Abstract
Lafora disease is a form of progressive myoclonic epilepsy with autosomal recessive transmission. Two genes have been identified so far: EPM2A and NHLRC1, and a third gene, concerning a pediatric onset subform, has been recently proposed. We report the case of a 23-year-old woman of Turkish origin with an unusual disease course. Clinical onset was at the age of 19 years with tonic–clonic seizures, followed by cognitive impairment; EEG was in favor of Lafora disease, and the mutation c.436G>A (a missense mutation substituting aspartic acid in asparagine) in the NHLRC1 gene confirmed this diagnosis. After 5 years of evolution, the patient only has moderate cognitive impairment. Some NHLRC1 mutations, particularly c.436G>A, are associated with a slower clinical course, but there are conflicting data in the literature. This case strengthens the hypothesis that the c.436G>A mutation in the NHLRC1 gene leads to less severe phenotypes and late-onset disease.
Keywords: Lafora disease, Progressive myoclonic epilepsy, EPM2A, EPM2B, NHLRC1
Lafora disease is a form of progressive myoclonic epilepsy with autosomic recessive transmission. Clinical signs include myoclonus, cognitive disorders, and generalized or occipital seizures. EEG recordings show slow background, anteroposterior dedifferentiation, and diffuse, irregular spike–waves, together with marked occipital spikes and photosensitivity. Pathology shows periodic acid Schiff (PAS)-positive Lafora bodies on skin or liver biopsy, but molecular biology is nowadays considered essential. Two genes have been identified so far, the first one is EPM2A [1], [2] with the 6q24 mutation present in 80% of cases. This gene codes for laforin, a dual specificity phosphatase that plays a part in the elimination process of intracellular glycosans. The second gene is NHLRC1 [3], also named EPM2B (with 6p22 as the most frequent mutation) which codes for malin, an ubiquitin ligase that plays a part in laforin destruction. A third gene (PRDM8, with the c.781T>C (Phe261Leu) mutation) has been recently reported that codes for a protein possibly interacting with laforin and malin and that could be expressed in a childhood-onset subform of Lafora disease [4].
We are reporting the case of a 23-year-old woman of Turkish origin. She had no particular medical history during childhood and adolescence. Her parents were cousins. At the age of 20, she experienced for the first time generalized tonic–clonic seizures during her fifth month of pregnancy, which were treated with lamotrigine and clobazam. Seizures persisted with a frequency of one per month until delivery. Cerebral MRI and standard blood tests were normal. EEG recordings showed a slow background with generalized spikes and spike–waves (Fig. 1). After delivery, levetiracetam was added because seizures were not well controlled. When she was 21, the patient presented the first signs of cognitive impairment. Neuropsychological evaluation showed a frontal syndrome associated with an anomic aphasia and memory impairment. Video-EEG made in 2010 showed theta waves and delta waves of large amplitude in anterior regions, with a 2-Hz frequency, which decreased with eyes opening and increased with hyperpnea, and short bursts of generalized spikes and spike–waves. Lamotrigine was stopped, levetiracetam was decreased, and zonisamide was introduced. One month after these modifications, seizure frequency decreased (she had one seizure between March and June 2010). Progressive myoclonic epilepsy was suggested. Mutation screening was performed in the EPM2A and NHLRC1 genes as previously reported [5]. The c.436>A substitution, leading to a change of a highly conserved aspartic acid to asparagine in codon 146 in the NHLRC1 gene at a homozygote state, was detected. At the age of 22, cognitive evaluation showed progressive worsening, with alteration of memory, language, attention, and executive functions.
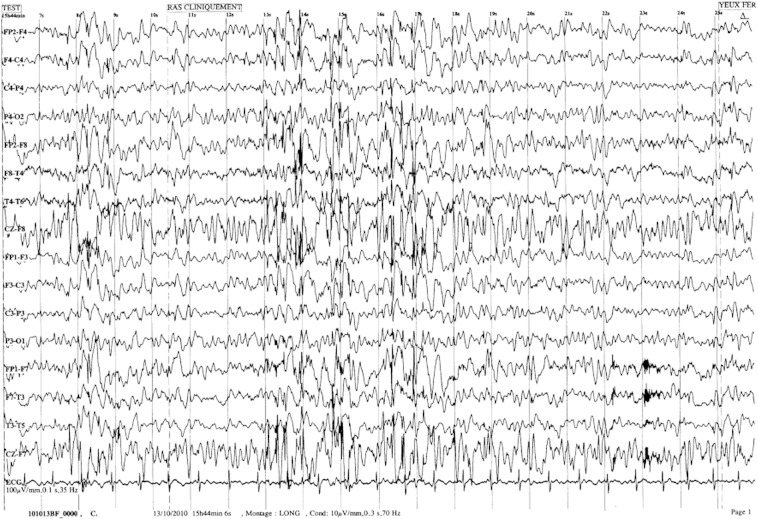
Fig. 1.
Mrs. C.'s EEG (10 μV/mm, 0.3 s, 70 Hz, longitudinal assembly). We can observe a slow background of theta frequency with bursts of diffuse spike and polyspike–waves.
The case is characterized by the late onset and slow disease progression: four years after the first signs, the patient is still able to walk, talk, and live at home. She never presented myoclonus. Because of this unusual clinical presentation, the diagnosis of Lafora disease was only raised because of the EEG pattern. NHLRC1 mutations are suggested to be associated with a later onset of the clinical signs and a slower disease course, as described by Gomez-Abad et al. [6] and Baykan et al. [7]. The p.Asp146Asn/D146N mutation found in our patient has been previously reported in eleven subjects in the literature (Table 1) [7], [8], [9], [10]. This mutation was found in another consanguineous Lafora family who did not originate from the same region of Turkey. In the family with four relatives reported by Baykan et al. [7], the mean age at onset was 19.5 years old. Another patient with slow disease course and without myoclonus was reported by Salar et al. [10]; this subject had the same mutation as our patient. The mean age at onset in patients with the D146N mutation reported in the literature is 18 years old; this mutation is the only one reported with such late ages at onset. However, there are conflicting data in the literature. Other authors reported cases of patients with the NHLRC1 mutation that were different from the c.436G>A mutation and which were associated with fast evolution and severe impairment [5], [8], [11].
Table 1.
Cases with the c.436G>A mutation reported in literature.
| Author | Age at onset | Origin | Myoclonus | Type of seizures | EEG | Sex | Clinical status at time of study | Seizure frequency | Age at the time of study |
|---|---|---|---|---|---|---|---|---|---|
| Lanoiselée | 19 | Turkish | − | GTCSs | Slow BG | W | Cognitive impairment | 1/month | 24 |
| Baykan | 19 | Turkish | + | GTCSs | Slow BG, generalized spike–waves and polyspike–waves | M | Mild myoclonus, GTCSs, moderate cognitive impairment | 1 to 4/year | 32 |
| Baykan | 21 | Turkish | + | GTCSs | Slow BG, generalized spike–waves and polyspike–waves | M | Mild myoclonus, GTCSs, moderate cognitive impairment | 1 to 4/year | 29 |
| Baykan | 17 | Turkish | + | GTCSs | Slow BG, generalized spike–waves and polyspike–waves | M | Moderate cognitive impairment | 1/year | 28 |
| Baykan | 21 | Turkish | + | GTCSs | Slow BG, generalized spike–waves and polyspike–waves | M | Minor myoclonus, GTCSs, slight cognitive decline | < 1/year | 25 |
| Salar | 30 | Turkish | − | GTCSs | Slow BG, diffuse spikes and waves | M | Dementia, extrapyramidal signs | 48 | |
| Couarch | 13 | Turkish | + | GTCSs, absence | Slow BG, diffuse and FT epileptic abnormalities, PS | W | Severe intellectual impairment, ataxia disabling myoclonus | 27 | |
| Couarch | 13 | Turkish | + | M | |||||
| Franceschetti | 15 | Italian | + | Myoclonus | M | Mild cognitive and motor impairment, preserved daily living activities, social interaction | 2 | ||
| Franceschetti | 18 | Italian | GTCSs | M | Mild cognitive and motor impairment, preserved daily living activities, social interaction | 28 | |||
| Franceschetti | 13 | Italian | Absence | W | Mild cognitive and motor impairment, preserved daily living activities, social interaction |
GTCSs: generalized tonic–clonic seizures.
PS: photosensitivity.
BG: background.
In healthy subjects, the laforin–malin complex plays a part in the protein targeting to glycogen (PTG) destruction process, so it acts as a brake in glycogen production. Couarch et al. [9] reported that NHLRC1 mutations result in glycogen accumulation. These mutations affect the malin–laforin interaction and the PTG ubiquitination. The slower disease course in patients with D146N mutations could be explained by a partial preservation of enzymatic activity of the D146N variant that is not observed with other mutations. Lafora disease presents an important genetic heterogeneity, and there may be cases with late onset and slower progression in association with NHLRC1 mutations, which may cause a partial loss of function. It must be noted, however, that EEG remains typical for Lafora disease even in such atypical cases.
Lafora disease is still a mysterious affection. This case report shows that the clinical signs of Lafora disease can be different from the usual description made in 1911 [12]; it is conceivable that some less severe cases remain unknown. Because of unusual presentations, EEG is sometimes the only clue that hints at the possibility of Lafora disease. The relationship between phenotype and genotype is still incompletely understood.
Conflict of interest
None of the authors has any conflict of interest to disclose.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Minassian B.A., Lee J.R., Herbrick J.A., Huizenga J., Soder S., Mungall A.J. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet. 1998;20:171–174. doi: 10.1038/2470. [DOI] [PubMed] [Google Scholar]
- 2.Serratosa J.M., Gomez-Garre P., Anta B., de Bernabé D.B., Lindhout D., Augustin P.B. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2) Hum Mol Genet. 1999;8(2):345–352. doi: 10.1093/hmg/8.2.345. [DOI] [PubMed] [Google Scholar]
- 3.Chan E.M., Young E.J., Ianzano L., Munteanu I., Zhao X., Christopoulos C.C. Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat Genet. 2003;35:125–127. doi: 10.1038/ng1238. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull J., Girard J.-M., Lohi H., Chan E.M., Wang P., Tiberia E. Early-onset Lafora body disease. Brain. 2012;135:2684–2698. doi: 10.1093/brain/aws205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesca G., Boutry-Kryza N., De Toffol B., Milh M., Steschenko D., Lemesle-Martin M. Novel mutations in EPM2A and NHLRC1 widen the spectrum of Lafora disease. Epilepsia. 2010;51(9):1691–1698. doi: 10.1111/j.1528-1167.2010.02692.x. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Abad C., Gomez-Garre P., Gutiérrez-Delicado E., Sayqi S., Michelucci R., Tassinari C.A. Lafora disease due to EPM2B mutations: a clinical and genetic study. Neurology. 2005;22:64(6):982–986. doi: 10.1212/01.WNL.0000154519.10805.F7. [DOI] [PubMed] [Google Scholar]
- 7.Baykan B., Striano P., Gianotti S., Bebek N., Gennaro E., Gurses C. Late onset and slow progressing Lafora disease in four siblings with EPM2B mutation. Epilepsia. 2005;46(10):1695–1697. doi: 10.1111/j.1528-1167.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 8.Franceschetti S., Gambardella A., Canafoglia L., Striano P., Lohi H., Gennaro E. Clinical and genetic findings in 26 Italian patients with Lafora disease. Epilepsia. 2006;47:640–643. doi: 10.1111/j.1528-1167.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 9.Couarch P., Santiago V., Gourfinkel-An I., Lesca G., Gataullina S., Fedirko E. Lafora progressive myoclonus epilepsy: NHLRC1 affects glycogen metabolism. J Mol Med. 2011;89(9):915–925. doi: 10.1007/s00109-011-0758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salar S., Yeni N., Gündüz A., Güler A., Gökçay A., Velioğlu S. Four novel and two recurrent NHLRC1 (EPM2B) and EPM2A gene mutations leading to Lafora disease in six Turkish families. Epilepsy Res. 2012;08:273–276. doi: 10.1016/j.eplepsyres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Brackmann F., Kiefer A., Agaimy A., Gencik M., Trollman R. Rapidly progressive phenotype of Lafora disease associated with a novel NHLRC1 mutation. Pediatr Neurol. 2011;44:475–477. doi: 10.1016/j.pediatrneurol.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Lafora G.R. Über das Vorkommen amyloider Körperchen im Innerender Ganglienellen. Virchows Arch. 1911;205:295–303. [Google Scholar]