Abstract
Background
Maintaining lean body mass (LBM) after a severe burn is an essential goal of modern burn treatment. An accurate determination of LBM is necessary for short- and longterm therapeutic decisions. The aim of this study was to compare 2 measurement methods for body composition, wholebody potassium counting (K count) and dual x-ray absorptiometry (DEXA), in a large prospective clinical trial in severely burned pediatric patients.
Methods
Two-hundred seventy-nine patients admitted with burns covering 40% of total body surface area (TBSA) were enrolled in the study. Patients enrolled were controls or received long-term treatment with recombinant human growth hormone (rhGH). Near-simultaneous measurements of LBM with DEXA and fat-free mass (FFM) with K count were performed at hospital discharge and at 6, 9, 12, 18, and 24 months post injury. Results were correlated using Pearson’s regression analysis. Agreement between the 2 methods was analyzed with the Bland-Altman method.
Results
Age, gender distribution, weight, burn size, and admission time from injury were not significantly different between control and treatment groups. rhGH and control patients at all time points postburn showed a good correlation between LBM and FFM measurements (R2 between 0.9 and 0.95). Bland-Altman revealed that the mean bias and 95% limits of agreement depended only on patient weight and not on treatment or time postburn. The 95% limits ranged from 0.1 ± 2.9 kg for LBM or FFM in 7- to 18-kg patients to 16.3 ± 17.8 kg for LBM or FFM in patients >60 kg.
Conclusions
DEXA can provide a sufficiently accurate determination of LBM and changes in body composition, but a correction factor must be included for older children and adolescents with more LBM. DEXA scans are easier, cheaper, and less stressful for the patient, and this method should be used rather than the K count.
Keywords: Body composition, Absorptiometry/Bone densitometry, Whole-body counting, Burns, Pediatrics
Introduction
Severe thermal injury leads to a change in patient metabolism that may persist for over 24 months after the initial event.1 The ensuing period of hypermetabolism and catabolism following a burn leads to impaired immune function, decreased wound healing, erosion of lean body mass (LBM), and hinders rehabilitative efforts.1-4 Reintegration into society is delayed and quality of life impaired. Strategies for attenuating this maladaptive response5-7 can be divided into nonpharmacologic and pharmacologic approaches. The nonpharmacologic approach includes early excision of burned skin and closure of wounds, pertinacious surveillance for and treatment of sepsis, early commencement of high-protein high-carbohydrate enteral feeding, elevation of the immediate environmental temperature to over 30°C; and enrollment into an aerobic/resistance exercise program. This integrative approach has been shown to improve outcome.6,8-10
Pharmacologic approaches to attenuate hypermetabolic response postburn can be divided into anticatabolic and anabolic drugs. Long-term supplementation studies at the Galveston Shriners Hospital have shown that rhGH decreases catabolism, increases protein synthesis, promotes muscle and bone growth, and shortens hospital stay.11 Skin-graft donor site healing in children with burns covering ≥ 40% of total body surface area (TBSA) was shown to improve by 20%-30%.5,12 Improved growth and recovery time were demonstrated following administration of rhGH over a 12-month period.13 Concern that treatment with rhGH could potentially increase scarring prompted a study showing that severely burned children who received rhGH during the acute hospital course did not develop increased scarring.14 In contrast, rhGHtreated patients had a decreased need for surgical interventions and reconstructive surgeries. The reason for using rhGH as a perturbation in this trial is that rhGH is criticized for increasing edema formation and not muscle. We therefore wanted to determine whether rhGH would change the correlation or bias when dual x-ray absorptiometry (DEXA) was used compared with whole-body potassium counting (K count), serving as a perturbationcontrol group.
In those patients with burns ≥40% TBSA, marked catabolism and loss of LBM almost always result in immune dysfunction, organ failure, and increased mortality.1,2 To modulate these deleterious sequelae, precise body composition measurements are mandatory to detect loss of muscle mass and to counteract these changes. Many different methods of determining body composition have been developed, including body density and volume measurements,15-17 dilution methods,18 bioelectrical impedance and conductance methods,19 wholebody counting and neutron activation analysis,20 DEXA,21-24 K count, 25 magnetic resonance imaging, and computed tomography.
K count is one of the most accurate methods for detecting fat-free mass (FFM) but is labor intensive, expensive, and time consuming. DEXA is better suited to determine body composition: DEXA is fast, is inexpensive, and uses low doses of radiation. The validity of DEXA in severe burns, however, has not been determined. The aim of this study was to determine whether DEXA can be used to effectively and accurately measure LBM in severely burned pediatric patients.
Materials and Methods
Two-hundred seventy-nine patients (144 receiving short-or long-term rhGH and 135 controls) with burns covering ≥40% of TBSA, admitted to our hospital over a 6-year period, were entered into the study. Prior to the study, each subject, parent, or child’s legal guardian had to sign a written informed consent form. This study was approved by the Institutional Review Board at the University of Texas Medical Branch, Galveston.
On admission, the extent and degree of burns were assessed and recorded on a standard Lund and Browder chart by the attending burn surgeon. Information recorded at the time of admission also included burn-related data (date and mechanism) as well as demographic data (age, gender, ethnicity). All thermally injured children with burns covering ≥40% of their TBSA who were admitted to our burn unit, consented to the study, and required at least 1 surgical intervention were included in this study. Patients were resuscitated according to the Galveston formula, receiving lactated Ringer’s solution in amounts of 5000 mL/m2 TBSA burned + 2000 mL/m2 TBSA burned, given in increments during the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision and open wounds were covered with autograft or allograft. After the first operative procedure, it took 5-10 days until the donor site was healed, and patients were then taken back to the operating room. This procedure was repeated until all open wound areas were covered with autologous skin.
All patients received the same nutrition according to a standardized protocol. The intake is calculated as 1500 kcal/m2 body surface + 1500 kcal/m2 area burn, or the need was assessed by measuring the resting energy expenditure, multiplied by 1.4 with weekly adjustments as previously published.23,26,27 The nutrition route of choice was enteral nutrition. Therefore, almost all patients received nutrition via a duodenal (Dobhoff) or nasogastric tube. Parenteral nutrition was only given in the rare instances when the patient did not tolerate any tube feeds. One patient group did not receive any anabolic intervention, and 1 group of patients received rhGH at a dose of 0.05 or 0.1 mg/kg/d.
Near-simultaneous DEXA and K-count assessments were carried out at hospital discharge and at 6, 9, 12, 18, and 24 months post injury.
Whole-Body Potassium Measurement (K Count)
This method uses the natural radioactive decay of a small fraction of the total number of potassium atoms in the body that are radioactive (called 40K). It was originally used for surveillance of workers who had come into contact with radioactive sources during weapons manufacture or research. Surprisingly, a constant peak in the results (attributable to the isotope 40K) occurred in each employee independent of exposure to radioactivity. Once it was linked to FFM, the method was used to determine body composition.28-30 Only a minute fraction of the total-body potassium is 40K (about 0.012%). It produces high-energy γ radiation at a rate of around 266,000 disintegrations per minute, and 89% of these disintegrations will result in the production of calcium-40 by emitting a β particle with no attendant γ radiation (maximum energy of 1.33 MeV); 11% produces the gas argon-40 by electron capture with emission of an energetic γ photon (maximum energy of 1.46 MeV). All of the β particles and approximately half of the γ rays are absorbed in the body. Because of the small amount of radiation that must be measured, specialized equipment and shielding are required for accurate assessment. The K counter located at the University of Texas Medical Branch was designed to measure potassium in subjects ranging from preterm infants to obese adults. The patient is positioned supine between 2 arrays of 32 NaI detectors (16 above and 16 below) that are inside a room constructed of 8-in-thick steel walls (Figure 1).
Figure 1.

Whole-body K-Counter used in this study at the University of Texas Medical Branch.
The counting process takes 15 minutes, and patients are monitored continuously via a video camera and intercom system the whole-body counter is calibrated with the use of a phantom before each measurement. Total body potassium may be used to calculate body cell mass31 and to estimate FFM.
DEXA
Measurements of LBM, bone mineral content, and fat mass were carried out using DEXA (QDR-4500W model, Hologic, Waltham, MA; Figure 2) and recorded using a pediatric software package. This system is specifically designed to have minimal mean error when measuring LBM in children. The machine is calibrated daily using a phantom. The measurement of different tissues in the body using the DEXA is based on the principle that the intensity of a beam of x-rays passing through a patient is related to the thickness, density, and chemical composition of the tissues traversed. This alteration in intensity is also dependent on the energy of the x-ray, which, when low, is subject to 2 principles: Compton scattering and the photoelectric effect.32-34 These account for the change in intensity of the transmitted x-ray beam as it passes through the patient. Therefore, once the change in intensity and the mass attenuation coefficients are known, bone mass and overlying soft tissue mass can be calculated. The scan takes 5 minutes and requires cooperation on the part of the patient since movement must be kept to a minimum to prevent anomalous readings. Therefore, the use of DEXA measurements in infants and neonates is limited, although possible if the patient is asleep and not moving.
Figure 2.
DEXA scanner used in this study at Shriners Hospitals for Children.
Statistical Analysis
The relationship between DEXA and K-count measurements was determined using linear regression. Significance of the relationship between DEXA and K count was assessed by F test on the slope coefficient. Pearson’s correlation coefficient was computed.
Agreement between the 2 measurement methods was assessed using the Bland-Altman method.35,36 For the graphic display, the average of each measurement pair performed by DEXA and K count was plotted against their difference. The mean of all differences (mean bias) and the upper and lower limits of agreement were then superimposed as parallel lines; the limits of agreement were estimated by mean difference ±1.96 standard deviation (SD) of the differences to provide the interval within which 95% of differences between measurements by the 2 methods are supposed to lie.
To provide a better limit of agreement estimate for a wide range of patient weights and FFM values, and taking into account the increasing standard deviation with increasing FFM, limits of agreement were adjusted to weight. A linear best-fit curve was calculated from all data points using the following linear equation:
The slope and intercept of the best-fit curve were then used to plot V-shaped limits of agreement for 5–65 kg FFM, using 5-kg intervals to calculate the mean bias and ±1.96 SD to plot the appropriate curves.
Results
Two-hundred seventy-nine patients (144 rhGH and 135 controls) with burns covering ≥40% TBSA were enrolled in the study. Age, gender distribution, weight, burn size, and time between injury and hospital admission were not significantly different between the control patients and those receiving rhGH (Table 1).
Table 1. Patient Demographics.
| Group | rhGH | Control |
|---|---|---|
| n | 144 | 135 |
| Age [years] | 9 ± 5 | 9 ± 5 |
| TBSA [%] | 62 ± 15 | 60 ± 14 |
| TBSA 3rd [%] | 48 ± 24 | 48 ± 22 |
| M:F Ratio | 4.7:1 | 4:1 |
| Burn Type | ||
| • Flame [%] | 82 | 85 |
| • Scald [%] | 14 | 12 |
| • Other [%] | 4 | 3 |
| Admission post injury [days] | 4 ± 6 | 5 ± 6 |
rhGH=recombinant human growth hormone; TBSA= total body surface area. Results are ± standard deviation.
The analysis of all data pairs taken together (controls and rhGH patients at all time points postburn) revealed a strong correlation between DEXA and K-count measurements (Figure 3A). A similar level of correlation was found when control patients (Figure 3B) and rhGH patients (Figure 3C) at all time points postburn were analyzed separately.
Figure 3.
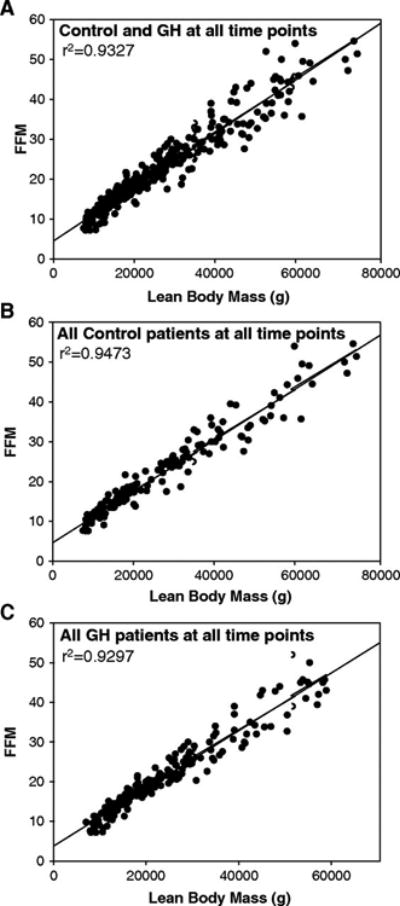
(A) Correlation of lean-body mass (LBM) with fat-free mass (FFM) for all patients (Control + Growth Hormone) across all time points (n=279). (B) Correlation of lean-body mass (LBM) with fat-free mass (FFM) for control patients across all time points. (C) Correlation of lean-body mass (LBM) with fat-free mass (FFM) for growth hormone patients across all time points (n=144).
To determine whether the time interval after burn injury had an effect on the correlation between the measurement methods, patients from both treatment groups were stratified into 6 different categories: time of discharge and 6, 9, 12, 18, and 24 months postburn. DEXA and K-count measurements strongly correlated at all time points (Figure 4).
Figure 4.
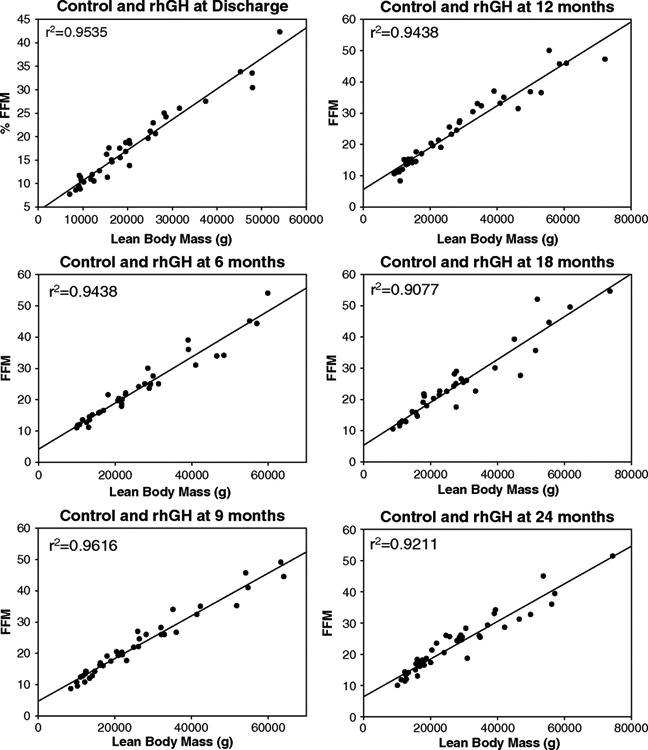
Correlation of lean-body mass (LBM) with fat-free mass (FFM) for all patients (Control + Growth Hormone) across different time points (n varies from 40 to 279).
Agreement between the 2 measurement methods was assessed with the Bland-Altman method. The mean bias ±95% limit of agreement (mean ± 1.96 SD) for all data pairs (controls, rhGH, all time points) ranged between −2.1 kg ± 6.3 kg and 17.7 kg ± 6.3 kg when using a constant SD, independent of patient weight (Figure 5A). Adjusted for smaller and larger weights, the limits of agreement were significantly lower for smaller children (10 kg LBM/FFM: mean bias -1.2 kg ± 3 kg 95% limit of agreement) than for adolescents and young adults (60 kg LBM/FFM: mean bias 16.3 kg ±17.8 kg; Figure 5B). A closer look at different patient weights with a stratification into 2 different weight groups (7-17.9 kg and 18-65 kg) revealed a very low bias in the smaller weight group (0.1 kg ± 2.9 kg, Figure 5C) but a larger bias that increased further with larger FFM in older children (Figure 5D).
Figure 5.
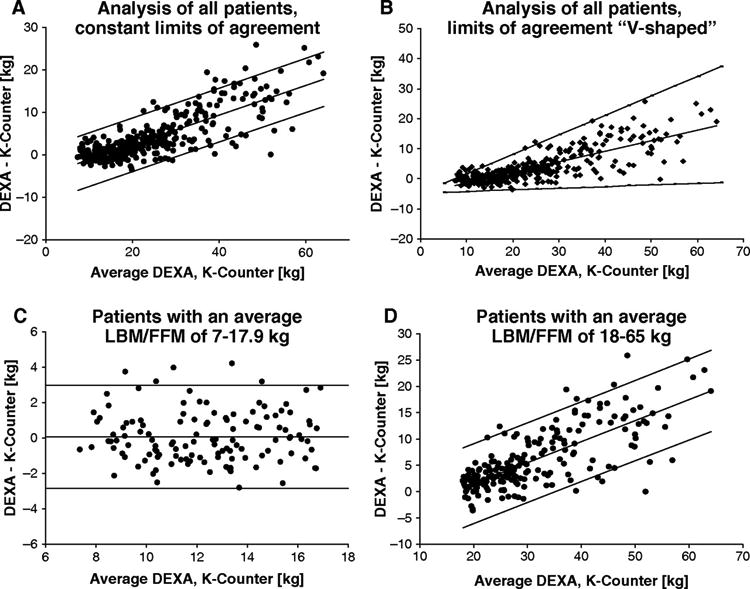
Agreement between lean body mass (LBM; DEXA) and fat-free mass (FFM; K count). The difference between each data pairs is plotted against their mean value (Bland-Altman method).35 Within the plot, the mean bias between the methods is expressed by the middle line and the upper and lower 95% limits of agreement by the respective upper and lower line. (A) Analysis of all patients, constant limits of agreement. (B) Analysis of all patients, limits of agreement V-shaped around the regression line of the differences (mean bias, equation: y = 0.3506x – 4.7275, R2 = 0.6551). (C) Patients with an average LBM/FFM of 7-17.9 kg. (D) Patients with an average LBM/FFM of 18-65 kg.
All of the aforementioned mean biases and limits of agreement only changed marginally when patients were divided into control and rhGH groups and separately examined at different time intervals postburn.
Discussion
Severe thermal injury leads to hypermetabolism and catabolism that persist for >24 months after the initial event.1 The consequences of hypermetabolism and catabolism are erosion of LBM, hyperglycemia, impaired immune function, decreased wound healing, and delayed rehabilitation and reintegration into society.6 Our group hypothesized that these detrimental responses can be attenuated, and rehabilitation and reintegration can be accelerated by using pharmacologic intervention and exercise.6,8-10 One of the main outcome variables for all of these studies is LBM. A substantial loss of LBM is deleterious to patient outcome. Therefore, precise measurements of body composition are essential to monitor patient recovery following thermal injury. Special logistic difficulties arise from the fact that burn patients in the early postburn phase are mostly intensive care inpatients and therefore are difficult to transport. Densitometry, isotope dilution methods, in vivo neutron activation analysis, DEXA, computed tomography, and magnetic resonance imaging are the most common methods used to measure body composition.37,38 Bioelectrical impedance analysis (BIA), a noninvasive method based on the electrical properties of tissues, has been used to assess body fluid compartments in critically ill patients and is described as an easy-to-use bedside tool.39 Another bedside technique is muscle ultrasound. The validity and correlation to DEXA in critically ill patients with multiple-organ failure were assessed in a small clinical study but proved to be only fair.40
The gold standard to determine FFM is whole-body potassium counting (K count). The K count, however, is associated with logistic and financial issues and is not well suited for intensive care patients. An alternative method to determine body composition is DEXA. This method is easier and faster to perform, with a 100-fold lower radiation exposure than a chest X ray. The use of DEXA to measure different body compartments (muscle, fat, bone) has been described and validated against gold standard methods in multiple clinical studies. Its use in severe burns, however, has not been determined. The aim of this study was to compare DEXA to K-count measurements in severely burned pediatric patients to determine whether DEXA can be used to accurately measure LBM.
Our data indicate that LBM (DEXA) and FFM (K-count) measurements correlate strongly and show an acceptable limit of agreement, as determined by the Bland-Altman analysis. One of the main criticisms has been that DEXA cannot differentiate water/edema from LBM and therefore gives false results, especially during the acute phase postburn.41 Water retention and edema formation play an important role during the acute phase postburn.42,43 Interestingly, in smaller children with less FFM, the mean bias between the methods was nearly zero, whereas it increased rapidly when the muscle mass surpassed 30 kg. Therefore, a correction must be in larger patients used that can be easily derived from Figure 5A. We found that the LBM measurements (DEXA) showed the same limits of agreement and mean bias as FFM (K-count) measurements across all time points and independent from a drug intervention that changes body composition. Because DEXA and K count strongly correlate, we suggest that DEXA accurately detects LBM and can differentiate edema from fat or muscle. The data indicate that this study is sufficiently powered and the results are thus valid.
The DEXA scanner located within our unit is easy to use and does not require any special shielding from the outside world. The patient can be scanned in the presence of a parent or caregiver, which significantly reduces the psychological stress for the patient. The K counter is housed in a darkened room that is shielded by steel and concrete. The patient is alone in the room during the scan (15 minutes) and can only communicate using a closedcircuit television and radio intercom. In our hospital, the patient must be moved a considerable distance to undertake the K-count scan.
All control patients in the above-mentioned time period who had simultaneous LBM and FFM measurements were included in the study. We then aimed at adding a perturbation that is known to alter body composition, somewhat serving as a positive control. We thus chose rhGH as an agent that alters body composition and, in addition, was criticized to increase whole-body edema. DEXA and K-count measurements correlated not only in control patients but also in control and rhGH patients, indicating that alterations in body composition can be detected by both methods. The Bland-Altman analysis method revealed that rhGH did not add any bias or error, indicating that LBM determined by DEXA is a valid end point for pharmacologic and nonpharmacologic trials.
In this study, a very close relationship between DEXA measurements of LBM and K-count measurements of FFM was shown. We have shown in this study that we are now able to accurately measure other parameters of body composition in pediatric patients. LBM can be accurately determined in severely burned children using the more user-friendly DEXA methodology instead of whole body potassium counting. We therefore advocate the use of DEXA measurements of body composition during the acute admission and during the reconstructive phase of burn care.
Conclusion
Evaluation of body composition in pediatric patients is comparable using both DEXA and K-count measurements. LBM and FFM measurements show no mean bias for smaller muscle masses, but a correction factor has to be applied when examining larger patients. Because DEXA scans are easier, cheaper, and less stressful for the patient, we recommend using this method rather than the K-count scan. Further studies are required to ascertain whether the accuracy of measurements seen here can be extrapolated to adult burn patients.
Acknowledgments
This study was supported by grants from the American Surgical Association, Shriners Hospitals for Children (8660), NIDRR (H133A020102), and NIGMS (R01-GM56687, T32 GM008256, and P50 GM60338).
The authors would like to acknowledge all clinical and scientific staff of the Galveston Shriners Hospital for their great support in conducting this study.
References
- 1.Jeschke MG, Przkora R, Suman OE, et al. Sex differences in the long-term outcome after a severe thermal injury. Shock. 2007;27:461–465. doi: 10.1097/01.shk.0000238071.74524.9a. [DOI] [PubMed] [Google Scholar]
- 2.Barrow RE, Przkora R, Hawkins HK, Barrow LN, Jeschke MG, Herndon DN. Mortality related to gender, age, sepsis, and ethnicity in severely burned children. Shock. 2005;23:485–487. [PubMed] [Google Scholar]
- 3.Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007;27:4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 4.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 5.Herndon DN, Barrow RE, Kunkel KR, Broemeling L, Rutan RL. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg. 1990;212:424–431. doi: 10.1097/00000658-199010000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 7.Przkora R, Herndon DN, Finnerty CC, Jeschke MG. Insulin attenuates the cytokine response in a burn wound infection model. Shock. 2007;27:205–208. doi: 10.1097/01.shk.0000238069.84826.1b. [DOI] [PubMed] [Google Scholar]
- 8.Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–1187. doi: 10.1016/s0039-6109(16)41685-3. [DOI] [PubMed] [Google Scholar]
- 9.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 10.Hart DW, Wolf SE, Chinkes DL, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–764. doi: 10.1097/01.TA.0000060260.61478.A7. [DOI] [PubMed] [Google Scholar]
- 11.Gore DC, Honeycutt D, Jahoor F, Wolfe RR, Herndon DN. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991;126:38–43. doi: 10.1001/archsurg.1991.01410250042006. [DOI] [PubMed] [Google Scholar]
- 12.Gilpin DA, Barrow RE, Rutan RL, Broemeling L, Herndon DN. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg. 1994;220:19–24. doi: 10.1097/00000658-199407000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przkora R, Herndon DN, Suman OE, et al. Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients. Ann Surg. 2006;243:796–803. doi: 10.1097/01.sla.0000219676.69331.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barret JP, Dziewulski P, Jeschke MG, Wolf SE, Herndon DN. Effects of recombinant human growth hormone on the development of burn scarring. Plast Reconstr Surg. 1999;104:726–729. doi: 10.1097/00006534-199909030-00017. [DOI] [PubMed] [Google Scholar]
- 15.Lim S, Joung H, Shin CS, et al. Body composition changes with age have gender-specific impacts on bone mineral density. Bone. 2004;35:792–798. doi: 10.1016/j.bone.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Shaw NJ, Fraser NC, Rose S, Crabtree NJ, Boivin CM. Bone density and body composition in children with growth hormone insensitivity syndrome receiving recombinant IGF-I. Clin Endocrinol (Oxf) 2003;59:487–491. doi: 10.1046/j.1365-2265.2003.01875.x. [DOI] [PubMed] [Google Scholar]
- 17.van der Sluis IM, Boot AM, Hop WC, De Rijke YB, Krenning EP, de Muinck Keizer-Schrama SM. Long-term effects of growth hormone therapy on bone mineral density, body composition, and serum lipid levels in growth hormone deficient children: a 6-year follow-up study. Horm Res. 2002;58:207–214. doi: 10.1159/000066262. [DOI] [PubMed] [Google Scholar]
- 18.Prelack K, Dwyer J, Sheridan R, et al. Body water in children during recovery from severe burn injury using a combined tracer dilution method. J Burn Care Rehabil. 2005;26:67–74. doi: 10.1097/01.bcr.0000150300.16237.47. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder D, Christie PM, Hill GL. Bioelectrical impedance analysis for body composition: clinical evaluation in general surgical patients. JPEN J Parenter Enteral Nutr. 1990;14:129–133. doi: 10.1177/0148607190014002129. [DOI] [PubMed] [Google Scholar]
- 20.Ryde SJ, Birks JL, Morgan WD, Evans CJ, Dutton J. A fivecompartment model of body composition of healthy subjects assessed using in vivo neutron activation analysis. Eur J Clin Nutr. 1993;47:863–874. [PubMed] [Google Scholar]
- 21.Gauglitz GG, Song J, Herndon DN, et al. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy KD, Thomas S, Mlcak RP, Chinkes DL, Klein GL, Herndon DN. Effects of long-term oxandrolone administration in severely burned children. Surgery. 2004;136:219–224. doi: 10.1016/j.surg.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995;126:252–256. doi: 10.1016/s0022-3476(95)70553-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Zhu S, Wang J, Pierson RN, Jr, Heymsfield SB. Wholebody skeletal muscle mass: development and validation of totalbody potassium prediction models. Am J Clin Nutr. 2003;77:76–82. doi: 10.1093/ajcn/77.1.76. [DOI] [PubMed] [Google Scholar]
- 26.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 27.Mlcak RP, Jeschke MG, Barrow RE, Herndon DN. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121–130. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80:649–680. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 29.Forbes GB, Hursh JB. Estimation of total body fat from potassium-40 content. Science. 1961;133:1918. [PubMed] [Google Scholar]
- 30.Kulwich R, Feinstein L, Anderson EC. Correlation of potassium-40 concentration and fat-free lean content of hams. Science. 1958;127:338–339. doi: 10.1126/science.127.3294.338-a. [DOI] [PubMed] [Google Scholar]
- 31.Moore FD, Olesen KH, McMurrey JD, Parker HV, Ball MR, Boyden CM. The Body Cell Mass and Its Supporting Environment: Body Composition in Health and in Disease. Philadelphia, PA: Saunders; 1963. [Google Scholar]
- 32.Pierson RN, Jr, Wang J, Thornton JC, et al. Bone mineral and body fat measurements by two absorptiometry systems: comparisons with neutron activation analysis. Calcif Tissue Int. 1995;56:93–98. doi: 10.1007/BF00296337. [DOI] [PubMed] [Google Scholar]
- 33.Rao PS, Gregg EC. Attenuation of monoenergetic gamma rays in tissues. Am J Roentgenol Radium Ther Nucl Med. 1975;123:631–637. doi: 10.2214/ajr.123.3.631. [DOI] [PubMed] [Google Scholar]
- 34.White DR, Peaple LH, Crosby TJ. Measured attenuation coefficients at low photon energies (9.88-59.32 keV) for 44 materials and tissues. Radiat Res. 1980;84:239–252. [PubMed] [Google Scholar]
- 35.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 36.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 37.Jebb SA, Elia M. Techniques for the measurement of body composition: a practical guide. Int J Obes Relat Metab Disord. 1993;17:611–621. [PubMed] [Google Scholar]
- 38.Plank LD, Hill GL. Energy balance in critical illness. Proc Nutr Soc. 2003;62:545–552. doi: 10.1079/pns2003259. [DOI] [PubMed] [Google Scholar]
- 39.Bracco D, Berger MM, Revelly JP, Schutz Y, Frascarolo P, Chiolero R. Segmental bioelectrical impedance analysis to assess perioperative fluid changes. Crit Care Med. 2000;28:2390–2396. doi: 10.1097/00003246-200007000-00034. [DOI] [PubMed] [Google Scholar]
- 40.Campbell IT, Watt T, Withers D, et al. Muscle thickness, measured with ultrasound, may be an indicator of lean tissue wasting in multiple organ failure in the presence of edema. Am J Clin Nutr. 1995;62:533–539. doi: 10.1093/ajcn/62.3.533. [DOI] [PubMed] [Google Scholar]
- 41.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delman K, Malek SK, Bundz S, Abumrad NN, Lang CH, Molina PE. Resuscitation with lactated Ringer’s solution after hemorrhage: lack of cardiac toxicity. Shock. 1996;5:298–303. [PubMed] [Google Scholar]
- 43.Shah A, Connolly CM, Kirschner RA, Herndon DN, Kramer GC. Evaluation of hyperdynamic resuscitation in 60% TBSA burn injured sheep. Shock. 2004;21:86–92. doi: 10.1097/01.shk.0000101666.49265.b4. [DOI] [PubMed] [Google Scholar]
- 44.Aili Low JF, Barrow RE, Mittendorfer B, Jeschke MG, Chinkes DL, Herndon DN. The effect of short-term growth hormone treatment on growth and energy expenditure in burned children. Burns. 2001;27:447–452. doi: 10.1016/s0305-4179(00)00164-9. [DOI] [PubMed] [Google Scholar]
- 45.Connolly CM, Barrow RE, Chinkes DL, Martinez JA, Herndon DN. Recombinant human growth hormone increases thyroid hormone-binding sites in recovering severely burned children. Shock. 2003;19:399–403. doi: 10.1097/01.shk.0000051758.08171.bc. [DOI] [PubMed] [Google Scholar]
- 46.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med. 2000;28:1578–1584. doi: 10.1097/00003246-200005000-00053. [DOI] [PubMed] [Google Scholar]
- 47.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study. Lancet. 1999;354:1789. doi: 10.1016/s0140-6736(99)02741-5. [DOI] [PubMed] [Google Scholar]
- 48.Przkora R, Barrow RE, Jeschke MG, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60:968–971. doi: 10.1097/01.ta.0000214580.27501.19. [DOI] [PubMed] [Google Scholar]
- 49.Thomas S, Wolf SE, Chinkes DL, Herndon DN. Recovery from the hepatic acute phase response in the severely burned and the effects of long-term growth hormone treatment. Burns. 2004;30:675–679. doi: 10.1016/j.burns.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996;12:45–51. doi: 10.1016/0899-9007(95)00017-8. [DOI] [PubMed] [Google Scholar]


