and Robinson 1987). Thus, any acute recovery from the impairing effects of alcohol on motor coordination might allow for some degree of recovery of driving ability as well. However, the current findings showed that acute tolerance to alcohol-impaired motor coordination was not accompanied by any acute tolerance to alcohol-impaired driving performance. Furthermore, correlational analyses showed that alcohol impairment of motor control was a strong predictor of driving impairment on the ascending limb, but not on the descending limb of the blood alcohol curve. Taken together, the findings suggest that motor control might be an important determinant of driving impairment early after drinking, during the rising phase of the blood alcohol curve, but when BAC begins to decline, other factors, such as fatigue or impaired attention, might play a greater role in determining driving impairment.
Another mechanism thought to relate to driving performance is the ability to exercise inhibitory control over inappropriate actions (Barry 1973; Fillmore et al. 2008). As such, the persistence of alcohol-induced disinhibition could play a potential role in impaired driving behavior. Results showed that alcohol impairment of inhibitory control did not diminish on the descending limb, and alcohol-induced disinhibition did not predict alcohol impairment of driving performance on either the ascending or descending limb. It is unclear why inhibitory control was not associated with driving performance in the current study. One possibility is that the specific type of inhibition measured (i.e., inhibition of a pre-potent motor response) does not play as large a role in driving performance as other forms of inhibition. For instance, attentional inhibition (i.e., the ability to ignore distracters in the environment) might be a stronger predictor of alcohol impairment of driving ability as the ability to tune out distracters within the driving scenario and focus on the task at hand is critical to optimal driving performance.
Prolonged disinhibition of behavior under alcohol might actually play a more important role in the individual's decision to drive after drinking. Indeed, the current findings showed that individuals exhibiting greater alcohol impairment of inhibitory control also self-reported greater willingness to drive following alcohol. The disinhibiting effects of alcohol can directly contribute to risky, impulsive decision-making in intoxicated individuals. As such, intoxicated individuals characterized by high levels of disinhibition might be more likely to exhibit drinking and driving behavior. This is especially important to consider given the time frame in which decisions to drive after drinking usually occur. Often, alcohol-impaired driving takes place after a drinking episode has been terminated (e.g., at the end of an evening when individuals are leaving a bar or party to return home). As this likely coincides with the descending limb of the blood alcohol curve, the drinker is likely to remain disinhibited by the drug, increasing the likelihood of a decision to drive after consuming alcohol. The current findings support this scenario in that alcohol-induced disinhibition predicted self-reported willingness to drive specifically as BAC was declining.
Another important finding of this study that should be considered in regard to the decision to drive while intoxicated is the marked decrease in drivers' subjective intoxication on the descending limb. This decrease in subjective intoxication from the ascending to descending limb was accompanied by an increase in the willingness to drive over the same time period, despite elevated BACs at the time of report, and greater willingness to drive was predicted by lower levels of subjective intoxication. Diminished reports of subjective intoxication and increased willingness to drive on the descending limb might be especially important to consider in populations displaying heightened instances of drinking and driving (e.g., binge drinkers, individuals with ADHD). Studies have shown that these populations report less subjective intoxication and a greater willingness to drive following an acute dose of alcohol (Beirness 1987; Marczinski and Fillmore 2009; Marczinski et al. 2008; Weafer et al. 2008; Weafer et al. 2009). Thus, there is some evidence to suggest that those who are more likely to drink and drive might actually underestimate self-intoxication levels, which could contribute to an increased willingness to drive under the influence. Interestingly, these populations at risk for driving while intoxicated also exhibit an increased sensitivity to the disinhibiting effects of alcohol (Marczinski et al. 2007; Weafer et al. 2009), which could also play a role in impulsive decisions to drive while intoxicated.
There are some potential limitations to this study. The sample size was relatively small, limiting generalizability to some degree. Also, ethical considerations prevent us from administering alcohol to individuals reporting a history of alcohol-related problems, including conviction for driving while intoxicated. It is possible that these individuals might exhibit even greater sensitivity to alcohol-induced disinhibition than those in the current sample, and this could be a major contributing factor to their decisions to drive after drinking. Additionally, the method that we chose to measure acute tolerance was based on comparisons between ascending and descending limbs of the blood alcohol curve following a single administration of alcohol. Although the mean BACs during testing were comparable across limbs, the rate of change in BAC during the battery, as well as the BAC range from start to finish, was variable between limbs. The rate of rise in BAC is usually swifter than the rate of descent, and these rate differences could affect impairment (e.g., Fillmore and Vogel-Sprott 1998). An alternative method to studying acute alcohol tolerance is to use intravenous ethanol infusion techniques designed to “clamp” the infusion rate to maintain a specific BAC (O'Connor et al. 1998). This would be a useful procedure to further investigate acute tolerance to impairment of driving performance. Finally, the present study focused on motor coordination and inhibitory control as potential factors related to driving impairment and willingness to drive. However, other skills, such as attention and working memory capacity, likely play important roles in determining driving impairment and decisions to drive as well. Such skills should be the focus of future research.
In conclusion, the study highlights the importance of examining alcohol impairment of individual skills related to driving performance and decisions to drive at later time points during a drinking episode. Acute tolerance can result in diminished impairment of some skills, but not others. Moreover, acute tolerance to the subjective effects of alcohol could reduce the ability to accurately appraise one's fitness to drive. As such, these findings have important implications, particularly in terms of education and prevention of intoxicated driving. Future policy changes could consider education regarding misperceptions of self-intoxication as well as information about the likelihood of sustained impairment of driving ability and inhibitory control in response to a dose of alcohol. Public awareness of these effects of alcohol and the time frame in which they occur has the potential to be quite influential in deterring driving under the influence.
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism Grants R01 AA018274, R01 AA012895, and F31 AA018584.
Footnotes
The authors have no conflict of interest to declare.
Contributor Information
Jessica Weafer, Department of Psychology, University of Kentucky, Kastle Hall, Lexington, KY 40506-0044, USA.
Mark T. Fillmore, Email: fillmore@uky.edu, Department of Psychology, University of Kentucky, Kastle Hall, Lexington, KY 40506-0044, USA.
References
- Barry H. Motivational and cognitive effects of alcohol. J Safety Res. 1973:200–221. [Google Scholar]
- Beirness DJ. Self-estimates of blood alcohol concentration in drinking-driving context. Drug Alcohol Depend. 1987;19:79–90. doi: 10.1016/0376-8716(87)90089-5. [DOI] [PubMed] [Google Scholar]
- Beirness D, Vogel-Sprott M. The development of alcohol tolerance: acute recovery as a predictor. Psychopharmacol (Berl) 1984;84:398–401. doi: 10.1007/BF00555220. [DOI] [PubMed] [Google Scholar]
- Cromer JR, Cromer JA, Maruff P, Snyder PJ. Perception of alcohol intoxication shows acute tolerance while executive functions remain impaired. Exp Clin Psychopharmacol. 2010;18:329–339. doi: 10.1037/a0019591. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn J. Compensating for alcohol-induced impairment: alcohol expectancies and behavioral disinhibition. J Stud Alcohol. 2002;63:237–246. doi: 10.15288/jsa.2002.63.237. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Social drinking history, behavioral tolerance and the expectation of alcohol. Psychopharmacol (Berl) 1996;127:359–364. doi: 10.1007/s002130050098. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: cognitive and pharmacokinetic factors. Alcohol Clin Exp Res. 1998;22:1476–1482. [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66(5):663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn JS, Harrison EL. Acute disinhibiting effects of alcohol as a factor in risky driving behavior. Drug Alcohol Depend. 2008;95:97–106. doi: 10.1016/j.drugalcdep.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger JA. Understanding driving: applying cognitive psychology to a complex everyday task. New York: Psychology Press; 2000. [Google Scholar]
- Haubenreisser T, Vogel-Sprott M. Reinforcement reduces behavioural impairment under an acute dose of alcohol. Pharmacol Biochem Behav. 1987;26:29–33. doi: 10.1016/0091-3057(87)90528-4. [DOI] [PubMed] [Google Scholar]
- Holloway FA. Low-dose alcohol effects on human behavior and performance. Alcohol Drugs Driv. 1995;11:39–56. [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Exp Clin Psychopharmacol. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Acute alcohol tolerance on subjective intoxication and simulated driving performance in binge drinkers. Psychol Addict Behav. 2009;23:238–247. doi: 10.1037/a0014633. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Harrison EL, Fillmore MT. Effects of alcohol on simulated driving and perceived driving impairment in binge drinkers. Alcohol Clin Exp Res. 2008;32:1329–1337. doi: 10.1111/j.1530-0277.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- Meda SA, Calhoun VD, Astur RS, Turner BM, Ruopp K, Pearlson GD. Alcohol dose effects on brain circuits during simulated driving: an fMRI study. Hum Brain Mapp. 2009;30:1257–1270. doi: 10.1002/hbm.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby E. Special Report Series Monograph No. 31. London: Medical Research Committee; 1919. Alcohol: its absorption into and disappearance from the blood under different conditions. [Google Scholar]
- Mitchell MC. Alcohol-induced impairment of central nervous system function: behavioral skills involved in driving. J Stud Alcohol Supp. 1985;10:109–116. doi: 10.15288/jsas.1985.s10.109. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Robinson C. Driving-related skills in impairment at low blood alcohol levels. Alcohol Drugs Traff Saf. 1987;T86:79–86. [Google Scholar]
- O'Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and elimination rate. Alcohol Clin Exp Res. 1998;22:202–210. [PubMed] [Google Scholar]
- Ostling EW, Fillmore MT. Tolerance to the impairing effects of alcohol on the inhibition and activation of behavior. Psychophar-macol (Berl) 2010;212:465–473. doi: 10.1007/s00213-010-1972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ NW, Harwood MK, Geller ES. Estimating alcohol impairment in the field: implications for drunken driving. J Stud Alcohol. 1986;47:237–240. doi: 10.15288/jsa.1986.47.237. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user's guide. Pittsburgh: Psychology Software Tools; 2002. [Google Scholar]
- Schweizer TA, Jolicoeur P, Vogel-Sprott M, Dixon MJ. Fast, but error-prone, responses during acute alcohol intoxication: effects of stimulus-response mapping complexity. Alcohol Clin Exp Res. 2004;28:643–649. doi: 10.1097/01.alc.0000121652.84754.30. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Guthrie S, Linnoila M. Effects of alcohol and other psychotropic drugs on eye movements: relevance to traffic safety. J Stud Alcohol. 1986;47:426–432. doi: 10.15288/jsa.1986.47.426. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M. Alcohol tolerance and social drinking: learning the consequences. Guilford; New York: 1992. [Google Scholar]
- Weafer J, Camarillo D, Fillmore MT, Milich R, Marczinski CA. Simulated driving performance of adults with ADHD: comparisons with alcohol intoxication. Exp Clin Psychopharmacol. 2008;16:251–263. doi: 10.1037/1064-1297.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT, Milich R. Increased sensitivity to the disinhibiting effects of alcohol in adults with ADHD. Exp Clin Psychopharmacol. 2009;17:113–121. doi: 10.1037/a0015418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens CM, Toplak ME, Wiesenthal DL. Cognitive failures as predictors of driving errors, lapses, and violations. Acc Anal Prev. 2008;40:1223–1233. doi: 10.1016/j.aap.2008.01.006. [DOI] [PubMed] [Google Scholar]