Abstract
A clean and sustainable deicing additive was prepared via the adsorption of acetate anions (Ac-) by magnesium (Mg) and aluminum (Al) calcined layered double hydroxide (Mg/Al-CLDH). Fourier transform infrared spectroscopy spectrums proved that Ac- had intercalated into LDH structure. X-ray diffraction patterns, scanning electron microscopy and transmission electron microscopy images showed that the intercalation spacing and platelet thickness of Mg and Al layered double hydroxide containing Ac- anions (Mg/Al-Ac- LDH) had been enlarged due to substitution of divalent CO3 2- anions by a larger quantity of monovalent Ac– anions. Differential scanning calorimetry tests testified that the insoluble Mg2/Al-Ac- LDH evidently decreased the freeze point (FP) of water to -10.68°C. X-ray photoelectron spectroscopy analyses confirmed that the Ac- were strongly confined by the metal layers of LDHs. FP test of asphalt mixtures confirmed that Mg/Al-Ac- LDHs reduced FP to -5.5°C. Immersion test results indicated that Mg/Al-Ac- LDH had a good deicing durability and Ac- did not released from asphalt mixture. Snow melting observation was conducted further testified that Mg/Al-Ac- LDH melted snow or ice sustainably.
Introduction
Ice may impose a potential risk for traffic safety, because tire skidding, brake failure or even losing control of direction are prone to take place on the pavement. Conventional prevention method is spraying chloride salts, which are including NaCl, CaCl2 or MgCl2. However, excessive use of chloride-based salt pollutes the surrounding environment and promotes corrosion in steel structure of vehicles, bridge and water pipes [1–5]. In addition, chloride anions would weaken the bonding strength between asphalt and aggregate, thereby affecting the service life of asphalt pavement [6, 7]. To mitigate these harmful effects, alternatives such as calcium-magnesium acetate (CMA) has been considered [8, 9]. Acetate-based salts have the similar melting snow or ice efficiency to that of chloride, and they are less harmful for the surrounding environment [10–12]. Nevertheless, it cannot melt snow or ice sustainably on the asphalt pavement, because rain or melted snow will wash the acetate anions (Ac-).
The method of incorporating the chloride deicing additives (Cl-DIA) in asphalt mixture has been developed since the last few decades [13–15]. Cl-DIA was added into asphalt mixture to play a role of self-melting snow or ice. Chloride component will gradually migrate from the interior structure of asphalt pavement due to the compression, vibration and wear from traffic load [16, 17]. However, the released chloride anions still pollute the surrounding environment and construction to some extent. Therefore, it is essential to develop a clean and durable deicing additive in the asphalt mixture.
Layered double hydroxides (LDHs) have attracted a great attention from many researchers owing to its anion exchangeable characteristic [18]. Its general formula of LDH is [M1-x 2+Mx 3+·(OH)2]x+Ax/n n-·mH2O, while M2 + is a divalent metal cation, M3 + is a trivalent metal cation, An- is an interlayer anion [19]. Previous papers have extensively reported that different types of anions can be intercalated into LDHs to inhibit or control their releasing process in aqueous solution [20–23]. In our preliminary test, we found that the freeze point of magnesium (Mg) and aluminum (Al) carbonate layered double hydroxide (Mg/Al-CO3 2- LDH) slurry is marginally lower than that of distilled water.
In this paper, magnesium (Mg) and aluminum (Al) acetate layered double hydroxide (Mg/Al-Ac- LDH) was prepared from Mg/Al-CO3 2- LDH by calcination recovery. The structure, component, and morphology of Mg/Al-Ac- LDH were characterized using Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). The effect of Mg/Al-Ac- LDH on decreasing freezing point (FP) of water was identified by differential scanning calorimetry (DSC) tests. Then, Mg/Al-Ac- LDH and Cl-DIA were added to asphalt mixtures to prepare Marshall specimen. The deicing properties of the mixtures were evaluated by FP measurement, immersion test and snow melting observation.
Material and Methods
Materials
The Mg/Al-CO3 2- LDH was provided by Jiangyin Ruilaw Chemical Co. LTD, Jiangsu, China. The structural formula of the respective LDH was Mg1-xAlx(OH)2(CO3)2/x·mH2O. The LDH samples were heated at 500°C for 4 h and the calcined Mg/Al-CO3 2- LDH sample is denoted as Mg/Al-CLDH. The physical and chemical properties of the samples are shown in Table 1. A commercial Cl-DIA was provided by Gezhouba Wuhan Road Materials Co.LTD, Hubei, China. Styrene—Butadiene—Styrene (SBS) modified asphalt that was produced by the China Best Modified Asphalt Co. LTD, Hubei, China and had a penetration of 48 dmm at 25°C, a ductility of 30 cm at 5°C, a softening point of 81°C, and a dynamic viscosity of 2.4 Pa.s at 135°C. Basalt and mineral filler (MF) (machine-glazed limestone) were obtained from Jingzhu, Hubei, China. All of the chemicals and reagents were analytical grade (Sinopharm Chemical Reagent Co., Ltd) and were used without further purification. Distilled water was used throughout the preparation and treatment processes.
Table 1. The physical and chemical properties of the Mg/Al-CLDHs.
| Sample | Molar ratio (Mg2+/Al3+) | Surface area(m2/g) | Average pore size(nm) | Pore volume(cm3g-1) |
|---|---|---|---|---|
| Mg2/Al-CLDH | 2:1 | 35.9 | 26.9 | 0.24 |
| Mg3/Al-CLDH | 3:1 | 33.5 | 25.6 | 0.22 |
| Mg4/Al-CLDH | 4:1 | 31.7 | 24.8 | 0.21 |
Preparation of Mg/Al-Ac- LDH
Mg/Al-Ac- LDH was prepared from Mg/Al-CO3 2- LDH by calcination recovery. First, 200g Mg/Al-CO3 2- LDH was calcined at 500°C for 2 h in a muffle furnace to remove CO3 2- anions. The cooling process was operated in a closed stored pan, which had to be evacuated air immediately by a vacuum pump to avoid carbon dioxide. Second, the calcined product and 500 ml of potassium acetate solution (concentration of 30 wt%) were mixed in a glass flask and the mixture was stirred for 2 h. Third, the mixture was filtered and the residue was dried at 105°C for 24 h and was grinded to obtain Mg/Al-Ac- LDH.
Characterizations
The FTIR spectra of Mg(2–4)/Al-CO3 2- LDH and the Mg(2–4)/Al-Ac- LDH were obtained using a Nexus FTIR spectrometer from Thermo Nicolet Corp. (New York, USA). The scan range was between 4000 cm-1 and 400 cm-1 with a resolution of 4 cm-1. XRD graphs of the Mg(2–4)/Al-CO3 2- LDH and the Mg(2–4)/Al-Ac- LDH were obtained using an X-ray diffractometer (D/MX-III A, Rigaku, Japan) with Cu Kα radiation (λ=0.15406 nm; 40 kV, 50 mA). The data were collected in step-scan mode at a scanning rate of 0.02°/s. The diffractograms were scanned from 5° to 20°. The freezing points of the 5 wt% slurries of Mg2/Al-Ac- LDH and Mg2/Al-CO3 2- LDH were determined using a DSC thermal analysis system (Perkin-Elmer 7 Series) that was equipped with a cooling accessory. Before recording the warming scans, a 5-mg slurry sample was sealed in an aluminum pan, cooled to -40°C in a nitrogen atmosphere and then heated to 20°C at a rate of 2°C /min. The freezing point of LDH slurry corresponds to the onset temperature of the DSC curve, and the peak area corresponds to the enthalpy of adsorption. The Mg2/Al-CO3 2- LDH and Mg2/Al-Ac- LDH powders were attached to stubs using a double-sided adhesive (carbon tape). The electrical conductivities of the samples were enhanced by sputtering the sample surfaces with a thin film of platinum using a sputter coating system (Baltec MED020, Vaduz, Liechtenstein). The sample morphologies were investigated by SEM (Quanta 250 FEG, FEI, Oregon, USA). TEM analyses were conducted using a JEM-2100F electron microscope (JEOL, Japan) at a 200-kV accelerating voltage.
Preparation of asphalt mixture with deicing additives
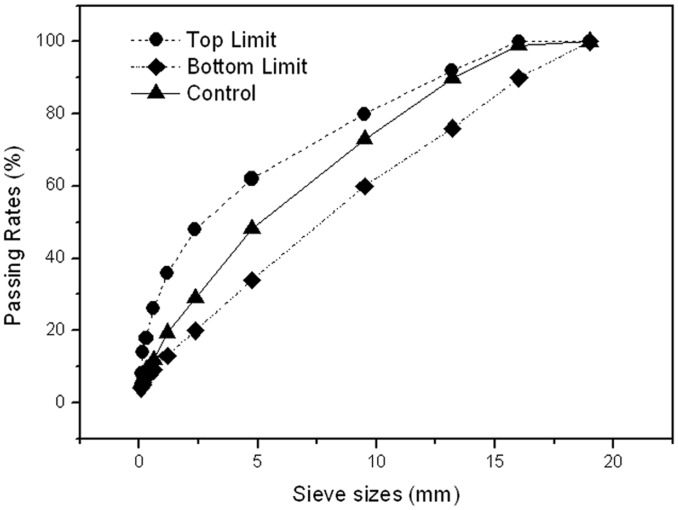
An AC-16 asphalt mixture is commonly used in the surface layer of asphalt pavement. According to the standard JTG F40–2004 (Technical Specification for Construction of Highway Asphalt Pavements, the Ministry of Transport of the People’s Republic of China), designed gradation of control mixture containing 6wt% MF is shown in Fig. 1.
Figure 1. The gradation of the control mixture.
The optimum asphalt content was determined to be 5.3 wt% from the Marshall test. The particle size distributions of the deicing additives (Mg2/Al-Ac- LDH and Cl-DIA) and the MF were given in Table 2. The particle sizes and density of Mg2/Al-Ac- LDH and Cl-DIA were similar to that of MF. Thus, in this experiment, the asphalt mixture was prepared by replacing the MF with equal weights of the two deicing additives.
Table 2. Particle size distribution of deicing additives and mineral filler.
| Sieve Size/mm | Passing Rate/% | ||||
|---|---|---|---|---|---|
| 1.18 | 0.6 | 0.3 | 0.15 | 0.075 | |
| Mg2/Al-Ac- LDH | 100 | 94.56 | 87.54 | 61.33 | 39.35 |
| Cl-DIA | 100 | 96.32 | 86.24 | 53.27 | 36.49 |
| MF | 100 | 97.58 | 88.94 | 62.79 | 43.56 |
Specimen preparation
The SBS modified asphalt was mixed with aggregates at 160°C for 90 s to obtain a well-distributed mixture. The Cl-DIA and MF were subsequently added to mixtures and mixed for 60 s. The hot mixtures were then placed in steel frames and compacted using 75 blows for each surface at 150°C to obtain the Marshall specimens (Φ101.6 mm×63.5 mm).
Evaluation of deicing properties of asphalt mixture
Freezing points of asphalt mixtures. Deicing additives are used to reduce the FPs of asphalt pavements. Therefore, it is essential to measure the FP of an asphalt mixture to evaluate the effect of deicing additive. The testing temperature range was from 5°C to -10°C. The following test method was used to determine the FPs. First, the Marshall specimen was placed in a refrigerator at 5°C for 1 h to achieve an equivalent temperature with environmental temperature. Then, 10 g of distilled water was added on the surface of the Marshall specimen and a plastic ring was firmly pasted around the edge by silicone adhesive to seal the water. A thermocouple with a precision of 0.1°C (TES-1310, by TES Electrical Electronic Co., LTD in Taiwan) was used to automatically record the temperature of the water film every 10 s with a data logger. The reflection peak point of output time-temperature curve was considered as the FP. Fig. 2 illustrates the scence picture for testing the FP of a Marshall specimen.
Figure 2. Scene picture for testing the FP of a Marshall specimen in a refrigerator.
Conductivity of immersion liquid. The conductivity of a liquid increases with the ion concentration [24], thus, the rate of ion release from a deicing asphalt mixture was determined by measuring the conductivity of the immersed liquid at different periods. The following measurement procedure was used. A Marshall specimen was completely immersed in 500 ml of deionized water at 25±1°C in a stainless steel container. The conductivity of the immersed liquid was then measured by a conductivity tester (DDS-11C, by Jingke Scientific Instrument Co., LTD in Shanghai, China) every 24 h and the immersed liquid was completely substituted with fresh water each day to simulate the washing cycles of real melted snow or rain. In addition, pavement abrasion caused by traffic load is considered as another critical factor to affect the amount of released ions. A mechanical abrasion by steel bush was applied on the Marshall specimen surface at a 5-days interval and the immersion test was conducted for a couple of days. Fig. 3 illustrates the scene picture of conductivity test.
Figure 3. Scene picture of measuring the conductivity of immersion test.
Freezing point of asphalt mixture after immersion. To evaluate the effect of rain on the ice-melting performance of an asphalt pavement that contains a deicing additive, the FPs of asphalt mixtures were measured after each immersion cycle.
Results and Discussion
FTIR
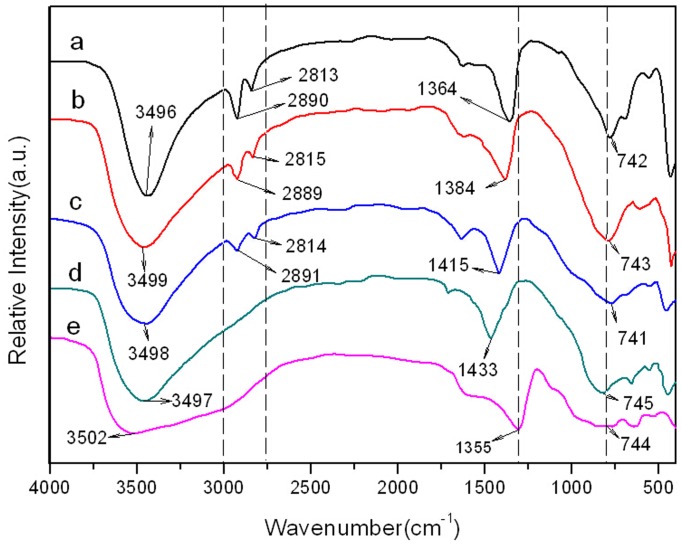
FTIR was used to identify whether Ac- anions have intercalated in the Mg/Al-CLDH. Fig. 4a, b and c show that the Mg(2–4)/Al-Ac- LDH samples exhibit two bands between 2750 and 3000 cm−1 assigned to the vibrational absorption of the methyl group, while Fig. 4d and e do not show any characteristic peaks in this range. It indicated that acetate anions (Ac-) had entered the LDH interlayer galleries of Mg(2–4)/Al-Ac- LDH. It is noteworthy that the intensities of the methyl peaks increased as the Mg/Al molar ratio decreased. This is because Mg2/Al-CLDH with the higher surface area than Mg3/Al-CLDH and Mg4/Al-CLDH (see in Table 1) can absorb larger amount of acetate anions. The peak at 1355 cm−1 in the spectrum of the Mg2/Al-CO3 2- LDH sample was attributed to the vibrational absorption of the interlayer CO3 2-, as reported previously [25]. After the calcination and adsorption of Ac-, this band shifted to a higher wavenumber. This is because a small amount of carbon dioxide has been absorbed by wet Mg(2–4)/Al-Ac- LDH during the drying process [26, 27]. For all FTIR spectra, the peaks appeared between 400 and 800 cm−1 were arisen from a superposition of the characteristic vibrations of Mg and Al oxides and the wide and extremely intense peaks at approximately 3500 cm−1 were attributed to hydrogen bonds.
Figure 4. FT-IR spectra.
(a) Mg2/Al-Ac- CLDH, (b) Mg3/Al-Ac- CLDH, (c) Mg4/Al-Ac-CLDH, (d) Mg2/Al-CLDH, (e) Mg2/Al-CO3 2- LDH.
XRD
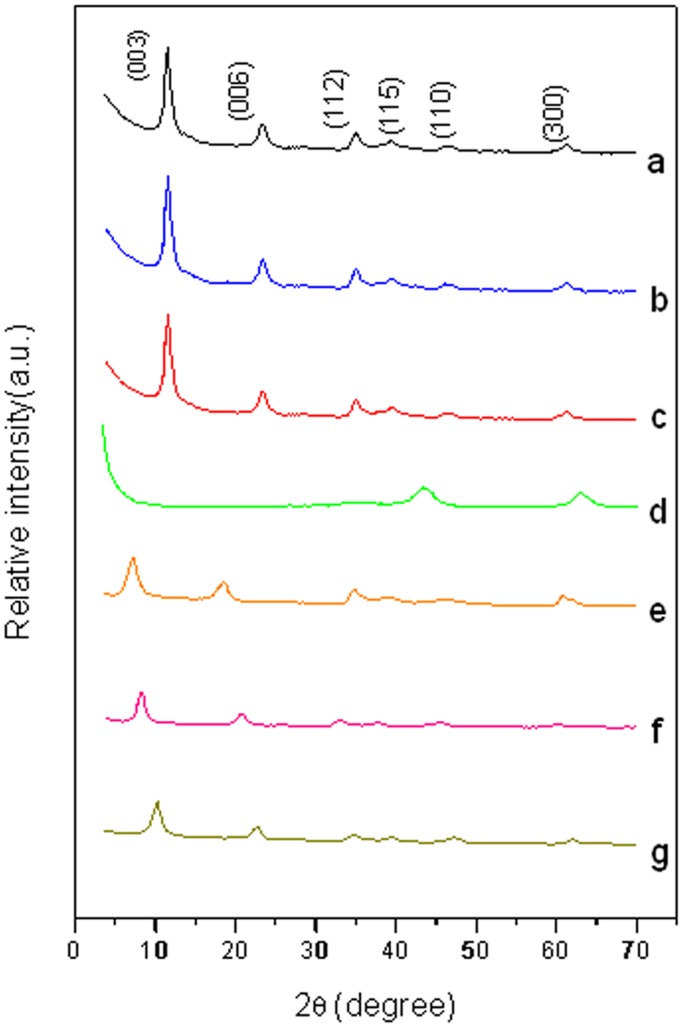
XRD can be used to analyze the crystal structure of Mg(2–4)/Al-CO3 2- LDH, Mg2/Al-CLDH and Mg(2–4)/Al-Ac- LDH. Fig. 5 (a, b, c) show that Mg(2–4)/Al-CO3 2- LDH have a series of sharp and strong diffraction peaks(003 and 006)at low 2θ values and small symmetric peaks (112, 115, 110 and 300) at high 2θ values. These peaks are typical reflections of the LDH metal layers. The interlayer distances of d (003) for Mg(2–4)/Al- CO3 2- LDH are both 7.56 Å, which can be calculated by the Bragg formula (nλ=2dsinθ). In this formula, n is an integer (n = 1), λ is the wavelength of incident wave (1.54nm) and d is the interlayer spacing. After calcination, the diffraction peaks (003 and 006) at a low 2θ values disappear and the peak area ratios are also changed (Fig. 5d), indicating that the layered structure is destroyed during calcinations. Fig. 5 e, f, and g reveal that (003), (006) and (112) diffraction peaks appeared again after calcinations. That means that Mg(2–4)/Al Ac- LDH have restored their layered structures after the adsorption of Ac- due to the “memory effect”. In addition, it can also be seen that the 2θ values of (003) peaks for Mg(2–4)/Al-Ac- LDH are 6.41, 9.12 and 10.63, corresponding to the interlayer distances of 13.77 Å, 9.69 Å and 8.32 Å. This result could be attributed to varying amount of Ac- among the metal cation layers, which are composed of divalent magnesium cations and trivalent aluminum cations. When the Mg/Al molar ratio decreased, the proportion of Al3+ increased and therefore the LDH metal cation layers can absorb more number of monovalent Ac-. As a result, the interlayer distance of LDH will be enlarged due to the increased amount of Ac-, which is in accordance with FTIR analysis. Further observation on the weakened intensity of the Mg(2–4)/Al-CLDH diffraction peaks after uptake of Ac- revealed that the crystallinity is decreased. This is owing to the calcination and rehydration procedure [28]. The diffraction angles and interlayer distances of LDHs are shown in Table 3.
Figure 5. XRD patterns.
(a) Mg2/Al-CO3 2- LDH, (b) Mg3/Al-CO3 2- LDH, (c) Mg4/Al-CO3 2- LDH, (d) Mg2/Al-LDH, (e) Mg2/Al-Ac- LDH, (f) Mg3/Al-Ac- LDH, (g) Mg3/Al-Ac- LDH.
Table 3. Diffraction angles at peak (003) and interlayer distances of LDHs.
| Specimen | Mg2/Al CO32-LDH | Mg3/Al CO32-LDH | Mg4/Al CO32-LDH | Mg2/Al-CLDH | Mg2/Al Ac- LDH | Mg3/Al Ac- LDH | Mg4/Al Ac- LDH |
|---|---|---|---|---|---|---|---|
| 2θ (degree) | 11.69 | 11.69 | 11.69 | ─ | 6.41 | 9.12 | 10.63 |
| Interlayer Distance(Å) | 7.56 | 7.56 | 7.56 | ─ | 13.77 | 9.69 | 8.32 |
DSC
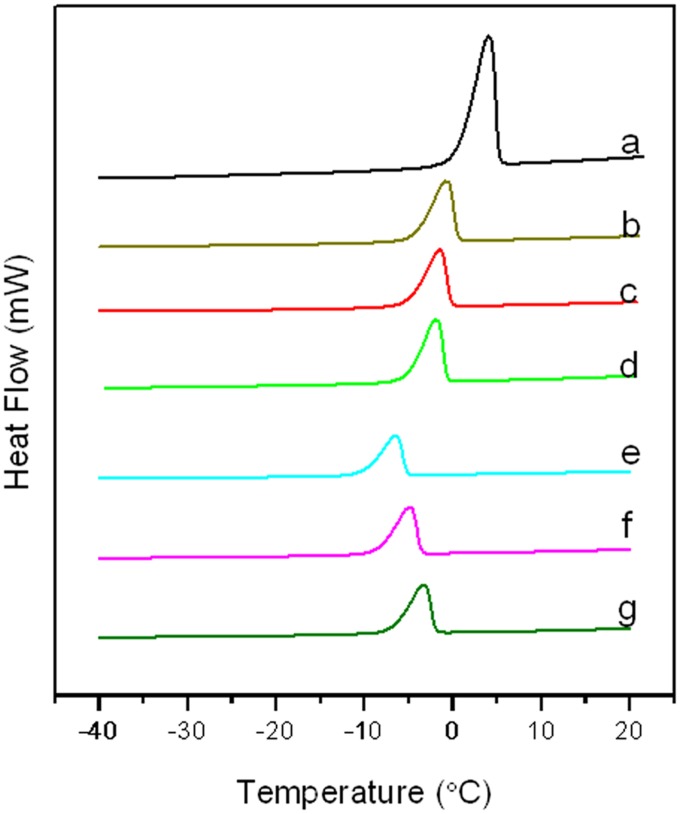
To indentify the effect of Mg2/Al-Ac- LDH and Mg(2–4)/Al-CO3 2- LDH on decreasing the FP of water, DSC was used to measure the FPs of their slurries. Fig. 6(b, c, and d) show that the FPs of the Mg(2–4)/Al-CO3 2- LDH of -3.48°C, -3.39°C, and -3.26°C were less than that of pure water (-2.53×10-2°C). Moreover, the endothermic peak areas (i.e., the enthalpy) of the Mg(2–4)/Al-CO3 2- LDH were 287.53 J/g, 293.47 J/g, and 298.76 J/g, which were less than that of pure water (375.68 J/g). That is, less heat was required to melt ice containing Mg(2–4)/Al-CO3 2- LDH powder than pure ice. These results indicate that Mg(2–4)//Al-CO3 2- LDH can marginally decrease the FP of water. Fig. 6 (e, f, and g) show that the FPs of the Mg(2–4)/Al-Ac- LDH decreased to -10.68°C, -8.98°C, and -7.13°C, and the corresponding enthalpies decreased to 107.36 J/g, 121.39 J/g, and 130.58 J/g. It is attributed to the increased amount of the acetate anions in the LDH metal layers, which is consistent with FTIR and XRD analysis. This result confirms that Mg2/Al-Ac- LDH has the better deicing capacity than the other two LDH deicers. The FPs and enthalpies of water and LDHs slurries are listed in Table 4.
Figure 6. DSC curves.
(a) pure water, (b) Mg2/Al-CO3 2- LDH, (c) Mg3/Al-CO3 2- LDH, (d) Mg4/Al-CO3 2- LDH, (e) Mg2/Al-Ac- LDH, (f) Mg3/Al-Ac- LDH, (g) Mg4/Al-Ac- LDH.
Table 4. Freeze points and enthalpies of water and LDHs slurries.
| Specimen | pure water | Mg2/Al CO32-LDH | Mg3/Al CO32-LDH | Mg4/Al CO32-LDH | Mg2/Al Ac- LDH | Mg3/Al Ac- LDH | Mg4/Al Ac- LDH |
|---|---|---|---|---|---|---|---|
| Freeze Point(°C) | -2.53×10-2 | -3.48 | -3.39 | -3.26 | -10.68 | -8.98 | -7.13 |
| Enthalpy(J/g) | 375.68 | 287.53 | 293.47 | 298.76 | 107.36 | 121.39 | 130.58 |
Morphology
The SEM photomicrographs for Mg2/Al-LDH and Mg2/Al-Ac- LDH are shown in Fig. 7. Fig. 7a depicts that the Mg2/Al-CO3 2- LDH crystallites consisted of homogeneous platelets and that the crystallites dimension ranged from 200 nm to 500 nm. Fig. 7a and c show that the distribution of Mg2/Al-Ac- LDH platelets was less uniform than that of the Mg2/Al-CO3 2- LDH platelets. This difference could be attributed to the decarbonation of Mg/Al-CO3 2- LDH during calcinations [29]. The release of CO3 2- anions could lead to the collapse of some metal cation layers, thereby rearranging the platelets. The “memory effect” of LDH [30] can be used to recovered Mg2/Al-Ac- LDH platelets by the intercalation of Ac- anions, which leads to defects and interconnected edges. Fig. 7b and d show that the thickness of each Mg/Al-CO3 2- LDH platelets was approximately 110 nm and that the thickness of the Mg2/Al-Ac- LDH platelets was about 172 nm. The crystal platelets were stacked with numerous metal cation layers; thus, the increase in the Mg2/Al-Ac- LDH platelet thickness was attributed to the enlarged interlayer distance among the metal cation layers. Fig. 7e confirms that the LDH sample is composed of hexagonal nanosheets with smooth surface, in agreement with the previous results [31]. Fig. 7f shows that the presence of an interlayer distance of about 0.76 nm(7.6 Å) is consistent with the distance of the (003) plane, corresponding with the X-ray analysis. It is noteworthy that the CLDH sample (Fig. 7g) nearly remains in the laminar shape of nanosheets with less regular shape and Fig. 7h further confirms that the intercalation of Ac- has enlarged the interlayer distance of Mg2/Al-Ac- LDH.
Figure 7. SEM and TEM images of the samples.
Mg2/l- CO3 2- LDH (a, b), Mg2/Al-Ac- LDH (c, d); TEM and high-resolution TEM images of the samples: Mg2/Al- CO3 2- LDH (e, f), Mg2/Al-Ac- LDH (g, h).
XPS
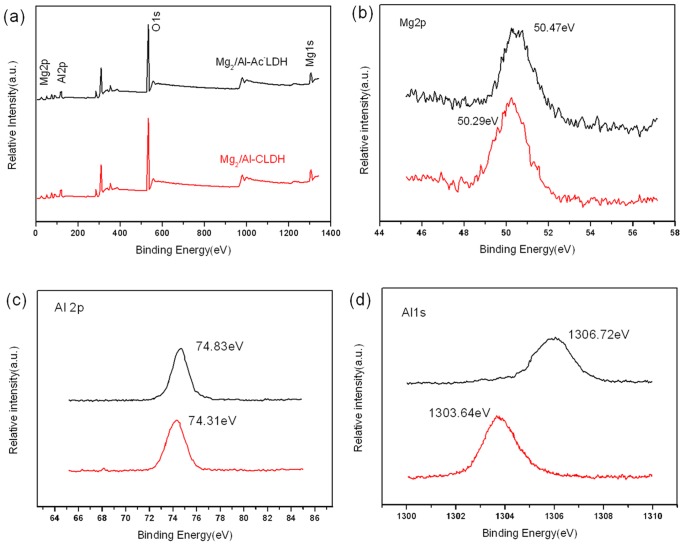
To indentify whether Ac- anions have been confined in the Mg2/Al-Ac- LDH, XPS was used to measure the binding energy of metal elements in Mg2/Al-CLDH and Mg2/Al-Ac- LDH. Fig. 8a shows the XPS survey spectra of the Mg2/Al-CLDH samples before and after the adsorption of Ac-. The peaks at binding energies of 50.29, 74.31, and 1303.64 eV were related to Mg 2p, Al 2p, and Al 1s before the adsorption of Ac-, respectively. Fig. 8b shows that the binding energy of Mg 2p increased from 50.29 eV before adsorption to 50.47 eV after adsorption, indicating that the interaction between the Mg cations and the acetate anions was strengthened. Fig. 8c and d show that the binding energies of Al 2p and Al 1p increased from 74.31 eV and 1303.64 eV to 74.83 eV and 1306.72 eV. That means the electronegativity of Mg2/Al-Ac- LDH is stronger than that of Mg2/Al-CLDH [28, 32, 33]. These positive shifts provided further evidence of the strengthening of the interactions between the Al cations and the acetate anions by adsorption. The aforementioned results show that the acetate anions entered into the host layer lattice of the Mg2/Al-CLDH during the rehydration process, and the anion interaction has a strong restricted effect on the motion of the acetate anions. Therefore, Mg2/Al-Ac- LDH can produce a sustainable decrease in the freezing point of water.
Figure 8. XPS survey spectra.
(a) and high-resolution XPS spectra o f Mg 2p (b), Al 2p (c) and Al 1s (d) of Mg2/Al-LDH before and after adsorption of Ac-.
Evaluation of the deicing properties of asphalt mixtures
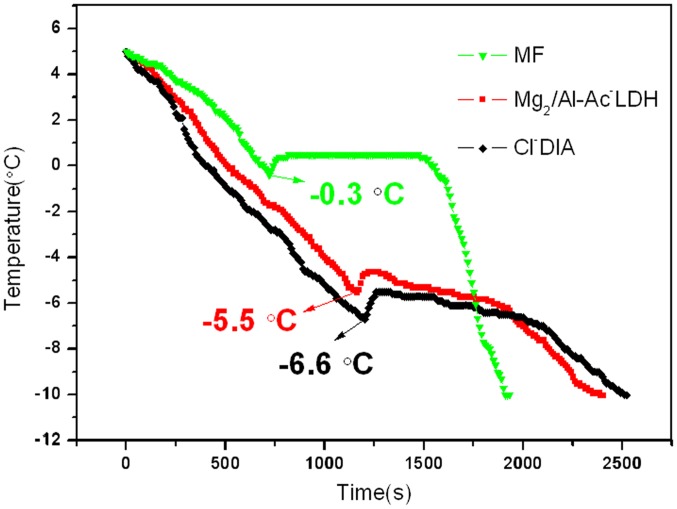
Freezing point of asphalt mixtures with deicing additives. FPs of asphalt mixtures with MF, Mg2/Al-Ac- LDH and Cl-DIA are shown in Fig. 9. Due to the supercooling process of water, there are both temperature reflection peaks in these curves [34]. In the MF group, asphalt mixture containing MF, the temperature of water film on the surface of MF asphalt mixture achieved the temperature reflection peak at -0.3°C after 720 s. Subsequently, a temperature plateau appeared near 0°C and eventually dropped down with prolonging time. In Mg2/Al-Ac- LDH and Cl-DIA groups, temperature of water film on the surface of Mg2/Al-Ac- LDH and Cl-DIA asphalt mixtures reduced to -5.5°C at 1150 s and -6.6°C at 1230 s, respectively. After then, the curves did not show a plateau but temperature kept going down. This can be attributed to that the FP of a liquid layer at the interface between asphalt mixture and ice continues to decrease because of decreasing volume of free liquid. Comparing these two freeze process, it can be found that FPs of Mg2/Al-Ac- LDH and Cl-DIA groups were apparently lower than that of MF group. Moreover, freeze process of Mg2/Al-Ac- LDH and Cl-DIA groups can be delayed. From the FP test results of asphalt mixtures containing Mg2/Al-Ac- LDH and Cl-DIA and MF, it testifies that Mg2/Al-Ac- LDH and Cl-DIA can both decrease the FPs of water films on the asphalt pavement and delay the freeze process.
Figure 9. Freezing points of asphalt mixtures containing deicing additives.
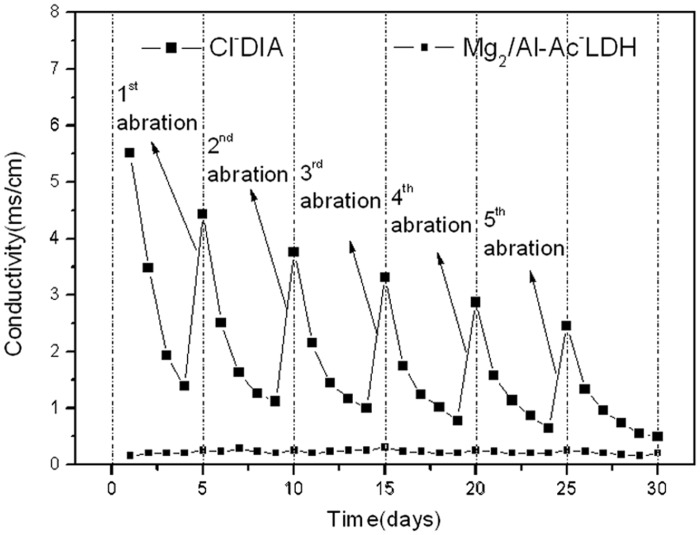
Immersion liquid conductivity. The conductivities of the immersion liquids for the Marshall specimen at different immersion times are shown in Fig. 10. After one day of immersion, the conductivity of the immersion liquid for the Mg2/Al-Ac- LDH and Cl-DIA groups were 0.31 ms/cm and 5.56 ms/cm, implying that ions were well confined in the Mg2/Al-Ac- LDH layered structure during the immersion. In the next three days, the conductivity of the immersion liquid for the Marshall specimen containing Mg2/Al-Ac- LDH remained nearly constant (0.3 ms/cm) but the conductivities of the immersed liquid for the Marshall specimen containing Cl-DIA were gradually decreased. It indicated that Mg2/Al-Ac- LDH had a good durability of fixing ions and the ions from Cl-DIA at the exterior of asphalt mixture was consuming. When the first mechanical abrasion was applied on the surface of asphalt mixture, the conductivity of immersion liquid for Cl-DIA group surged to 4.48 ms/cm but the conductivity of immersion liquid for Mg2/Al-Ac- LDH group was still unchanged. In addition, the conductivities of immersion liquids for the Cl-DIA group showed a declining zigzag trend in the following mechanical abrasion cycles due to decreasing residual salt in asphalt mixture. These results implied that Ac- anions in Mg2/Al-Ac- LDH were not washed under dynamic conditions and the amount of releasing ions from Cl-DIA was gradually decreased.
Figure 10. Conductivities of the immersion liquids of Marshall specimens containing deicing additives.
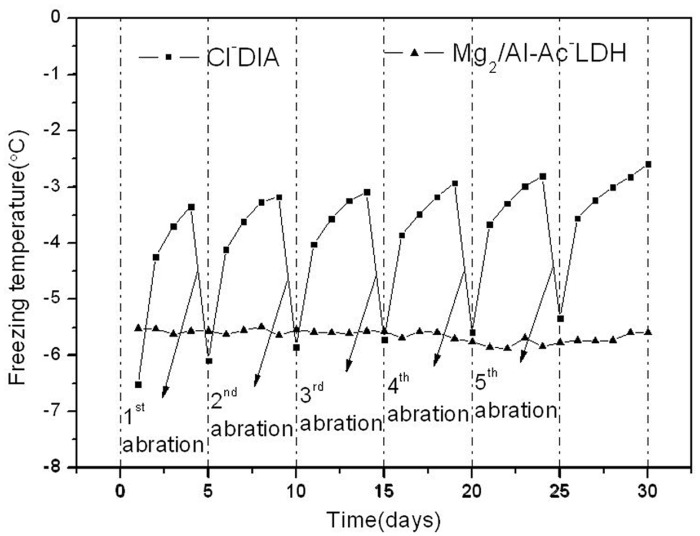
Freezing points of asphalt mixtures after immersion. FPs for the asphalt mixtures with Mg2/Al-Ac- LDH or Cl-DIA after immersion are shown in Fig. 11. The FP of the asphalt mixture with Cl-DIA increased from -6.5°C to -2.9°C after 4 days of immersion, showing that the deicing property of the Cl-DIA Marshall specimen had been significantly weakened. After the first mechanical abrasion, the FPs of Cl-DIA showed an increasing zigzag trend in the following mechanical abrasion cycles. It was interesting to underline that the freezing temperature of Mg2/Al-Ac- LDH and Cl-DIA asphalt mixture after the 4th mechanical abrasion were -5.5°C and -5.4°C, respectively. That indicated that the Mg2/Al-Ac- LDH had better deicing capability than Cl-DIA after a certain degree of mechanical abrasion. In contrast, the FP of the Marshall specimen containing Mg2/Al-Ac- LDH remained at around -5.5°C throughout the entire immersion process, implying that the deicing property of the asphalt mixture containing Mg2/Al-Ac- LDH did not change during the long time immersion. The above results testified that Mg2/Al-Ac- LDH had the sustainable anti-ice property.
Figure 11. Freezing points of Marshall specimens after immersion.
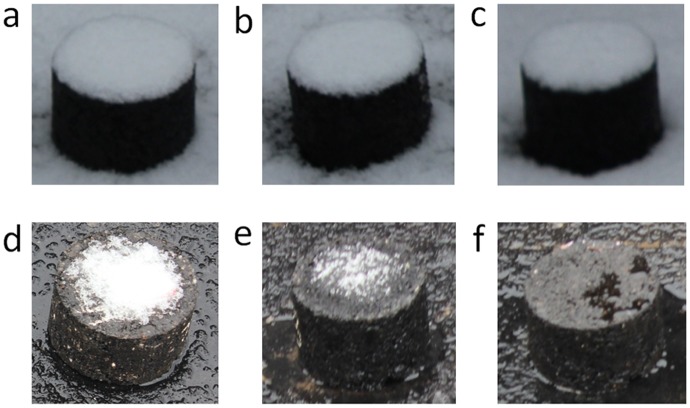
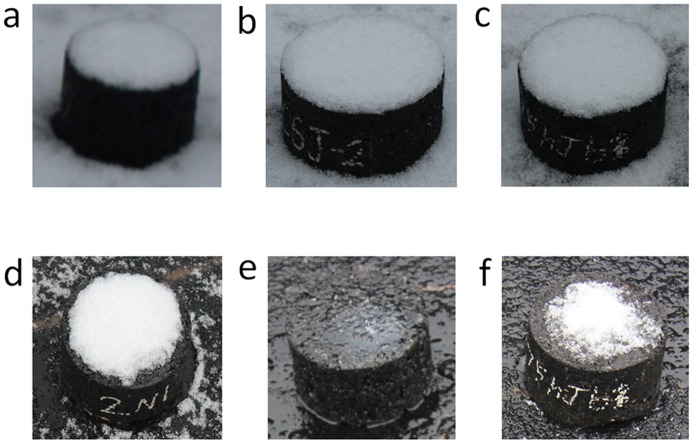
Snow melting observations. As is known, melting accumulated snow can effectively prohibit the icy road surface. In this test, Marshall specimens were placed outdoors before the snow. The first snow event occurred on the night of 6th, February in 2014 and the temperature was about -4〜-5°C during the observation process. Photos of the first snow melting observation are shown in Fig. 12. Obviously, the accumulated snow on surface of asphalt mixture containing Mg2/Al-Ac-LDH and Cl-DIA nearly melted. It testified that the Mg2/Al-Ac- LDH and Cl-DIA can melt snow in a short term. After that, the melted snow and rain washed the asphalt mixture several times in the next couple of days. The second snow event occurred on the morning of 12th, February in 2014 and the snow melting temperature was about -3〜-4°C. Photos of the second snow melting observation are shown in Fig. 13. It can be seen that asphalt mixture containing Mg2/Al-Ac- LDH melted snow evidently but the asphalt mixture containing Cl-DIA melted less snow than the former. This result can be explained by that the exposed Cl-DIA had been solved by melt snow or rain but the insoluble Mg2/Al-Ac- LDH can continuously play a role of melting snow. In practical asphalt pavement, traffic load is another key factor to melt snow or ice. Cl-DIA on the asphalt pavement will not be exhausted, because residual Cl-DIA from the interior layer of asphalt pavement keeps migrating to the road surface due to the mechanical abrasion. In other words, this static snow melting observation can only be used as an indirect approach to compare the effect of the melted snow or rain on melting-snow property of Mg2/Al-Ac- LDH and Cl-DIA.
Figure 12. Photos of the first snow melting observation.
(a). Asphalt mixture containing MF, (b). Asphalt mixture containing Mg2/Al-Ac- LDH, (c). Asphalt mixture containing Cl-DIA; (d). Asphalt mixture containing MF after 4 h, (e). Asphalt mixture containing Mg2/Al-Ac- LDH after 4 h, (f). Asphalt mixture containing Cl-DIA after 4 h.
Figure 13. Photos of the second snow melting observation.
(a). Asphalt mixture containing MF, (b). Asphalt mixture containing Mg2/Al-Ac- LDH, (c). Asphalt mixture containing Cl-DIA; (d). Asphalt mixture containing MF after 4 h, (e). Asphalt mixture containing Mg2/Al-Ac- LDH after 4 h, (f). Asphalt mixture containing Cl-DIA after 4 h.
Conclusions
On the basis of the characterizations of Mg2/Al-Ac- LDH and its deicing property, the main findings and conclusions can be drawn.
(a) FTIR spectra confirmed that Ac- had intercalated into Mg-Al LDH structure. XRD patterns showed that the interlayer distance of Mg2/Al-Ac- LDH was larger than those of Mg/Al-CO3 2- LDH and other Mg(3,4)/Al-Ac- LDHs, implying that a larger quantity of monovalent Ac- anions was required to compensate for the lost divalent CO3 2- anions. DSC curves demonstrated that Mg2/Al-Ac- LDH significantly reduced the FP of water. SEM showed that the Mg2/Al-Ac- LDH platelets were thicker than that the of Mg2/Al-CO3 2- LDH platelets and TEM further confirmed that the interlayer distance of Mg2/Al-Ac- LDH had been enlarged due to that double-layer distance of monovalent Ac- anions was wider than single-layer distance of divalent CO3 2- anions. XPS spectra proved that Ac- anions were strongly confined in LDH structure.
(b) The conductivity of the immersion liquid for the Marshall specimen showed that Mg2/Al-Ac- LDH constrained the Ac- anions in the Marshall specimens. FPs of the asphalt mixtures indicated that Mg2/Al-Ac- LDH had a better anti-ice durability than Cl-DIA. Snow melting observation further testified that Mg2/Al-Ac- LDH sustainably melted snow or ice and did not pollute environment.
Acknowledgments
This work is financially supported by the Ministry of Transport of the People’s Republic of China (Grant No. 2013 318 800 020) and the Independent Innovation Foundation of Wuhan University of Technology (Grant No: 145201016). The authors gratefully acknowledge them.
Data Availability
All relevant data are within the paper.
Funding Statement
This work is financially supported by the Ministry of Transport of the People’s Republic of China (grant No. 2013 318 800 020, URL: http://www.moc.gov.cn/ and the Independent Innovation Foundation of Wuhan University of Technology (grant No: 145201016, URL: http://cxjj.whut.edu.cn/).
References
- 1. Bryson GM, Barker AV (2002) Sodium accumulation in soils and plants along Massachusetts roadsides. Commun Soil Sci Plan 33:67–78. 10.1081/CSS-120002378 [DOI] [Google Scholar]
- 2. Fay L, Shi XM (2012) Environmental impacts of chemicals for snow and ice control: State of the knowledge. Water Air Soil Pollut 223(5):2751–2770. 10.1007/s11270-011-1064-6 [DOI] [Google Scholar]
- 3. Shi XM, Fay L, Yang ZX, Nguyen TA, Liu YJ (2009) Corrosion of deicers to metals in transportation infrastructure: Introduction and recent developments. Corros Rev 27(1–2):23–52. [Google Scholar]
- 4. Cunningham MA, Snyder E, Yonkin D, Ross M, Elsen T (2008) Accumulation of deicing salts in soils in an urban environment. Urban Ecosys 11:17–31. 10.1007/s11252-007-0031-x [DOI] [Google Scholar]
- 5. Gałuszka A, Migaszewski ZM, Podlaski R, Dołęgowska S, Michalik A (2011) The influence of chloride deicers on mineral nutrition and the health status of roadside trees in the city of Kielce. Poland Environ Monit Assess 176: 451–464. 10.1007/s10661-010-1596-z [DOI] [PubMed] [Google Scholar]
- 6. Feng DC, Yi JY, Wang DS, Chen LL (2010) Impact of salt and freeze—thaw cycles on performance of asphalt mixtures in coastal frozen region of China. Cold Reg Sci Technol 62(1):34–41. 10.1016/j.coldregions.2010.02.002 [DOI] [Google Scholar]
- 7. Starck P, Löfgren B (2007) Influence of de-icing agents on the viscoelastic properties of asphalt mastics. J Mater Sci 42(2):676–685. 10.1007/s10853-006-0316-0 [DOI] [Google Scholar]
- 8. Locke CE, Kennelley KJ, Boren MD, Luster V (1987) A study of corrosion properties of a new deicer, calcium magnesium acetate. Transport Res Rec 1113:30–38. [Google Scholar]
- 9. Palmer DA (1987) Formates as alternative deicers. Transport Res Rec 1127:34–36. [Google Scholar]
- 10. Bang SS, Johnston D (1998)Environmental effects of sodium acetate/formate deicer, Ice Shear. Arch. Environ Contam Toxic 35:580–7. 10.1007/s002449900419 [DOI] [PubMed] [Google Scholar]
- 11. Manning DG, Crowder LW (1989) Comparative field study of the operational characteristics of calcium magnesium acetate and rock salt. Transport Res Rec 1246:18–26. [Google Scholar]
- 12. Hellstén PP, Salminen JM, Jørgensen KS, Nystén TH (2005) Use of potassium formate in road winter deicing can reduce groundwater deterioration. Environ Sci Technol 39:5095–5100. 10.1021/es0482738 [DOI] [PubMed] [Google Scholar]
- 13. Jones PH (1981) Snow and ice control and the transport environment. Environ Conserv 8:33–38. 10.1017/S0376892900026655 [DOI] [Google Scholar]
- 14. Ninomiya H, Takeichi K, Kawamura K (1993) Performance Evaluation of Pavement with Deicing Materials. Transportation Research Record 1387:151–155. [Google Scholar]
- 15. Szatkowski WS, Swanson LH (1986)Assessment of Verglimit (an anti-frost agent) in UK surfacing trials. Res Rep Transport Road Res 84:1–11. [Google Scholar]
- 16. Wang ZJ, Li FP, Ma R, Yan EH (2013) Experiment on Road Performance of Asphalt Mixture with Automatic Long-term Snowmelt Agent. Int J Pavement Res Technol 6(4):372–378. [Google Scholar]
- 17. Tan YQ, Sun RR, Guo M, Zhong Y, Zhou SW (2013) Research on deicing performance of asphalt mixture containing salt. China J Highway Transport 26:23–29. [Google Scholar]
- 18. Miyata S (1983) Anion-exchange properties of hydrotalcite-like compounds. Clay Miner 31:305–311. 10.1346/CCMN.1983.0310409 [DOI] [Google Scholar]
- 19. Cavani F, Trifirò F, Vaccari A (1991) Hydrotalcite-type anionic clays: Preparation, properties and applications. Cataly Today 11:173–301. 10.1016/0920-5861(91)80068-K [DOI] [Google Scholar]
- 20. Dong LJ, Ge CL, Qin PY, Chen Y, Xu QH (2014) Immobilization and catalytic properties of candida lipolytic lipase on surface of organic intercalated and modified MgAl-LDHs. Solid State Sci 31:8–15. 10.1016/j.solidstatesciences.2014.02.006 [DOI] [Google Scholar]
- 21. Gao XR, Lei LX, O’Hare D, Xie J, Gao PR, Chang T (2013) Intercalation and controlled release properties of vitamin C intercalated layered double hydroxide. J Solid State Chem 203:174–180. 10.1016/j.jssc.2013.04.028 [DOI] [Google Scholar]
- 22. Huang ZJ, Wu PX, Zhang X, Wang XR, Zhu NW, et al. (2012) Intercalation of Fe(III) complexes into layered double hydroxides: Synthesis and structural preservation. Appl Clay Sci 65:87–94. 10.1016/j.clay.2012.05.007 [DOI] [Google Scholar]
- 23. Chuang YH, Liu CH, Tzou YM, Chang JS, Chiang PN, et al. (2010) Comparison and characterization of chemical surfactants and bio-surfactants intercalated with layered double hydroxides (LDHs) for removing naphthalene from contaminated aqueous solutions. Colloid Surface A 366(1–3):170–177. 10.1016/j.colsurfa.2010.06.009 [DOI] [Google Scholar]
- 24. Liu ZZ, Xing ML, Chen SF, He R, Cong PL (2014) Influence of the chloride-based anti-freeze filler on the properties of asphalt mixtures. Constr Build Mater 51:133–140. 10.1016/j.conbuildmat.2014.06.094 [DOI] [Google Scholar]
- 25. Mameri N, Yeddou AR, Lounici H, Belhocine D, Grib H, et al. (1998) Defluoridation of septentrional Sahara water of North Africa by electrocoagulation process using bipolar aluminium electrodes. Water Res 32(5):1604–1612. 10.1016/S0043-1354(97)00357-6 [DOI] [Google Scholar]
- 26. Zou Y, Ali´rio ER (2002) Hydrotalcite-like compounds as adsorbents for carbon dioxide. Energ Convers Manage 43(14): 1865–1876. 10.1016/S0196-8904(01)00125-X [DOI] [Google Scholar]
- 27. Di Cosimo JI, Dıez VK, Xu M, Iglesia E, Apesteguıa CR (1998) Structure and surface and catalytic properties of Mg-Al basic oxides. J Cataly 178(2):499–510. 10.1006/jcat.1998.2161 [DOI] [Google Scholar]
- 28. Zhou JB, Cheng Y, Yu JG, Liu G (2011) Hierarchically porous calcined lithium/aluminum layered double hydroxides: facile synthesis and enhanced adsorption towards fluoride in water. J Mater Chem 21(48):19353–19361. 10.1039/c1jm13645c [DOI] [Google Scholar]
- 29. Iyi N, Matsumoto T, Kaneko Y, Kitamura K (2004) Deintercalation of carbonate ions from a hydrotalcite-like compound: enhanced decarbonation using acid-salt mixed solution. Chem Mater 16:2926–2932. 10.1021/cm049579g [DOI] [Google Scholar]
- 30. Wong F, Buchheit RG (2004) Utilizing the structural memory effect of layered double hydroxides for sensing water uptake in organic coatings. Prog in Org Coat 51:91–102. 10.1016/j.porgcoat.2004.07.001 [DOI] [Google Scholar]
- 31. Zhou JB, Yang SL, Yu JG, Shu Z (2011) Novel hollow microspheres of hierarchical zinc—aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water. J Hazard Mater. 192:1114–1121. 10.1016/j.jhazmat.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 32. Dussaulta L, Dupina JC, Dumitriub E, Aurouxc A, Guimona C (2005) Microcalorimetry, TPR and XPS studies of acid—base properties of NiCuMgAl mixed oxides using LDHs as precursors. Thermochim Acta. 434(1–2): 93–99. 10.1016/j.tca.2005.01.012 [DOI] [Google Scholar]
- 33. Cairon O, Dumitriu E, Guimon C (2007) Acido-basicity of Mg-Ni-Al Mixed Oxides from LDH Precursors: A FTIR and XPS Study. J Phys Chem. 111, 8015–8023. [Google Scholar]
- 34. Felice G, Filippo M, Giovanni P, Sara F, Massimo P (2012) Effectiveness of sodium chloride-based anti-icing filler in asphalt mixtures. Constr Build Mater 30:174–179. 10.1016/j.conbuildmat.2011.12.036 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.