Abstract
Objective
Identifying risk factors for hardware removal in patients undergoing mandibular reconstruction with vascularized osseous free flaps remains a challenge. The purpose of this study is to identify potential risk factors, including osteocutaneous radial forearm versus fibular flap, for need for removal and to describe the fate of implanted hardware.
Study Design
Case series with chart review.
Setting
Academic tertiary care medical center.
Subjects and Methods
Two hundred thirteen patients undergoing 227 vascularized osseous mandibular reconstructions between the years 2004 and 2012. Data were compiled through a manual chart review, and patients incurring hardware removals were identified.
Results
Thirty-four of 213 evaluable vascularized osseous free flaps (16%) underwent surgical removal of hardware. The average length of time to removal was 16.2 months (median 10 months), with the majority of removals occurring within the first year. Osteocutaneous radial forearm free flaps (OCRFFF) incurred a slightly higher percentage of hardware removals (9.9%) compared to fibula flaps (6.1%). Partial removal was performed in 8 of 34 cases, and approximately 38% of these required additional surgery for removal.
Conclusion
Hardware removal was associated with continued tobacco use after mandibular reconstruction (P = .03). Removal of the supporting hardware most commonly occurs from infection or exposure in the first year. In the majority of cases the bone is well healed and the problem resolves with removal.
Keywords: mandible, hardware removal, osseous free flap, reconstruction
Introduction
Vascularized osseous free flaps have become the standard of care for oromandibular reconstruction secondary to malignancy, osteoradionecrosis, or trauma. Osteocutaneous free flaps restore important 3-dimensional anatomic relationships and repair large bony and soft tissue defects that would otherwise leave the patient with an unacceptable functional and cosmetic outcome. Although cosmesis is a concern for the reconstructive head and neck surgeon, restoration of speech and swallowing and maintenance of oral competence are perhaps more important outcomes. Mandibular stability is essential to the aforementioned goals, and advancements in reconstructive plating systems have optimized fixation of osteotomized segments. These advancements include the replacement of steel plates with titanium, slimmer plate profiles with increased malleability, and enhanced stability with the introduction of locking screws.1,2
Despite improvements in hardware and surgical techniques, hardware complications and failures persist. Hardware-related complication rates are approximately 15%, with hardware exposure and extrusion cited as the most common complication (Figure 1).1,3,4 Other complications include plate fracture, infection, and malunion, and the majority of these complications occur within the first year after reconstruction.2,5 This additional morbidity to the patient can often result in multiple clinic visits and potentially another trip to the operating room for hardware removal. Most of the time, prior to removal, conservative treatment options such as observation, antibiotic therapy, debridement and primary closure, and hyperbaric oxygen treatments are attempted. However, hardware removal rates are approximately 15%.1 The ability to identify factors that lead to removal, therefore, may guide surgical decision making and discussion with patients at the time of initial placement as well as expedite definitive treatment if a hardware complication still occurs. Furthermore, the rate of hardware removal associated with osteocutaneous radial forearm flaps compared to fibula flaps is not well defined.
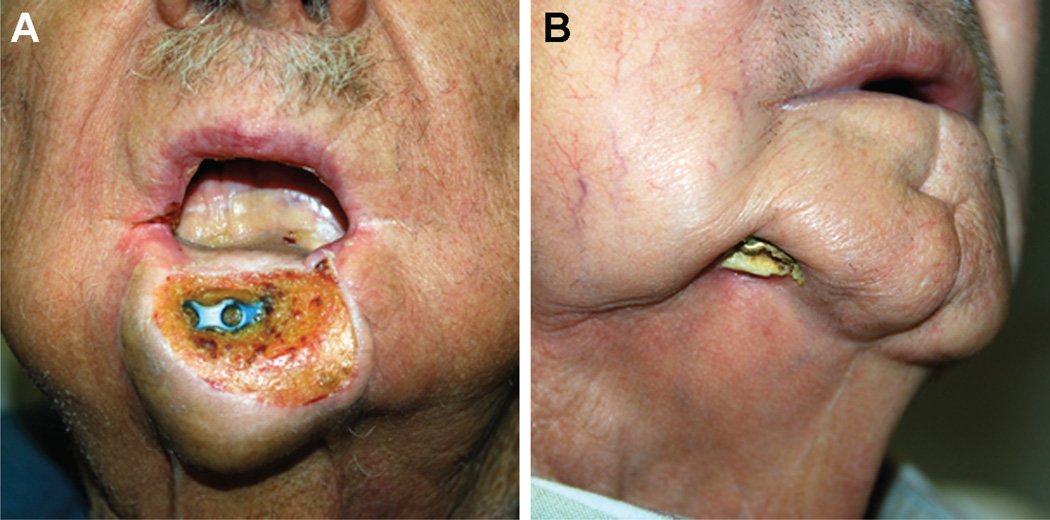
Figure 1.
Patient examples of osteocutaneous radial forearm free flaps with hardware exposure (A, B).
To this end, we sought to identify the incidence of hardware removal and potential risk factors for eventual hardware removal in patients undergoing mandibular reconstruction with vascularized osseous free flaps.
Methods
After University of Alabama at Birmingham (UAB) Institutional Review Board approval, a retrospective reviewof medical records for all patients presenting to UAB for a radius or fibula osteocutaneous free flap reconstruction by a single surgeon between September 2004 and October 2012 was performed. A total of 229 patients receiving 260 free flaps were identified; 14 patients received 2 osteocutaneous flaps and 1 patient received 3. Thirty-one cases were excluded from analysis for the following reasons: 22 were identified as non-mandible reconstructions, 3 were children younger than 18 years of age, 2 patients died in the immediate postoperative time period, and 2 patients received a total of 4 flaps within the same hospitalization. In the remaining 229 cases, 40 patients receiving 48 flaps were identified as having hardware removal. Patients in this group all had an operative note in their medical records indicating that hardware removal was performed. Six patients receiving 6 flaps were excluded from the removal group for the following reasons: 3 had hardware removal because of postoperative flap failure; in 1 patient, removal was planned but the procedure was discontinued as the patient was found to have extensive, unresectable disease; in 1 case, hardware was removed by and the patient followed up with the oral and maxillofacial surgery service; 1 patient’s removal was unplanned and decided intraoperatively with the proper consent from family. Therefore, 34 patients (receiving 42 flaps) incurred a hardware removal and only the flap that incurred the removal was analyzed for relevant information. One hundred seventy-nine patients (receiving 185 flaps) were included in the final comparison group of those who did incur a hardware removal. In this group, only the first flap performed on the patient was included for analysis.
For all patients, demographic information such as age, race, and sex was collected. The following clinical factors were collected: American Society of Anesthesiologists (ASA) preoperative comorbidity classification6 (determined by the lead author based on recorded comorbidities), date of surgery, date of discharge, length of stay, indication for flap (oral cavity cancer, recurrent oral cavity cancer, osteoradionecrosis, or other—trauma, skin cancer, salivary malignancy, osteosarcoma, ameloblastoma, odontogenic cyst, bisphosphonate necrosis, oropharyngeal cancer, osteomyelitis secondary to fracture, and repair or reconstruction of a previous surgery), radiation treatment status (postoperatively, previously, none, not recorded, or both pre- and postoperatively), tobacco use status at the time of surgery, tobacco use in the postoperative period, and date of last UAB otolaryngology follow-up visit.
Surgical fixation was performed in the following way: Mandibular hardware was bent intraoperatively to obtain appropriate contour, and bicortical locking screws were used on the native mandible and monocortical locking screws on the bone flap. At least 3 screws were placed on the native mandible and 2 placed on each segment of the bone flap. Synthes (West Chester, Pennsylvania) mandibular hardware was used with rare exception throughout the study period. Other surgery-related information such as type of osteocutaneous free flap (radius or fibula), flap location (anterior, lateral, or both), type of plate used (2.0 mm, 2.4mm, other, or not recorded), number of osteotomies, if 2 plates were used for fixation, postoperative complications (within the first 30 days), date of exposure (intraoral or external) if one occurred, and time to exposure was obtained. Hardware exposure was often associated with some evidence of infection prior to exposure and subsequently, purulent drainage would develop at the exposed site. The extent of this was inconsistently documented and therefore recorded in the manner described. Information such as time to removal, reason for removal, whether or not bony union was present at time of removal, what was removed, was a second hardware removal necessary, and if the reason for removal resolved was gleaned from the records of the failure patients (n = 34). Length of stay was calculated from the date of surgery to the discharge date, time to exposure was calculated from the date of surgical placement to the date of the first clinic note denoting exposure, and time to removal was calculated from the date of surgical placement to the date of removal. Follow-up at UAB was 6 months, at which point the patients could return to their local otolaryngologist for continued care and follow-up. Follow-up in the hardware removal group was calculated from the date of surgery to the date of last UAB otolaryngology encounter.
Statistical analysis was performed using commercially available statistical software (SAS, version 9.3; SAS Institute Inc, Cary, North Carolina). Descriptive statistics were summarized as means with standard deviation (SD) or medians and categorical data as frequency and percentages. Characteristics were compared between the patients whoexperienced hardware removal and those who did not by t tests for means and chi-square test for frequency data. Six month, 1 year, and 2 year removal rates were determined for the hardware removal group and the trend in time to removal displayed on a line graph. A P value of\.05 was deemed statistically significant.
Results
The study included a total of 213 patients who underwent mandibular defect reconstruction with either a radial or fibular vascularized osseous flap. In the collective patient population, the majority were Caucasian (78%) and male (68%), presenting with oral cavity cancer (43%) as the most common indication for free flap. The next most common indication for free flap was the “other” category (22%), which included trauma, oropharyngeal cancer, salivary, odontogenic, and skin malignancies, and repairs of previous surgeries performed elsewhere. Recurrent oral cavity cancer was considered separately and comprised 20%, while osteoradionecrosis was the indication for the remaining 15%. Mean age was 58.8 years (range, 18–97 years). Just over half of osseous free flaps performed were radial (52%), and lateral mandibular defects were most commonly reconstructed (66%). Average length of stay was 8.5 days (SD = 3.55, range, 2–24 days). Approximately 81% of patients were determined to be ASA class 1 or 2 preoperatively, and 76% of patients were current or former tobacco users at the time of presentation. Hypertension was the most commonly noted comorbidity, seen in approximately 51%, but there was no significant difference between the 2 groups (P = .37).
Of the 213 flaps evaluated, overall exposure (external or intraoral) rate was 19% (n = 41; 21 patients in the non hardware removal group and 20 in the hardware removal group). Exposures were initially managed with debridement and local flap coverage unless the patient was noted to have recurrent or metastatic disease, in which case no additional procedures were performed. It should be noted that the majority of the non-hardware removal exposure group did have recurrent or metastatic disease, needed a new free flap, or they declined further intervention and therefore did not undergo a removal procedure. Only 8 of these 21 patients could be managed conservatively with debridement and local flap or mucosal coverage, 6 hyperbaric oxygen treatments. For the 21 exposures observed in the non-hardware removal group, the average time to exposure was 8.6 months (SD = 14, median = 3.7, range, 0.6–60 months).
It was found that 34 (16%) patients incurred a hardware removal. Clinical and surgical factors were then assessed between the removal group and non-removal group (Tables 1 and 2). There were no significant differences between the groups relating to gender, race, or age, although younger patients had a higher rate of removal that trended toward significance (P = .08). We identified a significant difference in hardware removal in patients who admitted to postoperative tobacco use (P = .03) and preoperative tobacco also demonstrated a trend toward removal, with a P value of .07.
Table 1.
Demographic and clinical factors of patients receiving osteocutaneous flaps.
| Factor | Hardware failure (n = 34) Mean (SD) |
No hardware failure (n = 179) Mean (SD) |
P |
|---|---|---|---|
| Age (years) | 56.5 (12.7) | 61.1 (14.3) | .08 |
| Range, 19–80 | Range, 18–97 | ||
| N (%) | N (%) | ||
| Gender | |||
| Male | 21 (62) | 124 (69) | .39 |
| Female | 13 (38) | 55 (31) | |
| Race | |||
| Caucasian | 28 (82) | 137 (76) | .49 |
| African American | 6 (18) | 30 (17) | |
| Other | 0 (0) | 3 (2) | |
| Not recorded | 0 (0) | 9 (5) | |
| ASA classification | |||
| 1 | 6 (17) | 22 (12) | .73 |
| 2 | 23 (68) | 121 (68) | |
| 3 | 5 (15) | 34 (19) | |
| 4 | 0 (0) | 2 (1) | |
| Tobacco use preoperative | |||
| No | 8 (23) | 37 (21) | .07 |
| Current tobacco user | 22 (65) | 82 (46) | |
| Former tobacco user | 3 (9) | 54 (30) | |
| Not recorded | 1 (3) | 6 (3) | |
| Tobacco use postoperative | |||
| Yes | 8 (23) | 28 (16) | .03 |
| No | 25 (74) | 111 (62) | |
| Unknown | 2 (3) | 40 (22) |
Table 2.
Tumor characteristics and surgical factors of patients receiving osteocutaneous flaps.
| Factor | Hardware failure (n = 34) N (%) |
No hardware failure (n = 179) N (%) |
P |
|---|---|---|---|
| Indication for flap | |||
| Oral cavity cancer | 18 (53) | 73 (41) | .40 |
| Recurrent oral cancer | 5 (15) | 38 (21) | |
| ORN | 6 (17) | 25 (14) | |
| Other | 5 (15) | 43 (24) | |
| Free flap | |||
| Radius | 21 (62) | 90 (50) | .22 |
| Fibula | 13 (38) | 89 (50) | |
| Flap location | |||
| Anterior | 11 (32) | 47 (26) | .54 |
| Lateral | 22 (65) | 119 (67) | |
| Both | 1 (3) | 13 (7) | |
| Plate used | |||
| 2 mm | 31 (91) | 144 (96) | .19 |
| 2.4 mm | 3 (9) | 4 (3) | |
| Both | 0 (0) | 2 (1) | |
| Two plates used | |||
| Yes | 3 (9) | 14 (8) | .84 |
| No | 31 (91) | 165 (92) | |
| Number of osteotomies | |||
| 0 | 22 (65) | 94 (52) | .52 |
| 1 | 9 (26) | 55 (31) | |
| 2 | 3 (9) | 28 (16) | |
| 3 | 0 (0) | 2 (1) | |
| Postoperative complications | |||
| None | 25 (74) | 140 (78) | .54 |
| 1 or more | 9 (26) | 39 (22) | |
| Radiation status | |||
| Postoperative | 12 (35) | 58 (32) | .37 |
| Previous | 10 (30) | 60 (33) | |
| None | 12 (35) | 46 (26) | |
| Unknown | 0 (0) | 12 (7) | |
| Preoperative and postoperative | 0 (0) | 3 (2) | |
| Length of stay (days) | 8.4 (3.4) | 8.6 (3.7) | .82 |
When comparing flap characteristics, we noted that osteocutaneous radial forearm free flaps (OCRFFF) did incur a slightly higher percentage of hardware removals (9.9%) compared to fibular flaps (6.1%). However, this was nonsignificant (P = .22). Interestingly, anterior reconstructions did not differ from lateral reconstructions with regards to need for a hardware removal. In the majority of cases (82%), a 2.0 mm profile plate was used for fixation and was not a significant factor, nor was the use of 2 or more plates. The number of osteotomies was also not significantly different between groups. The majority of patients did not experience any postoperative flap-related complications (78%). In the remaining 22% of patients with a complication, the most common postoperative complications were wound infection or fistula formation (50%) and free flap vascular insufficiency (31%), with some patients experiencing more than 1 complication. Radiation status was similar between groups, with a fairly even split between history of prior radiation and postoperative adjuvant radiation.
Of those who had hardware removal, the average time to removal was 16.2 months (SD = 15.5, range, 2.6–67), with a median of 10 months (Figure 2). Reasons for removal were characterized as malunion, exposure, infection, or pain. Fifteen patients had more than 1 reason for removal. Exposure and infection were the most common reasons, seen in 20 cases and 17 cases, respectively. Pain was documented in 7 cases and malunion in 6. Of the 20 that exposed, the average time to exposure was 16.4 months (SD = 18.2, median = 9, range, 0.8–66.6 months). At the time of removal, bony union was found to be present in 69%. With respect to fibula free flap compared to osteocutaneous radial forearm free flap, there was found to be no significant difference in malunions (P = .15). However, more OCRFFFs (n = 9) did have malunion at the time of removal compared to fibulas (n = 3). In most cases (59%), the whole plate was removed while 23% underwent partial removal and 18% underwent removal of the hardware as well as the flap. Of the 8 patients undergoing partial removal, 3 patients required a second operation for further hardware removal. In 65% of cases, the reason for removal was resolved upon removal, while 32% experienced continued symptoms. This information could not be determined in 1 patient as follow-up was not available. Average follow-up for this group was 25.6 months (SD = 19.5, median = 17.5, range, 3.6–83).
Figure 2.
Over 50% of hardware removal cases occurred within the first year after reconstruction. Another 21% occurred within the second year, and a slow decline is seen in the number of removal cases thereafter.
Discussion
Understanding the fate of implanted hardware and the conditions associated with its removal are critical to appropriate counseling and postoperative planning. Here we present data on our experience with hardware-related complications and hardware removal. We identified perioperative tobacco use as a risk factor for hardware removal in patients undergoing vascularized osseous free flap. Our overall hardware exposure rate was 19%, and the rate of removal was 16%. These data are consistent with other reports, and we found the median time to removal was 10 months and the average time to be 16.2 months. The whole plate was removed in most cases; however, in those who underwent partial removal, 38% required a second hardware removal procedure. We also found a tendency to more hardware removals in radial forearm bone flaps compared to fibulas. Considering the not infrequent percentage of patients developing exposure and/or requiring plate removal, we contend that this information should be stated in the informed consent process.
Our 16% removal rate is similar to other studies in which plate complication and removal rates hover around 15%.1,3,7,8 However, differences across studies in hardware and free flaps used make a true comparison difficult. The majority of patients in this study were reconstructed using a 2.0 mm plate and all patients were reconstructed with OCRFFF or fibular free flap. The 2.0 mm locking reconstruction plate system has previously been shown to have a lower complication rate, and the use of both OCRFFF and fibulas has been validated for single-stage mandibular reconstruction.2,4,5 It is therefore striking that a low rate of hardware complications persists despite advancements in hardware and reconstructive techniques. Perhaps then, it is the complicated nature of the patient population selected for mandibular reconstruction with an osteocutaneous free flap that affects hardware removal. The 2 most common indications for free flap reconstruction seen in this study were oral cavity carcinoma (new diagnosis or recurrent) and osteoradionecrosis. In the former, tumor recurrence is a constant threat in the postoperative setting, and this has been associated with an increase in plate-related complications.9 In the latter, an association with higher flap failure rates and malunion has been demonstrated.10 This demonstrates some of the problems encountered in this patient population that may contribute to hardware complications and removals.
In the current series, slightly more hardware removals occurred in the OCRFFFs than the fibula free flaps. This was a nonsignificant finding, but we believe that the quality of the bone stock and the relative thinness of the skin paddle provided by the OCRFFF compared to the fibula promote hardware exposure11,12 and therefore necessitate eventual removal. Interestingly, anterior reconstructions were not associated with an increased removal rate in our series. Compared to lateral mandibular defects, there is significantly more gravitational tension on the soft-tissue envelope in anterior reconstructions. Furthermore, lateral defects of less than 5 cm can often be managed using a bridging plate alone.13 Therefore, the lack of increased hardware removals in anterior mandibular reconstructions may be because fibular free flap is the flap of choice for this area, which was associated with a lower removal rate. In light of data showing high rates of anterior plate complications in those reconstructed with a plate and soft tissue flap,14 our data support the continued use of osteocutaneous free flaps unless patient comorbidities or disease status indicate otherwise. Previous radiation therapy also did not prove to be associated with hardware removal. This is consistent with other reports that radiation therapy is not associated with an increased risk for plate related complications.9,15 Postoperative tobacco use, though, was higher in the hardware removal group, and this was statistically significant (P = .03). This finding is not entirely surprising as the negative effects of nicotine on wound healing have been well documented.16 Thus, continued tobacco use can and likely does contribute to hardware complications and removals. Time to removal was similar to a previously published study by Knott et al1 in which they found a median of 11.5 months.
Limitations to this study are those primarily associated with a retrospective study, and the low number of removals limited our ability to reveal any statistically significant relationships compared to the much larger no removal group. Lastly, we did not limit our patient population to those with a predetermined follow-up period. All patients were included in the analysis because the potential for a hardware-related complication and removal is high in the first 6 months, as evidenced by our 29.4% removal rate at 6 months. However, this does restrain our ability to accurately assess any further complications incurred in the patients with less than 6 months follow-up.
Conclusion
Hardware removal was associated with continued tobacco use after mandibular reconstruction. Removal of the supporting hardware most commonly occurs from infection or exposure in the first year. In the majority of cases the bone is well healed and the problem resolves with removal.
Acknowledgments
Sponsorships: None.
Funding source: Work was supported by grants from NIH (T32CA091078).
Footnotes
This article was presented at the 2013 AAO-HNSF Annual Meeting & OTO EXPO; September 29–October 3, 2013; Vancouver, BC, Canada.
Author Contributions
Kristine E. Day, conception and design, collection and interpretation of data, drafting and revising of manuscript, final approval of manuscript; Renee Desmond, conception and design, analysis and interpretation of data, drafting and revising of manuscript, final approval of manuscript; J. Scott Magnuson, conception and design, analysis and interpretation of data, revising of manuscript, final approval of manuscript; William R. Carroll, conception and design, analysis and interpretation of data, revising of manuscript, final approval of manuscript; Eben L. Rosenthal, conception and design, analysis and interpretation of data, revising of manuscript, final approval of manuscript.
Disclosures
Competing interests: None.
References
- 1.Knott PD, Suh JD, Nabili V, et al. Evaluation of hardware-related complications in vascularized bone grafts with locking mandibular reconstruction plate fixation. Arch Otolaryngol Head Neck Surg. 2007;133:1302–1306. doi: 10.1001/archotol.133.12.1302. [DOI] [PubMed] [Google Scholar]
- 2.Militsakh ON, Wallace DI, Kriet JD, Girod DA, Olvera MS, Tsue TT. Use of the 2.0-mm locking reconstruction plate in primary oromandibular reconstruction after composite resection. Otolaryngol Head Neck Surg. 2004;131:660–665. doi: 10.1016/j.otohns.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Farwell DG, Kezirian EJ, Heydt JL, Yueh B, Futran ND. Efficacy of small reconstruction plates in vascularized bone graft mandibular reconstruction. Head Neck. 2006;28:573–579. doi: 10.1002/hed.20455. [DOI] [PubMed] [Google Scholar]
- 4.Arganbright JM, Tsue TT, Girod DA, et al. Outcomes of the osteocutaneous radial forearm free flap for mandibular reconstruction. JAMA Otolaryngol Head Neck Surg. 2013;139:168–172. doi: 10.1001/jamaoto.2013.1615. [DOI] [PubMed] [Google Scholar]
- 5.Militsakh ON, Werle A, Mohyuddin N, et al. Comparison of radial forearm with fibula and scapula osteocutaneous free flaps for oromandibular reconstruction. Arch Otolaryngol Head Neck Surg. 2005;131:571–575. doi: 10.1001/archotol.131.7.571. [DOI] [PubMed] [Google Scholar]
- 6.Reid BC, Alberg AJ, Klassen AC, Koch WM, Samet JM. The American Society of Anesthesiologists’ class as a comorbidity index in a cohort of head and neck cancer surgical patients. Head Neck. 2001;23:985–994. doi: 10.1002/hed.1143. [DOI] [PubMed] [Google Scholar]
- 7.Hidalgo DA, Pusic AL. Free-flap mandibular reconstruction: a 10-year follow-up study. Plast Reconstr Surg. 2002;110:438–449. doi: 10.1097/00006534-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Arcas JM, Arias J, Del Castillo JL, et al. The fibula osteomyocutaneous flap for mandible reconstruction: a 15-year experience. J Oral Maxillofac Surg. 2010;68:2377–2384. doi: 10.1016/j.joms.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson RE, Schuller DE, Forrest LA, Mountain RE, Ali T, Young D. Factors involved in long- and short-term mandibular plate exposure. Arch Otolaryngol Head Neck Surg. 1997;123:217–222. doi: 10.1001/archotol.1997.01900020107016. [DOI] [PubMed] [Google Scholar]
- 10.Sweeny L, Lancaster WP, Dean NR, et al. Use of recombinant bone morphogenetic protein 2 in free flap reconstruction for osteonecrosis of the mandible. J Oral Maxillofac Surg. 2012;70:1991–1996. doi: 10.1016/j.joms.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean NR, Wax MK, Virgin FW, Magnuson JS, Carroll WR, Rosenthal EL. Free flap reconstruction of lateral mandibular defects: indications and outcomes. Otolaryngol Head Neck Surg. 2012;146:547–552. doi: 10.1177/0194599811430897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virgin FW, Iseli TA, Iseli CE, et al. Functional outcomes of fibula and osteocutaneous forearm free flap reconstruction for segmental mandibular defects. Laryngoscope. 2010;120(suppl 4):S190. doi: 10.1002/lary.21654. [DOI] [PubMed] [Google Scholar]
- 13.Arden RL, Rachel JD, Marks SC, Dang K. Volume-length impact of lateral jaw resections on complication rates. Arch Otolaryngol Head Neck Surg. 1999;125:68–72. doi: 10.1001/archotol.125.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Deleyiannis FW, Rogers C, Ferris RL, Lai SY, Kim S, Johnson J. Reconstruction of the through-and-through anterior mandibulectomy defect: indications and limitations of the double-skin paddle fibular free flap. Laryngoscope. 2008;118:1329–1334. doi: 10.1097/MLG.0b013e3181734f60. [DOI] [PubMed] [Google Scholar]
- 15.Futran ND, Urken ML, Buchbinder D, Moscoso JF, Biller HF. Rigid fixation of vascularized bone grafts in mandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1995;121:70–76. doi: 10.1001/archotol.1995.01890010056010. [DOI] [PubMed] [Google Scholar]
- 16.Rinker B. The evils of nicotine: an evidence-based guide to smoking and plastic surgery. Ann Plast Surg. 2013;70:599–605. doi: 10.1097/SAP.0b013e3182764fcd. [DOI] [PubMed] [Google Scholar]