Abstract
Background
Family histories of atopy, as well as histories of atopic dermatitis and food allergy, are important risk factors for an infant to have asthma. Although atopic sensitization appears to contribute to the development of asthma, it is unclear when the airways become involved with the atopic process and whether airway function relates to the atopic characteristics of the infant.
Objective
We sought to evaluate whether atopic infants without prior episodes of wheezing have increased expired nitric oxide (eNO) levels and heightened airway reactivity.
Methods
Infants with eczema were recruited, and atopic status was defined by specific IgE levels to foods or aeroallergens and total IgE levels. eNO, forced expiratory flow at 75% exhaled volume (FEF75), and airway reactivity to inhaled methacholine were measured in sedated infants. Airway reactivity was quantified by using the provocative concentration to decrease FEF75 by 30%.
Results
Median age for the 114 infants evaluated was 10.7 months (range, 2.6–19.1 months). Infants sensitized to egg or milk compared with infants sensitized to neither egg nor milk had lower flows (FEF75: 336 vs 285 mL/s, P < .003) and lower lnPC30 (mg/mL) provocative concentrations to decrease FEF75 by 30% (−0.6 vs −1.2, P < .02) but no difference in eNO levels. Infants with total serum IgE levels of greater than 20 IU/mL had higher eNO levels compared with infants with IgE levels of 20 IU/mL or less (14.6 vs 11.2 ppb, P < .023) but no difference in forced flows or airway reactivity.
Conclusions
Our findings suggest that atopic characteristics of the infant might be important determinants of the airway physiology of forced expiratory flows, airway reactivity, and eNO.
Keywords: Atopy, eczema, airway reactivity
Family histories of atopy, as well as the presence of atopic dermatitis or food allergy, particularly to egg and milk, are important risk factors for an infant to have asthma.1-4 Although atopic sensitization appears to contribute to the development of asthma, it is unclear when the airways become involved with the atopic process. Heightened airway reactivity and increased levels of expired nitric oxide (eNO) are physiologic characteristics of children and adults with asthma. Among children and adults, increasing levels of eNO correlate with heightened airway reactivity,5-9 although these physiologic airway measurements can be influenced by factors other than atopic airway inflammation.10-14 Several studies have evaluated whether the airway characteristics of heightened airway reactivity and increased levels of eNO are present early in life before the onset of asthma. Young et al,15 as well as the members of our laboratory,16 have reported that among healthy infants without prior episodes of wheezing, airway reactivity was greater in those infants with a family history of asthma or allergy compared with that seen in infants with a family history negative for these factors. Follow-up of the Perth infants found that heightened airway reactivity as an infant was associated with a diagnosis of asthma at 6 years of age.17 In a small group of infants and toddlers with and without a history of wheezing, Wild-haber et al18 reported that family history of parental atopy was associated with higher levels of eNO. Frey et al19 reported that at 1 month of age, a history of maternal atopic disease modified the effects of prenatal risk factors on eNO. In addition, among infants with a history of maternal atopy, eNO levels measured at 1 month of age were associated with an increased risk of respiratory symptoms in the subsequent year of life.20 Cumulatively, these studies support the significance of a family history of atopy as a risk factor for heightened airway reactivity and increased levels of eNO very early in life. However, it has not been determined whether these airway characteristics relate directly to the atopic status of the infant. In addition, no study has evaluated whether very early in life, before the onset of wheezing, heightened airway reactivity is associated with increased levels of eNO. We hypothesized that the airways are part of the atopic process, even among infants who have not had episodes of wheezing. Using a cohort of infants with eczema and a history negative for wheezing or lower respiratory tract illness, we evaluated the relationships between airway function (eNO, forced expiratory flows, and reactivity) and allergic status.
Methods
Subjects
Infants with a history of eczema were recruited from general pediatric community-based clinics and from community-based advertisements for a longitudinal study of the relationship between airway function, atopic status, the development of recurrent wheezing, and a diagnosis of asthma at 5 years of age. Physiologic and immunologic evaluation will be obtained on entry, at 1 year's follow-up, and at 5 years of age. Subjects were excluded for premature birth (<36 weeks' gestation), congenital malformations of the cardiorespiratory system, history of lower respiratory tract illness, or wheezing. Testing was performed while the infant was sleeping after receiving 50 to 100 mg/kg chloral hydrate orally. The institutional review board approved the study, and informed consent was obtained from parents. All subjects were evaluated at James Whitcomb Riley Hospital for Children, Indianapolis, Indiana.
eNO levels were measured at a constant expiratory flow of 25 mL/s, as previously described.21 Infants breathed through a mask with a nasal and oral compartment. After several inflations to 30 cm H2O, which inhibited inspiratory effort, forced expiration was maintained through the oral compartment at 25 mL/s while NO was sampled with a chemiluminescence analyzer.
Airway reactivity was assessed from changes in forced expiratory flows using the raised-volume rapid thoracic compression technique, as previously described.16,22 Subjects with baseline airway function of less than 2 SDs of predicted value did not undergo methacholine challenge. Forced expiratory maneuvers were repeated 2 minutes after each increasing concentration of inhaled methacholine (0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5.0, and 10 mg/mL). The challenge was stopped if forced expiratory flow at 75% of exhaled volume (FEF75) decreased by more than 30% from baseline. After the last methacholine dose, infants inhaled 5 mg of nebulized albuterol. Airway reactivity was quantified by using the methacholine concentration required to decrease FEF75 by 30% (PC30) from baseline. The concentration to achieve PC30 was obtained by means of interpolation between 2 doses of methacholine on the dose-response curve.
Dermatitis and atopic status
At the time of testing, the degree of eczema was quantified using a dermatitis scoring system, SCORAD,23 which is based on the distribution of body surface involvement (face, upper limbs, trunk, and lower limbs), intensity (erythema, edema, oozing, excoriation, and lichenification), and subjective items (pruritus and insomnia); the maximum score is 100. After application of topical anesthetic cream (LMX 4; Ferndale Laboratories, Ferndale, Mich) to the antecubital fossa, a 5-mL sample of venous blood was obtained for measurement of total serum IgE and allergen-specific IgE (Immune Tech, Inc, Menlo Park, Calif) levels to food (egg white, milk, and wheat), perennial allergens (cat and house dust mite), and aeroallergens (timothy grass, Bermuda grass, ragweed, Alternaria species, and cedar). Parents completed a respiratory history questionnaire related to family history of asthma/allergy and exposure to tobacco smoking.
Statistical analysis
Differences between groups were compared by using the Student t test for age and the Wilcoxon rank sum test for SCORAD values. Comparisons for categorical variables were performed with Pearson χ2 tests. For analyses in which 20% or more of the cells had expected counts of less than 5, the 2-sided Fisher exact test was used. Associations between eNO and total serum IgE levels and between PC30 and total serum IgE levels were examined by using the Spearman rank order correlation coefficient. Differences between groups for measures of airway function were tested with analysis of covariance after adjusting for height, sex, race, family smoking, immediate family history of allergy or asthma, and the interaction between family smoking and an immediate family history of allergy or asthma. A log transformation was applied to PC30 to satisfy the equal variance assumption. Pearson correlation coefficients were used to describe the relationship between eNO and PC30 when subjects were divided by parental history of asthma or allergy and parental history of tobacco smoking. SAS version 9.1.3 software (SAS Institute, Inc, Cary, NC) was used to conduct all of the analyses.
Results
The median age for the 114 infants recruited was 10.7 months (range, 2.6–19.1 months). The study population was evenly divided by sex (51% male subjects) and race (46% white and 53% African American). Measurements of eNO were obtained in 107 infants, measurements of baseline forced expiratory flows were obtained in 114 infants, and measurements of airway reactivity were obtained in 90 infants. Failure to obtain physiologic measurements most often resulted from the infant awakening before completion of testing. Ten subjects did not undergo bronchial challenge because their baseline values for FEF75 were greater than 2 SDs less than predicted value.
Before 2 years of age, sensitization to food is more common than sensitization to aeroallergens.2,24-27 Because egg and milk are the most frequent allergens for atopic sensitization during infancy and sensitization with either of these antigens is an important risk factor for the subsequent development of asthma as an older child, we grouped infants as sensitized to egg or milk versus infants not sensitized to either of these allergens. Table I summarizes the demographics for these 2 groups. There were no significant differences for age, length, or sex; however, the sensitized group had a significantly greater number of nonwhite subjects, who were primarily African American (76%). The 2 groups did not differ in percentage of mothers or fathers with a history of allergy or asthma, and there was no significant difference for exposure to maternal or paternal tobacco smoking. The egg- or milk-sensitized infants had higher total serum IgE levels; however, they did not differ in their dermatitis score (SCORAD) at the time of pulmonary function testing. Fig 1 summarizes the comparison of baseline forced expiratory flow (FEF75), airway reactivity (lnPC30), and eNO level; values are adjusted for height, sex, race, family smoking, immediate family history of allergy or asthma, and the interaction between family smoking and an immediate family history of allergy or asthma. The group of infants sensitized to either egg or milk had significantly lower baseline forced expiratory flows (Fig 1, A). There were no significant differences between the groups for forced vital capacity or forced expiratory volume in 0.5 seconds. In addition, egg- or milk-sensitized infants had significantly lower PC30 (heightened airway reactivity) values compared with infants sensitized to neither egg nor milk (Fig 1, B). There was no significant difference between the mean values of eNO for the 2 groups (Fig 1, C). When atopic sensitization was expanded to positive for any of the 10 antigens, there were no significant differences between nonsensitized and sensitized infants for FEF75 (334 ± 17 vs 309 ± 13, P = .184), lnPC30 (−0.8 ± 0.2 vs −0.7 ± 0.2, P = 0.840), or eNO (11.6 ± 1.3 vs 12.7 ± 1.0, P =.476).
Table I. Demographics: sensitization to egg or milk.
| Negative for egg and milk (n = 73) | Positive for egg or milk (n = 41) | P value | |
|---|---|---|---|
| Age (mo), mean ± SE | 10.8 ± 0.5 | 10.6 ± 0.7 | .875 |
| Length (cm) | 72.6 ± 0.7 | 72.1 ± 1.0 | .689 |
| Sex (% male) | 48 | 51 | .737 |
| Race (% white) | 58 | 24 | .001 |
| Maternal asthma/allergy (%) | 44 | 41 | .760 |
| Paternal asthma/allergy (%) | 28 | 27 | .91 |
| Maternal smoking (%) | 18 | 15 | .663 |
| Paternal smoking (%) | 21 | 24 | .634 |
| IgE-specific antigen (%) | |||
| Food (any) | 8 | 100 | .0001 |
| Mite | 14 | 34 | .010 |
| Cat | 23 | 44 | .022 |
| Pollen allergen | 26 | 29 | .709 |
| SCORAD | 10.3 ± 0.9 | 10.5 ± 1.2 | .540 |
| IgE >20 IU/mL | 22 | 54 | .001 |
Fig 1.
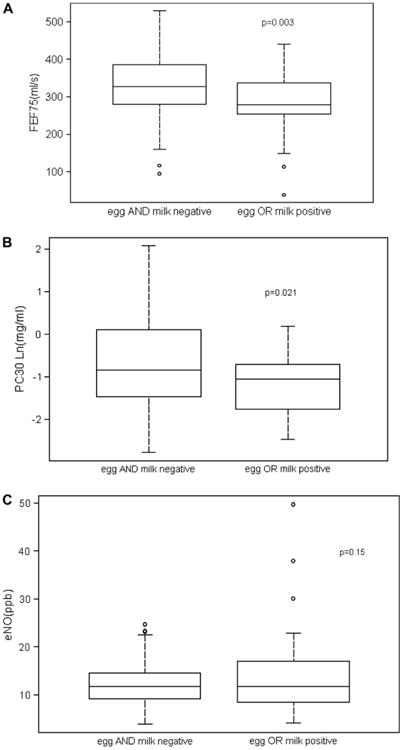
Comparison of infants sensitized to either egg or milk and those infants not sensitized to either egg or milk. Box plots illustrate distribution of data. A, Mean ± SE forced expiratory flows (FEF75) were significantly less in the infants sensitized to egg or milk (336 ± 12 vs 280 ± 16 mL/s, P < .003). B, Mean ± SE PC30 values were significantly less in infants sensitized to egg or milk (−0.6 ± 0.2 vs −1.2 ± 0.2 mg/mL, P < .023). C, Mean ± SE eNO levels were not significantly different for the 2 groups (11.6 ± 1.0 vs 13.8 ± 1.3 ppb, P = .15).
The infants with sensitivity to egg or milk had significantly increased levels of total serum IgE, which has been used to define atopy. By using the criteria of a total serum IgE level of greater than 20 IU/mL, a value greater than 2 SDs above normal for this age,28 we compared baseline airway function, airway reactivity, and eNO level in groups divided by total serum IgE level. The group of infants with increased IgE levels had significantly higher values for eNO (Fig 2); however, there were no significant differences in FEF75 and PC30 values. With all infants combined, increasing total serum IgE levels correlated with increasing levels of eNO; however, the correlation did not achieve statistical significance (r = 0.17, P =.076).
Fig 2.
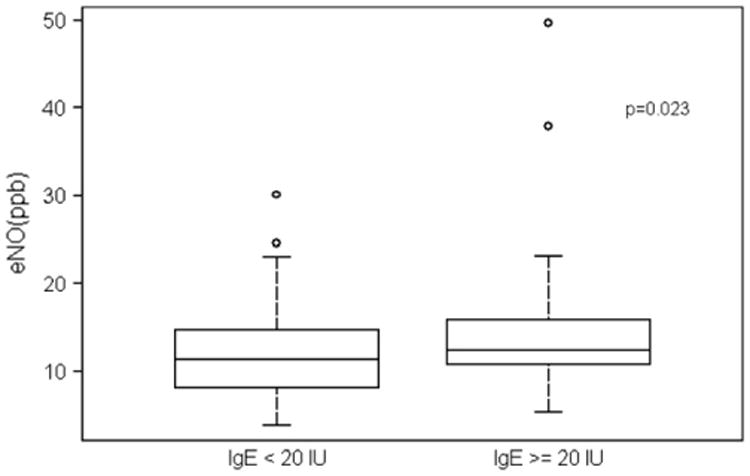
Box plots illustrate distribution of eNO. Mean ± SE values were significantly greater (11.2 ± 1.0 vs 14.6 ± 1.3 ppb, P < .023) for infants with IgE levels of 20 IU/mL or greater versus those with IgE levels of less than 20 IU/mL.
Because older children and adults with asthma demonstrate a significant relationship between airway reactivity and eNO levels, we evaluated whether a similar relationship exists among our infants without previous wheezing. Fig 3 shows that infants with higher values for eNO had lower PC30 values (greater airway reactivity), although the correlation was weak (r = −0.26, P = .02). This relationship between airway reactivity and eNO level was not modified for infants characterized as atopic, either by sensitization to milk or egg or by having a total IgE level of greater than 20 IU. We evaluated whether parental history of asthma/allergy, tobacco smoking, or infant's atopic status modified the relationship between airway reactivity and eNO level. Table II summarizes the correlations between eNO level and PC30 value when subjects are divided by parental history of asthma or allergy and parental history of tobacco smoking. For infants with a positive history for maternal allergy/asthma, increasing eNO levels were associated with more negative PC30 values (greater airway reactivity), whereas there was no significant relationship for those infants with histories negative for maternal asthma/allergy. Paternal history of asthma/allergy had the opposite effect. In addition, among infants not exposed to tobacco smoking, higher eNO levels were associated with lower PC30 values, whereas there was no relationship for infants exposed to either maternal or paternal tobacco smoking.
Fig 3.
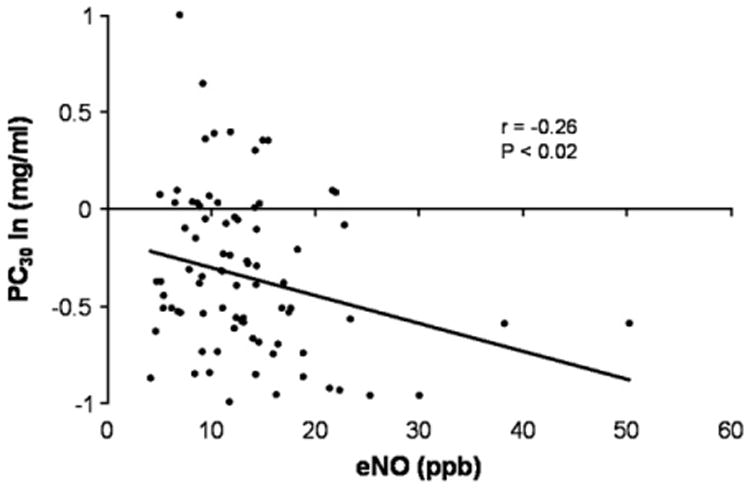
Increasing eNO levels (in parts per billion) correlated with lower PC30 values (in milligrams per milliliter) or heightened airway reactivity (r = −0.26, P = .02).
Table II. Relationship between airway reactivity (lnPC30) and eNO.
| Dependent | Independent | Subgroup | r | P value | |
|---|---|---|---|---|---|
| lnPC30 | eNO | Maternal asthma/allergy | No | −0.17 | .272 |
| lnPC30 | eNO | Maternal asthma/allergy | Yes | −0.40 | .015 |
| lnPC30 | eNO | Paternal asthma/allergy | No | −0.30 | .020 |
| lnPC30 | eNO | Paternal asthma/allergy | Yes | −0.23 | .297 |
| lnPC30 | eNO | Parental smoking | No | −0.27 | .039 |
| lnPC30 | eNO | Parental smoking | Yes | −0.14 | .548 |
Means and hypothesis tests are adjusted for height, sex, race, family smoking, immediate family history of allergy or asthma, and interaction between family smoking and family history of allergy or asthma.
Discussion
Our study found that among infants with eczema but without prior lower respiratory tract infection or wheezing, sensitization to egg or milk was associated with lower baseline airway function and heightened airway reactivity but no difference in eNO levels. When infants were characterized by an increased level of total serum IgE, they had higher levels of eNO but no difference in baseline airway function or airway reactivity. We also found that among our group of infants, there was a weak correlation between increasing levels of eNO and heightened airway reactivity. These findings suggest that early in life, before the occurrence of recurrent respiratory symptoms, sensitization to egg or milk and increased total serum IgE level are associated with the physiologic airway characteristics of heightened airway reactivity and increased eNO levels.
Our finding of lower airway function in infants with sensitization to milk or egg but before the onset of wheezy illnesses suggests that atopic sensitization to these antigens during infancy might contribute to lower airway function at this very young age. In addition to lower baseline airway function, our infants sensitized to egg or milk had heightened airway reactivity. There was a greater percentage of African American subjects in our egg- or milk-sensitized group compared with the nonsensitized group. Studies in older children suggest that African American subjects are at greater risk for allergen sensitization, and genetic studies have suggested racial differences in genes associated with asthma.29-31 Because we did not find that PC30 or eNO values were related to race and differences in baseline airway function and airway reactivity in the group sensitized to egg or milk persisted after adjusting for race, we believe that the observed differences were primarily related to sensitization to egg or milk.
After our assessment of baseline airway function, we evaluated airway reactivity to inhaled methacholine, and we did not assess the response to an inhaled bronchodilator. Therefore it is unclear whether the lower baseline airway function of the infants sensitized to egg or milk reflects increased baseline airway tone, another index of heightened airway responsiveness, or fixed smaller-sized airway caliber. Previous studies that assessed airway function in infants before wheezing have found that lower airway function was associated with exposure to tobacco smoking; however, these studies also did not evaluate whether the lower airway function persisted after an inhaled bronchodilator.16,32 Our egg- or milk-sensitized infants did not differ from nonsensitized infants for exposure to parental tobacco smoking. In addition, we adjusted for history of smoking exposure in our analyses, and therefore we believe that our observed differences in airway function are caused by atopic sensitization rather than exposure to tobacco smoke. Young et al,15 as well as members of our laboratory,16 have reported that among infants without prior episodes of wheezing, airway reactivity was heightened among those infants with family history of asthma. Our current findings of heightened airway reactivity in infants sensitized to egg or milk associates heightened airway reactivity to atopic sensitization of the infant rather than to family history.
Our finding that sensitization to egg or milk among infants was associated with lower airway function and higher reactivity should be interpreted with some caution because we examined associations between several airway function measures and several definitions of allergic status. Thus we performed multiple statistical tests, increasing the probability that a significant result could occur by chance. Our findings should be interpreted in light of this, as well as how they relate to previous studies and their biologic plausibility.
Our finding that early atopic sensitization to egg or milk is an important determinant of airway function is consistent with the findings in older children. Illi et al33 reported that 7-year-old children with asthma had lower airway function and heightened airway reactivity if they had atopic sensitization before 3 years of age. Similarly, Lowe et al34 found that 3-year-old children with atopic sensitization had lower airway function than nonsensitized children. In both of these studies of children, no measurements of lung function were obtained during infancy; therefore it is unclear whether the lower airway function or heightened airway reactivity was present earlier in life. In those studies early sensitization before 3 years of age referred to aeroallergens, whereas our findings in infants refer to sensitization to egg or milk. However, atopic sensitization to food most often occurs during infancy and before sensitization to aeroallergens.2,25-27 In addition, food sensitization before 2 years of age is a risk factor for the development of asthma as an older child, whereas inhalant sensitization before 2 years of age without concurrent food sensitization conferred no increased risk for the development of asthma.1-4,24 Therefore our findings of lower airway function and heightened airway reactivity in infants sensitized to egg or milk suggest that the airways are involved in the atopic process very early in life, even before the onset of wheezing.
Our infants sensitized to egg or milk had lower airway function and higher airway reactivity; however, they did not have higher levels of eNO. When our infants were grouped by an increased level of total serum IgE, those infants with increased total serum IgE levels had higher eNO levels than those infants with lower IgE levels. Among older children and adults, increased eNO levels are associated with increased total serum IgE levels, allergic sensitization, and risk of asthma in preschool children.35-38 Previous studies of infants have reported that the level of eNO was influenced by parental history of asthma or allergy19,39; however, we are not aware of studies that have related the infant's level of eNO directly to a measurement obtained from the infant, such as total serum IgE.
Previous studies of infants have assessed either eNO levels or airway reactivity; however, we are not aware of studies in infants that have measured both of these physiologic airway characteristics early in life. Among our group of infants, there was a weak correlation between increased levels of eNO and lower PC30 values (heightened airway reactivity), which is consistent with studies of older children.5,7,9 The weaker correlation we observed between eNO levels and airway reactivity among our infants than reported for older children might reflect that our infants have not yet had episodes of wheezing and that with increasing age there is increased atopic sensitization, particularly to aeroallergens, and increased levels of total IgE. Our findings suggest that very early in life, before the onset of wheezing and lower respiratory tract illnesses, factors other than atopic inflammation might also be important determinants of airway reactivity and eNO level. These factors can include the mechanical properties of the airway wall, NO metabolism within the airway, and such clinical covariants as obesity and gastroesophageal reflux. In addition, the relationship between eNO level and airway reactivity might reflect atopy rather than asthma per se; eNO levels correlate with airway reactivity among atopic subjects but not among nonatopic subjects.5,6,9 When our group of infants was divided as atopic versus nonatopic based on either sensitization to egg or milk or based on total serum IgE level, we did not find a significant relationship between eNO levels and airway reactivity. However, for infants with a maternal history positive for asthma or allergy, increasing eNO levels did correlate with increasing airway reactivity, whereas there was no correlation for infants having a negative history for maternal asthma or allergy. Exposure to tobacco smoking also modified the relationship between eNO levels and airway reactivity, which is consistent with previous reports that exposure to tobacco smoking affects eNO levels.40,41 Cumulatively, our findings in infants highlight the complex relationships among atopy, exposure to tobacco smoking, airway reactivity, and eNO levels early in life.
There are several limitations to our study. Although obtaining measurements of airway physiology in approximately 100 infants, which is a large number of subjects for studies in this age group, it remains relatively small compared with studies in older cooperative children and adults. This number of subjects makes it difficult to separate out complex interactions; this is particularly true when looking at subsets of the children, as in Table II.
In addition, 10 of our subjects did not undergo bronchial challenge because their baseline airway function was greater than 2 SDs less than normal. These 10 subjects did not differ from all of the other infants for age, total IgE level, number of IgE-antigen positive test results, or the percentage positive to egg or milk, dermatitis score, and sex. Another limitation of our study was that we enrolled only infants with eczema; there were no subjects without any dermatitis. We assumed that within this group of infants with eczema there would be atopic and nonatopic subjects, which would enable us to assess the relationship between atopy and physiologic airway characteristics. There is support for 2 variants of eczema, atopic and nonatopic42; subjects with the nonatopic eczema have a lower risk for asthma than subjects with atopic eczema.10,43
Lastly, we recruited infants with a history negative for previous lower respiratory tract illness or wheezing; however, this criterion does not exclude the potential of clinically mild lower respiratory tract illnesses, which was thought to be an upper respiratory tract illness. Although the observed associations in the present study are significant after controlling for parental atopy, the current cross-sectional analysis does not yield information on the temporal relationship between changes in lung function and IgE level. Therefore it still remains unclear whether sensitization is the causal factor in the coexistence of lung function and sensitization because they might still reflect that some children inherit concurrent existing diseases caused by a common underlying factor rather than the result of an underlying atopic march. Additional insights will be obtained from this cohort as they are followed longitudinally and we evaluate changes in the patterns of sensitization and lung function.
In summary, our findings indicate that infants with atopic sensitization to egg or milk have lower baseline airway function and heightened airway reactivity before the development of lower respiratory tract illness or wheezing. In addition, infants with an increased total serum IgE level have increased levels of eNO. These findings suggest that certain characteristics of an infant's atopic status might be important determinants of airway function early in life.
Clinical implications.
Our findings suggest that sensitization to milk or egg might be an important determinant of airway function early in life. An increased total serum IgE level was associated with higher eNO levels but not with airway function.
Acknowledgments
Supported by National Institutes of Health grant no. HL54062.
Abbreviations used
- eNO
Expired nitric oxide
- FEF75
Forced expiratory flow at 75% exhaled volume
- NO
Nitric oxide
- PC30
Provocative concentration to decrease FEF75 by 30%
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.Bergmann RL, Edenharter G, Bergmann KE, Forster J, Bauer CP, Wahn V, et al. Atopic dermatitis in early infancy predicts allergic airway disease at 5 years. Clin Exp Allergy. 1998;28:965–70. doi: 10.1046/j.1365-2222.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- 2.Hattevig G, Kjellman B, Bjorksten B. Clinical symptoms and IgE responses to common food proteins and inhalants in the first 7 years of life. Clin Allergy. 1987;17:571–8. doi: 10.1111/j.1365-2222.1987.tb02053.x. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes HL, Sporik R, Thomas P, Holgate ST, Cogswell JJ. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol. 2001;108:720–5. doi: 10.1067/mai.2001.119151. [DOI] [PubMed] [Google Scholar]
- 4.Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: Follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95:1179–90. doi: 10.1016/s0091-6749(95)70074-9. [DOI] [PubMed] [Google Scholar]
- 5.Franklin PJ, Turner SW, Le Souef PN, Stick SM. Exhaled nitric oxide and asthma: complex interactions between atopy, airway responsiveness, and symptoms in a community population of children. Thorax. 2003;58:1048–52. doi: 10.1136/thorax.58.12.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin PJ, Stick SM, Le Souef PN, Ayres JG, Turner SW. Measuring exhaled nitric oxide levels in adults: the importance of atopy and airway responsiveness. Chest. 2004;126:1540–5. doi: 10.1378/chest.126.5.1540. [DOI] [PubMed] [Google Scholar]
- 7.Leuppi JD, Downs SH, Downie SR, Marks GB, Salome CM. Exhaled nitric oxide levels in atopic children: relation to specific allergic sensitisation, AHR, and respiratory symptoms. Thorax. 2002;57:518–23. doi: 10.1136/thorax.57.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silkoff PE, McClean PA, Slutsky AS, Caramori M, Chapman KR, Gutierrez C, et al. Exhaled nitric oxide and bronchial reactivity during and after inhaled beclomethasone in mild asthma. J Asthma. 1998;35:473–9. doi: 10.3109/02770909809071000. [DOI] [PubMed] [Google Scholar]
- 9.Steerenberg PA, Janssen NAH, de Meer G, Fischer PH, Nierkens S, Van Loveren H, et al. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax. 2003;58:242–5. doi: 10.1136/thorax.58.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohme M, Wickman M, Lennart NS, Svartengren M, Wahlgren CF. Family history and risk of atopic dermatitis in children up to 4 years. Clin Exp Allergy. 2003;33:1226–31. doi: 10.1046/j.1365-2222.2003.01749.x. [DOI] [PubMed] [Google Scholar]
- 11.Brusasco V, Crimi E, Pellegrino R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax. 1998;53:992–8. doi: 10.1136/thx.53.11.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depalo A, Carpagnano GE, Spanevello A, Sabato R, Cagnazzo MG, Gramiccioni C, et al. Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med. 2008;263:70–8. doi: 10.1111/j.1365-2796.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 13.Ricciardolo FLM, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–65. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 14.Ricciardolo FLM, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol. 2004;113:610–9. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Young S, Lesouef PN, Geelhoed GC, Stick S, Turner DJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991;324:1168–73. doi: 10.1056/NEJM199104253241704. [DOI] [PubMed] [Google Scholar]
- 16.Tepper RS, Williams-Nkomo T, Martinez T, Kisling J, Coates C, Daggy J. Parental smoking and airway reactivity in healthy infants. Am J Respir Crit Care Med. 2005;171:78–82. doi: 10.1164/rccm.200406-711OC. [DOI] [PubMed] [Google Scholar]
- 17.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163:37–42. doi: 10.1164/ajrccm.163.1.2005013. [DOI] [PubMed] [Google Scholar]
- 18.Wildhaber JH, Hall GL, Stick SM. Measurements of exhaled nitric oxide with the single-breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med. 1999;159:74–8. doi: 10.1164/ajrccm.159.1.9805021. [DOI] [PubMed] [Google Scholar]
- 19.Frey U, Kuehni C, Roiha H, Cernelc M, Reinmann B, Wildhaber JH, et al. Maternal atopic disease modifies effects of prenatal risk factors on exhaled nitric oxide in infants. Am J Respir Crit Care Med. 2004;170:260–5. doi: 10.1164/rccm.200307-1002OC. [DOI] [PubMed] [Google Scholar]
- 20.Latzin P, Kuehni CE, Baldwin DN, Roiha HL, Casaulta C, Frey U. Elevated exhaled nitric oxide in newborns of atopic mothers precedes respiratory symptoms. Am J Respir Crit Care Med. 2006;174:1292–8. doi: 10.1164/rccm.200606-782OC. [DOI] [PubMed] [Google Scholar]
- 21.Martinez T, Weist A, Williams T, Clem C, Silkoff PE, Tepper RS. Assessment of exhaled nitric oxide kinetics in healthy infants. Am J Respir Crit Care Med. 2002;165:A486. doi: 10.1152/japplphysiol.00758.2002. abstract. [DOI] [PubMed] [Google Scholar]
- 22.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, et al. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161:353–9. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 23.Pucci N, Novembre E, Cammarata MG, Bernardini R, Monaco MG, Calogero C, et al. Scoring atopic dermatitis in infants and young children: distinctive features of the SCORAD index. Allergy. 2005;60:113–6. doi: 10.1111/j.1398-9995.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- 24.Illi S, von ME, Lau S, Nickel R, Niggemann B, Sommerfeld C, et al. The pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin Immunol. 2001;108:709–14. doi: 10.1067/mai.2001.118786. [DOI] [PubMed] [Google Scholar]
- 25.Lopez N, de Barros-Mazon S, dos Santos Vilela MM, Condino Neto A, Ribeiro JD. Are immunoglobulin E levels associated with early wheezing?: a prospective study in Brazilian infants. Eur Respir J. 2002;20:640–5. doi: 10.1183/09031936.02.00219302. [DOI] [PubMed] [Google Scholar]
- 26.Nickel R, Kulig M, Forster J, Bergmann R, Bauer CP, Lau S, et al. Sensitization to hen's egg at the age of twelve months is predictive for allergic sensitization to common indoor and outdoor allergens at the age of three years. J Allergy Clin Immunol. 1997;95:613–7. doi: 10.1016/s0091-6749(97)70021-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang IJ, Lin YT, Yang YH, Chen CL, Tsai YH, Chiang BL, et al. Correlation between age and allergens in pediatric atopic dermatitis. Ann Allergy Asthma Immunol. 2004;93:334–8. doi: 10.1016/S1081-1206(10)61391-9. [DOI] [PubMed] [Google Scholar]
- 28.Sherrill DL, Stein R, Halonen M, Holberg CJ, Wright A, Martinez FD. Total serum IgE and its association with asthma symptoms and allergic sensitization among children. J Allergy Clin Immunol. 1999;104:28–36. doi: 10.1016/s0091-6749(99)70110-7. [DOI] [PubMed] [Google Scholar]
- 29.Barnes KC, Grant AV, Hansel NN, Gao P, Dunston GM. African Americans with asthma: genetic insights. Proc Am Thorac Soc. 2007;4:58–68. doi: 10.1513/pats.200607-146JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celedon JC, Sredl D, Weiss ST, Pisarski M, Wakefield D, Cloutier M. Ethnicity and skin test reactivity to aeroallergens among asthmatic children in Connecticut. Chest. 2004;125:85–92. doi: 10.1378/chest.125.1.85. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson MD, Sellins S, Grube E, Schroer K, Gupta J, Wang N, et al. Aeroallergen sensitization in healthy children: racial and socioeconomic correlates. J Pediatr. 2007;151:187–91. doi: 10.1016/j.jpeds.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145:1129–35. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 33.Illi S, von Mutius E, Lau S, Nickel R, Gruber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113:925–31. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 34.Lowe LA, Woodcock A, Murray CS, Morris J, Simpson A, Custovic A. Lung function at age 3 years: effect of pet ownership and exposure to indoor allergens. Arch Pediatr Adolesc Medi. 2004;158:996–1001. doi: 10.1001/archpedi.158.10.996. [DOI] [PubMed] [Google Scholar]
- 35.Saito J, Inoue K, Sugawara A, Yoshikawa M, Watanabe K, Ishida T, et al. Exhaled nitric oxide as a marker of airway inflammation for an epidemiologic study in schoolchildren. J Allergy Clin Immunol. 2004;114:512–6. doi: 10.1016/j.jaci.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 36.Simpson A, Custovic A, Pipis S, Adisesh A, Faragher B, Woodcock A. Exhaled nitric oxide, sensitization, and exposure to allergens in patients with asthma who are not taking inhaled steroids. Am J Respir Crit Care Med. 1999;160:45–9. doi: 10.1164/ajrccm.160.1.9809091. [DOI] [PubMed] [Google Scholar]
- 37.Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112:883–92. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Moeller A, Diefenbacher C, Lehmann A, Rochat M, Brooks-Wildhaber J, Hall G, et al. Exhaled nitric oxide distinguishes between subgroups of preschool children with respiratory symptoms. J Allergy Clin Immunol. 2008;121:705–9. doi: 10.1016/j.jaci.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Wildhaber JH, Hall GL, Stick SM. Measurements of exhaled nitric oxide with the single-breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med. 1999;159:74–8. doi: 10.1164/ajrccm.159.1.9805021. [DOI] [PubMed] [Google Scholar]
- 40.Franklin PJ, Turner S, Mutch R, Stick SM. Parental smoking increases exhaled nitric oxide in young children. Eur Respir J. 2006;28:730–3. doi: 10.1183/09031936.06.00007206. [DOI] [PubMed] [Google Scholar]
- 41.Hall GL, Reinmann B, Wildhaber JH, Frey U. Tidal exhaled nitric oxide in healthy, unsedated newborn infants with prenatal tobacco exposure. J Appl Physiol. 2002;92:59–66. doi: 10.1152/jappl.2002.92.1.59. [DOI] [PubMed] [Google Scholar]
- 42.Kusel MM, Holt PG, de Klerk N, Sly PD. Support for 2 variants of eczema. J Allergy Clin Immunol. 2005;116:1067–72. doi: 10.1016/j.jaci.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 43.Wuthrich B, Schmid-Grendelmeier P. Natural course of AEDS. Allergy. 2002;57:267–8. doi: 10.1034/j.1398-9995.2002.1n3572.x. [DOI] [PubMed] [Google Scholar]


