Abstract
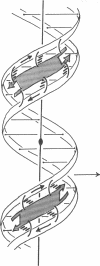
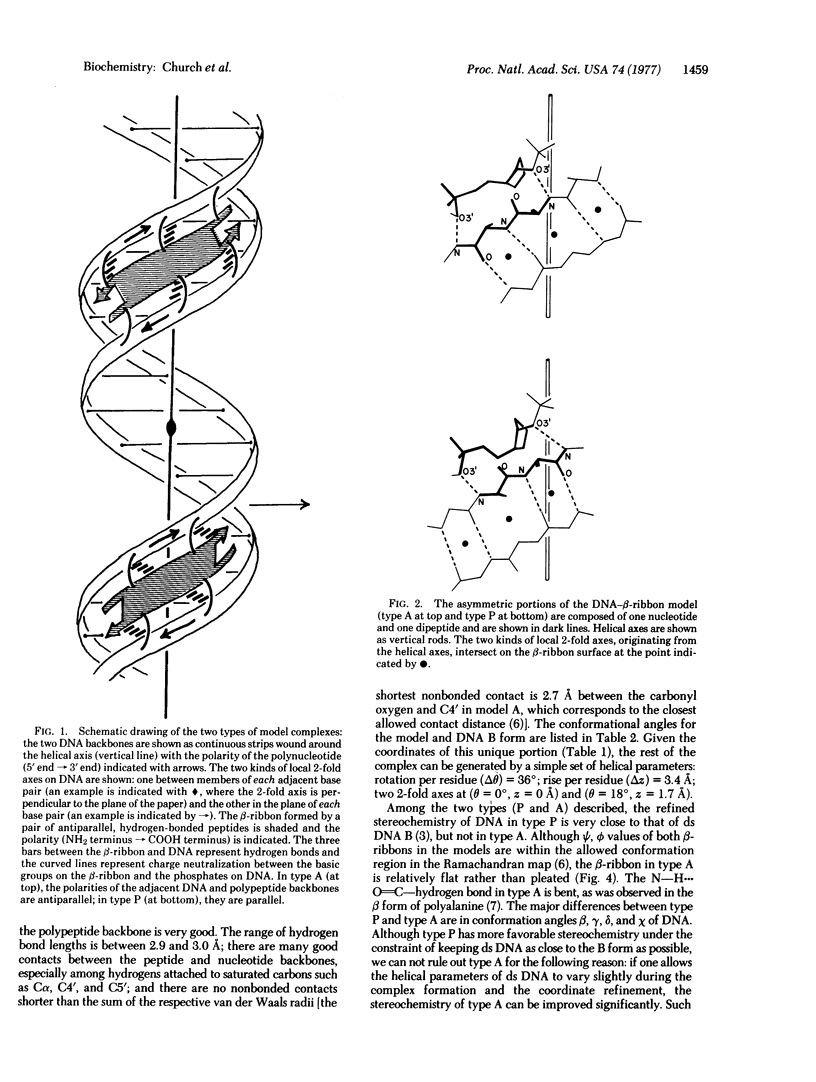
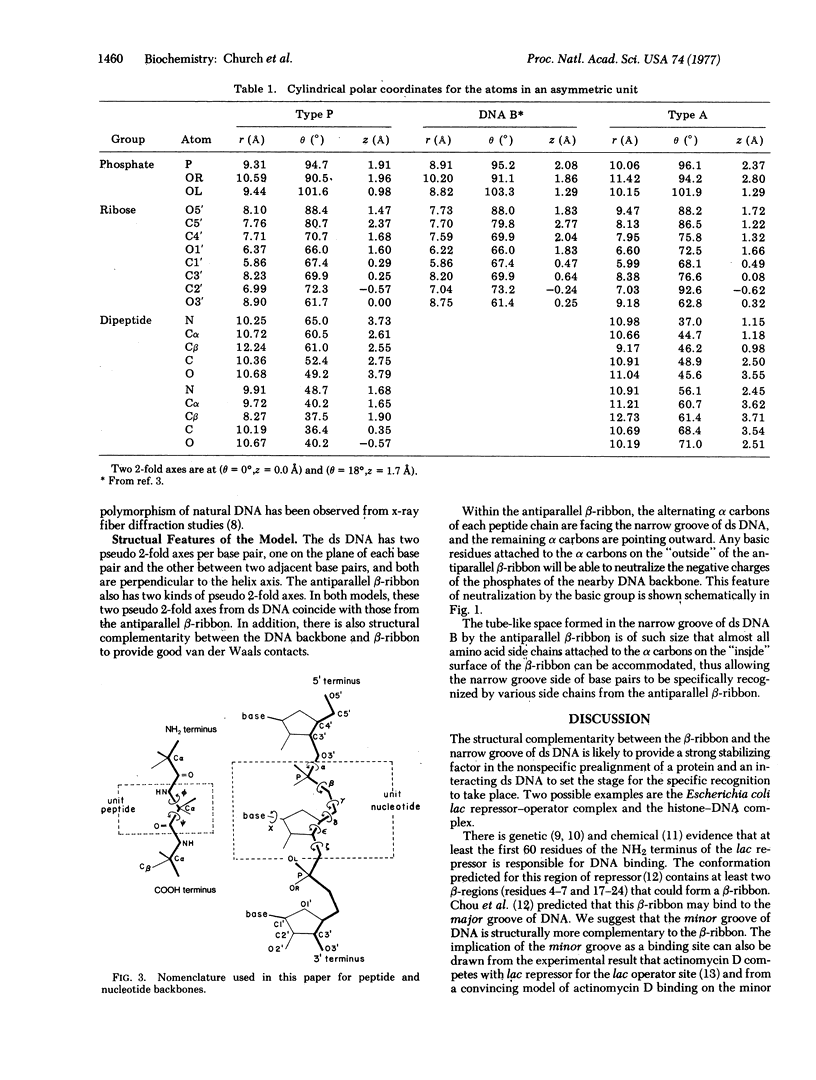
A model for the complex between double-stranded DNA and a beta-ribbon (a two-stranded antiparallel beta-sheet) of proteins is proposed as one of the possible modes of structural recognition between DNA and proteins. In this model, the contact occurs on the narrow groove of DNA, and the symmetry elements as well as the repeat distances of DNA and the beta-ribbon coincide, thus providing favorable complementary contacts.
Full text
PDF
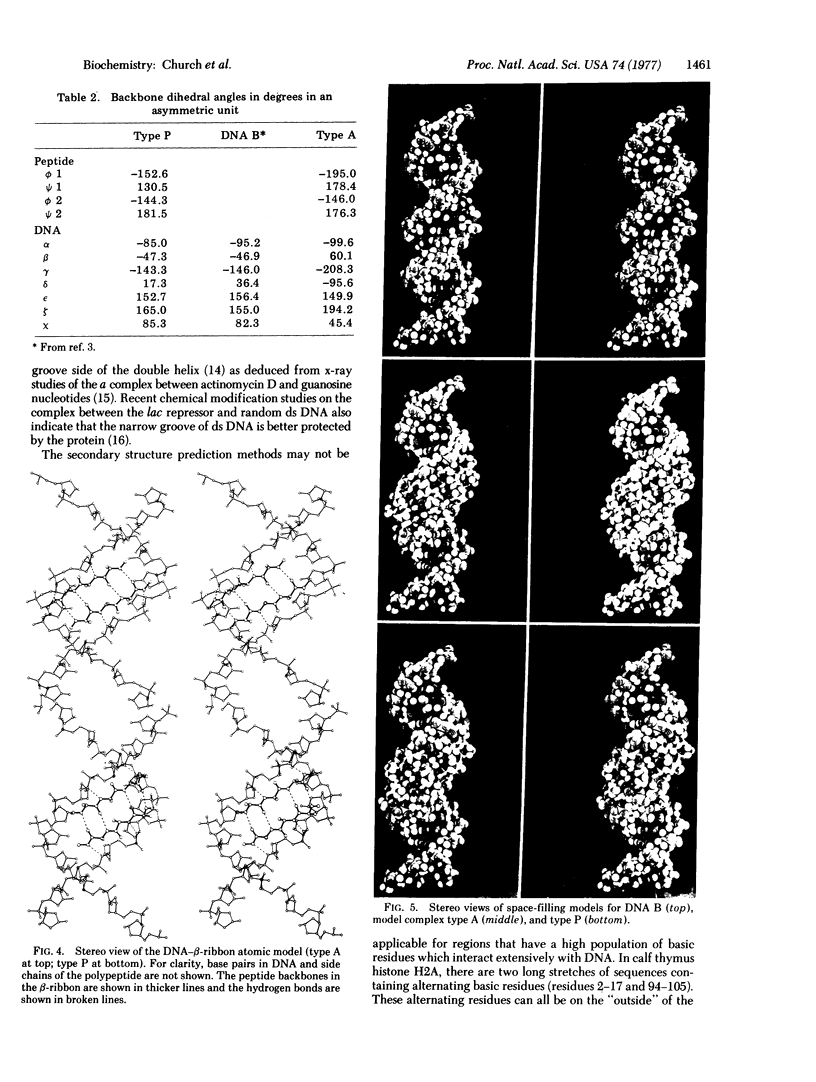

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K., Beyreuther K., Fanning E., Geisler N., Gronenborn B., Klemm A., Müller-Hill B., Pfahl M., Schmitz A. How lac repressor binds to DNA. Nature. 1972 Jun 9;237(5354):322–327. doi: 10.1038/237322a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Dover S. D. Refinement of bond angles of an alpha-helix. J Mol Biol. 1967 Nov 28;30(1):209–212. doi: 10.1016/0022-2836(67)90253-7. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Bram S., Tougard P. Polymorphism of natural DNA. Nat New Biol. 1972 Oct 4;239(92):128–131. doi: 10.1038/newbio239128a0. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J. A proposed model for interaction of polypeptides with RNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):283–287. doi: 10.1073/pnas.71.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C. Conformation of twisted beta-pleated sheets in proteins. J Mol Biol. 1973 Apr 5;75(2):295–302. doi: 10.1016/0022-2836(73)90022-3. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Adler A. J., Fasman G. D. Conformational prediction and circular dichroism studies on the lac repressor. J Mol Biol. 1975 Jul 25;96(1):29–45. doi: 10.1016/0022-2836(75)90180-1. [DOI] [PubMed] [Google Scholar]
- Jain S. C., Sobell H. M. Stereochemistry of actinomycin binding to DNA. I. Refinement and further structural details of the actinomycin-deoxyguanosine crystalline complex. J Mol Biol. 1972 Jul 14;68(1):1–20. doi: 10.1016/0022-2836(72)90258-6. [DOI] [PubMed] [Google Scholar]
- Kolchinsky A. M., Mirzabekov A. D., Gilbert W., Li L. Preferential protection of the minor groove of non-operator DNA by lac repressor against methylation by dimethyl sulphate. Nucleic Acids Res. 1976 Jan;3(1):11–18. doi: 10.1093/nar/3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Files J. G., Weber K. Lac repressor. Specific proteolytic destruction of the NH 2 -terminal region and loss of the deoxyribonucleic acid-binding activity. J Biol Chem. 1973 Jan 10;248(1):110–121. [PubMed] [Google Scholar]
- Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Jain S. C. Stereochemistry of actinomycin binding to DNA. II. Detailed molecular model of actinomycin-DNA complex and its implications. J Mol Biol. 1972 Jul 14;68(1):21–34. doi: 10.1016/0022-2836(72)90259-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Platt T., Ganem D., Miller J. H. Altered sequences changing the operator-binding properties of the Lac repressor: colinearity of the repressor protein with the i-gene map. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3624–3628. doi: 10.1073/pnas.69.12.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]