Abstract
Seizures are known to occur in Creutzfeldt–Jakob disease (CJD). In the setting of a rapidly progressive condition with no effective therapy, determining appropriate treatment for seizures can be difficult if clinical morbidity is not obvious yet the electroencephalogram (EEG) demonstrates a worrisome pattern such as status epilepticus. Herein, we present the case of a 39-year-old man with CJD and electrographic seizures, discuss how this case challenges conventional definitions of seizures, and discuss a rational approach toward treatment. Coincidentally, our case is the first report of CJD in a patient with Stickler syndrome.
Keywords: Epilepsy, Seizures, Creutzfeldt–Jakob disease, Status epilepticus, Stickler syndrome
1. Introduction
While rare, there are increasing reports of seizures occurring in patients with Creutzfeldt–Jakob disease (CJD) [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. When there is obvious clinical morbidity, the decision to treat seizures is straightforward. However, in cases where clinical morbidity is not obvious yet the electroencephalogram (EEG) demonstrates a worrisome pattern, treatment decisions become difficult, especially in a rapidly progressive condition for which there is no effective disease-modifying therapy. Neurologists and epileptologists will increasingly face this question as the index of suspicion for seizures in CJD rises, as more inpatients undergo long-term EEG monitoring, and as the formal definition of a seizure continues to evolve. To highlight these issues, we discuss the case of a patient with CJD and electrographic seizures. Coincidentally, our case is also the first ever report of CJD occurring in a patient with Stickler syndrome, a rare autosomal dominant connective tissue disorder [15].
2. Case report
A 39-year-old right-handed man was seen at a tertiary otolaryngology center for worsening lightheadedness. This symptom had developed over one month after his son was diagnosed with Stickler syndrome type I, of which the patient was a carrier as proven by genetic testing. Specifically, the patient was heterozygous for a 2-base-pair mutation (AG) on exon 41 of the COL2A1 gene which led to a frameshift mutation, a subsequent premature downstream termination codon, and, ultimately, a null allele. The patient's only possible clinical manifestation was myopia.
His lightheadedness was initially attributed to anxiety related to his recent diagnosis and myopia. Nevertheless, his symptoms worsened, and he sought medical attention within days because he started experiencing “foggy thinking” and his wife reported episodic word-finding difficulty. Neurological examination showed no focal deficits, but there was “mild cognitive slowing”. He was ultimately discharged without intervention.
Within days, however, he returned and was admitted because of inability to care for himself. Complete blood count, electrolyte levels, glucose level, renal and liver function, erythrocyte sedimentation rate, and C-reactive protein level were all within normal laboratory range. Serum toxicology, infectious disease, and paraneoplastic antibody panels were negative. A urine toxicology panel was similarly negative. Cerebrospinal fluid examination was unremarkable, including a negative assay for 14-3-3 protein. Serum thyroid peroxidase antibodies were elevated (up to 812 IU/mL), prompting an empiric week-long trial of daily high-dose intravenous methylprednisolone for possible steroid responsive encephalopathy with autoimmune thyroiditis; however, he showed no clinical improvement.
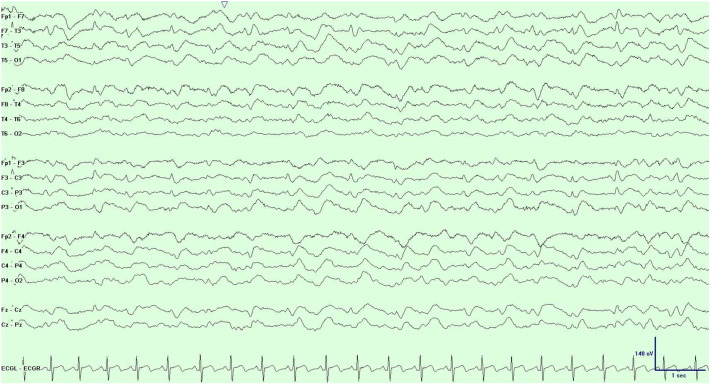
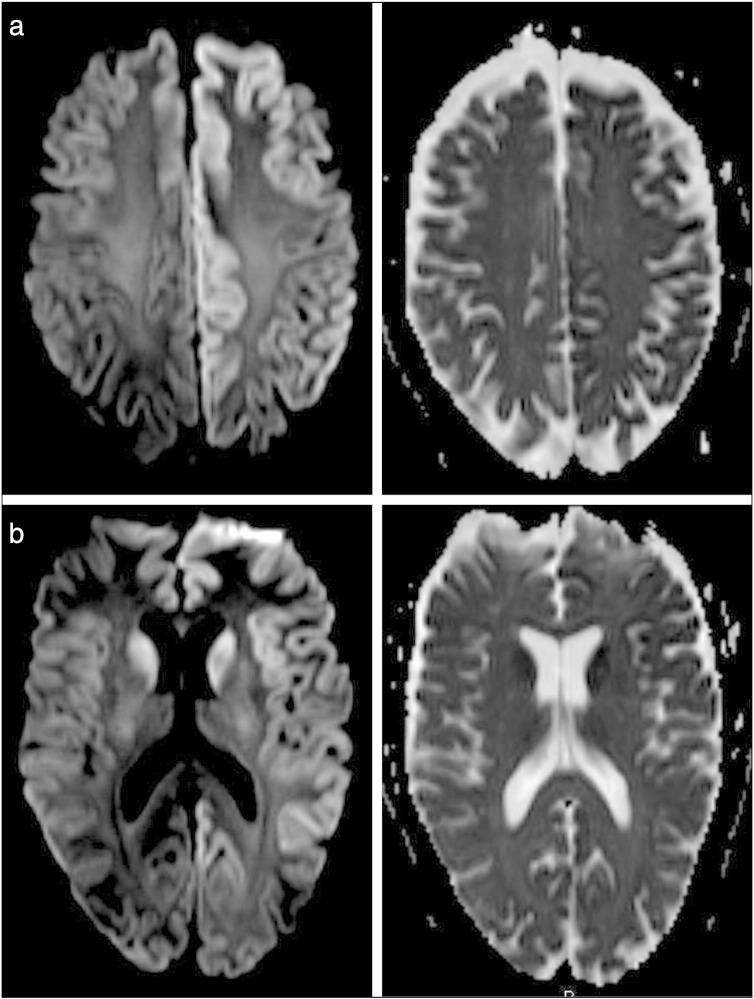
Because the patient had unexplained altered mental status, he underwent long-term EEG monitoring at the start of admission which revealed sharp generalized periodic discharges with a triphasic morphology at a frequency of 1 Hz with greater amplitude on the left (Fig. 1). Brain MRI demonstrated prominent diffusion restriction in the cortical ribbon of the left hemisphere and in the caudate nuclei bilaterally (Fig. 2).
Fig. 1.
EEG displayed in bipolar montage using the 10–20 system. Interictal generalized periodic 1-Hz triphasic sharp waves.
Fig. 2.
a. DWI and ADC images of MRI with DWI demonstrating diffusion restriction in the cortical ribbon diffusely within the left hemisphere. b. DWI and ADC images of MRI with DWI demonstrating diffusion restriction in the bilateral caudate nuclei and the cortical ribbon diffusely within the left hemisphere.
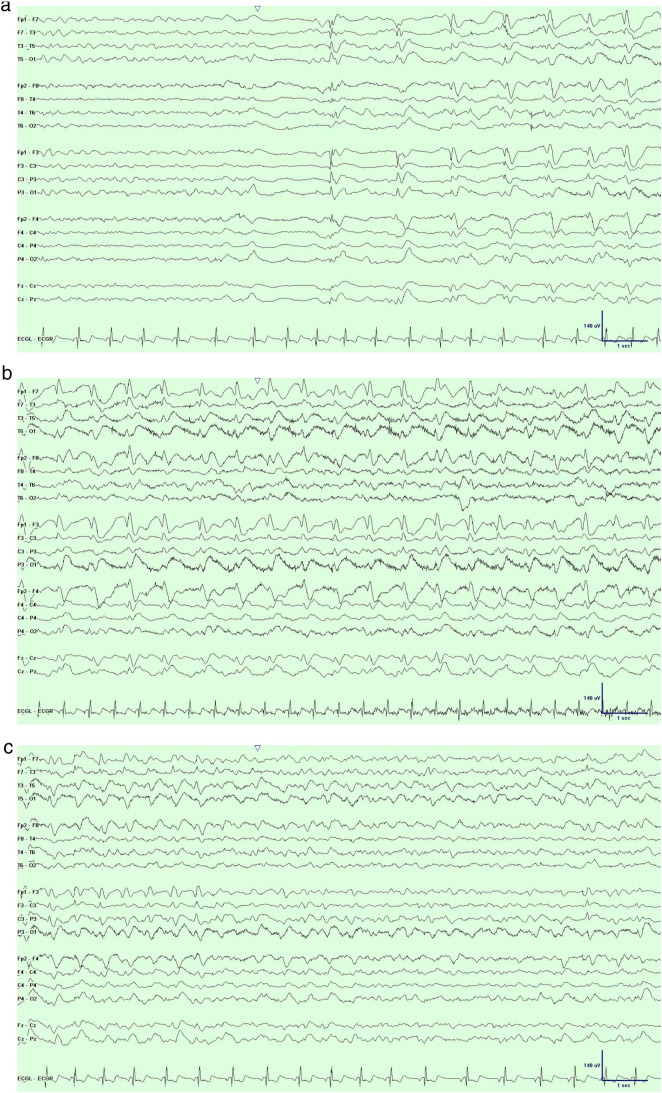
Over a 2- to 3-week period, he developed progressive lightheadedness, sleep disturbances, and cognitive difficulty to the point where he was only able to blink once for yes and twice for no. He also exhibited an exaggerated startle response and became bedridden. With worsening mental status, a repeat EEG disclosed recurrent runs lasting up to 60 s of spike and wave activity arising from a quiescent background and evolving in frequency from 0.5 Hz to a maximum of 2 Hz before terminating abruptly (Fig. 3). As there was no definite clinical correlate, this pattern was thought to represent an electrographic (subclinical) seizure.
Fig. 3.
a. Onset of electrographic seizure with generalized spike–wave complexes evolving out of quiescent background and increasing in frequency from 0.5 Hz to 1 Hz. b. In the midst of the same electrographic seizure with generalized spike–wave complexes at a frequency of 2 Hz. c. End of the same electrographic seizure with termination of generalized spike–wave complexes and resumption of background activity.
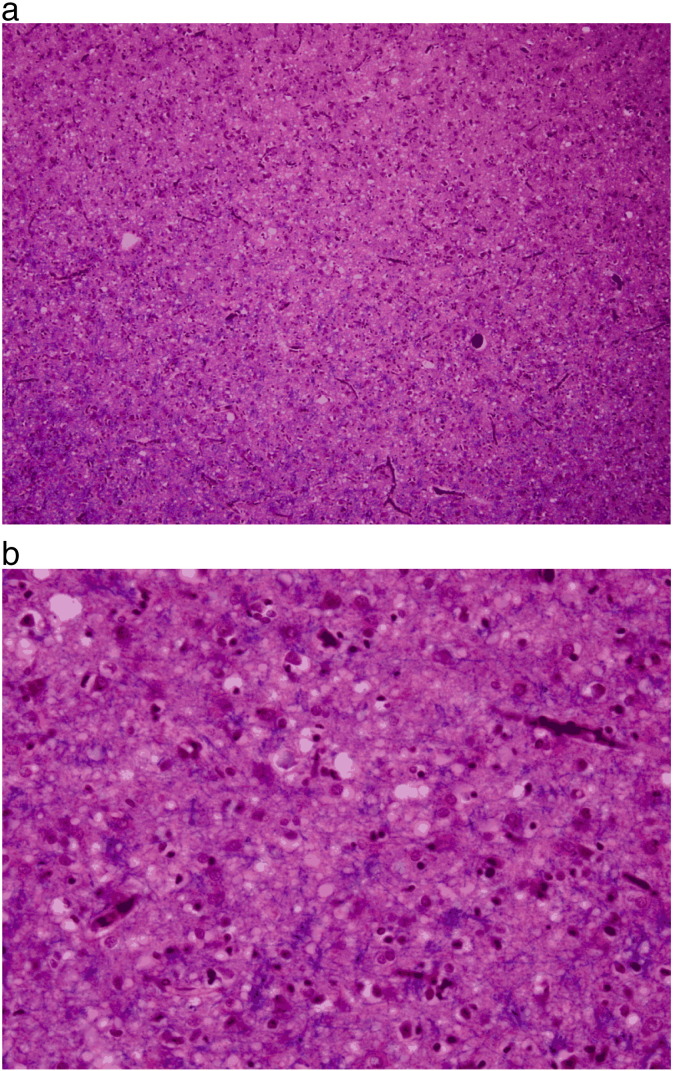
Based on the clinical diagnosis of CJD and his rapid clinical deterioration, his family refused further treatment, and he died one month after hospital admission. Autopsy confirmed the diagnosis of sporadic CJD (Fig. 4).
Fig. 4.
a. Frontal cortex (LH&E stain) showing marked spongiform change at 10 × magnification. b. Frontal cortex (LH&E stain) showing marked spongiform change, vacuoles, and reactive gliosis at 40 × magnification.
3. Discussion
Our case highlights two main issues in the treatment of seizures in CJD. Firstly, the question arises over whether the EEG patterns in question are truly seizures. In case reports, the criteria used for diagnosing seizures in CJD have varied. Some have defined seizures as EEG patterns with a definite clinical correlate — most often motoric and focal as in epilepsia partialis continua [1], [2], [3], [4]. Others have defined seizures based on EEG patterns concomitant with altered mental status [5], [6], [7], [8], [9], [10]. Of these reports, one study further required combined electroclinical improvement (i.e., improved mental status) resulting from antiepileptic drug (AED) administration [5], while two utilized the evolution of the electrographic pattern as an additional diagnostic criterion [6], [7].
We chose to define seizure in our case report based on accepted EEG-based criteria for an electrographic seizure [16], [17]. Our patient had electrographic events all without a clinical correlate. These events lasted longer than 10 s and increasingly evolved in frequency from 0.5 Hz to 2 Hz. It is worth noting, however, that modern EEG criteria for defining ictal events have largely been developed in the context of critical care monitoring. Whether they are relevant in a progressive degenerative disease remains a topic for debate.
The second issue highlighted by this case is whether electrographic seizures should be treated. No AEDs have ever been shown to alter the terminal trajectory of CJD [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Only two reports have noted transient clinical improvement with AEDs [5], [11]. Other reports of seizures in CJD using pharmacological agents (e.g., propofol, midazolam) geared toward electrographic burst suppression did not result in clinical improvement — not even transiently [6], [7], [8]. In our patient, treating his electrographic seizures seemed unlikely to alter his relentless downhill course and was rejected by his family.
Our case suggests that given the terminal trajectory of CJD at the present time, treating seizures in CJD requires first a concerted and thoughtful attempt to define seizures as rigorously as possible. Once a seizure has been firmly identified, then there must be judicious consideration of the clinical risks and benefits of any AED intervention. In CJD, much like any other medical condition, not all seizures are alike, not all seizures merit treatment, and there is no substitute for clinical acumen.
Disclosure
None of the authors have conflicts of interest to report. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Marcus C. Ng, Email: mcng@mgh.harvard.edu.
M. Brandon Westover, Email: mwestover@partners.org.
Andrew J. Cole, Email: acole1@partners.org.
References
- 1.Aiguabella M., Falip M., Veciana M., Bruna J., Palasi A., Corral L. Refractory nonconvulsive status epilepticus in Creutzfeldt–Jakob disease. Epileptic Disord. 2010;12:239–242. doi: 10.1684/epd.2010.0318. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld M.Y., Talianski-Aronov A., Soffer D., Korczyn A.D. Generalized convulsive status epilepticus in Creutzfeldt–Jakob disease. Seizure. 2003;12:403–405. doi: 10.1016/s1059-1311(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee K., Haight E., Olejniczak P. Epilepsia partialis continua in Creutzfeldt–Jakob disease. Acta Neurol Scand. 2000;102:398–402. doi: 10.1034/j.1600-0404.2000.102006398.x. [DOI] [PubMed] [Google Scholar]
- 4.Aronyk K., Petito F., Solomon G.E. Partial elementary motor seizures as the first symptom of Creutzfeldt–Jakob disease. Ann Neurol. 1984;15:210–211. doi: 10.1002/ana.410150220. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa K., Yoshihashi H., Suzuki Y., Oishi M., Kamei S. Case of probable Creutzfeldt–Jakob disease presenting with complex partial seizure following sleeplessness and cognitive impairment. Geriatr Gerontol Int. 2011;11:229–232. doi: 10.1111/j.1447-0594.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 6.Espinosa P.S., Bensalem-Owen M.K., Fee D.B. Sporadic Creutzfeldt–Jakob disease presenting as nonconvulsive status epilepticus case report and review of the literature. Clin Neurol Neurosurg. 2010;112:537–540. doi: 10.1016/j.clineuro.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Cohen D., Kutluay E., Edwards J., Peltier A., Beydoun A. Sporadic Creutzfeldt–Jakob disease presenting with nonconvulsive status epilepticus. Epilepsy Behav. 2004;5:792–796. doi: 10.1016/j.yebeh.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Rossetti A.O., Dunand M. Creutzfeldt–Jakob disease: evolution from nonconvulsive status epilepticus, through SIRPIDs, to generalized periodic discharges. Clin Neurophysiol. 2007;118:2533–2536. doi: 10.1016/j.clinph.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Torre J.L., Solar D.M., Astudillo A., Cereceda R., Acebes A., Calatayud M.T. Creutzfeldt–Jakob disease and non-convulsive status epilepticus: a clinical and electroencephalographic follow-up study. Clin Neurophysiol. 2004;15:316–319. doi: 10.1016/j.clinph.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Rees J.H., Smith S.J., Kullmann D.M., Hirsch N.P., Howard R.S. Creutzfeldt–Jakob disease presenting as complex partial status epilepticus: a report of two cases. J Neurol Neurosurg Psychiatry. 1999;66:406–407. doi: 10.1136/jnnp.66.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro J.M., Shujaat A., Wang J., Chen X. Creutzfeldt–Jakob disease presenting as refractory nonconvulsive status epilepticus. J Intensive Care Med. 2004;19:345–348. doi: 10.1177/0885066604269771. [DOI] [PubMed] [Google Scholar]
- 12.Lowden M.R., Scott K., Kothari M.J. Familial Creutzfeldt–Jakob disease presenting as epilepsia partialis continua. Epileptic Disord. 2008;10:271–275. doi: 10.1684/epd.2008.0216. [DOI] [PubMed] [Google Scholar]
- 13.Schrooten M., De Vooght W., Weckhuysen S., Van Paesschen W., Van Damme P. Normalization of 14-3-3 in CJD. Acta Neurol Belg. 2008;108:64–66. [PubMed] [Google Scholar]
- 14.Cokgor I., Rozear M., Morgenlander J.C. Seizures and Creutzfeldt–Jakob disease. A case report and series review. N C Med J. 1999;60:108–109. [PubMed] [Google Scholar]
- 15.Lieberfarb R.M., Levy H.P., Rose P.S., Wilkin D.J., Davis J., Balog J.Z. The Stickler syndrome: genotype/phenotype correlation in 10 families with Stickler syndrome resulting from seven mutations in the type II collagen gene locus COL2A1. Genet Med. 2003;5:21–27. doi: 10.1097/00125817-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Chong D.J., Hirsch L.J. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- 17.Young G.B., Wang J.T., Connolly J.F. Prognostic determination in anoxic–ischemic and traumatic encephalopathies. J Clin Neurophysiol. 2004;21:379–390. [PubMed] [Google Scholar]