Abstract
By examining the literature concerning early intervention with antipsychotic medications, and how it affects long-term morbidity, this article will review the concept that early intervention with antipsychotic medications improves the long-term course of schizophrenia. It also looks at the potential long-term effects of discontinuing antipsychotic medications early in the course of schizophrenia. It appears that early intervention with antipsychotic medications decreases some of the long-term morbidity associated with schizophrenia. Some of the implications of this finding are discussed in the context of both clinical practice and clinical research.
“For simple weak-mindedness, which signifies a kind of recovery with defect, it is distinctly enlightening and often confirmed by experience that, sooner or later, a fresh exacerbation of the disease may follow and bring about a higher degree and another form of dementia.”
(Kraepelin, 1971, p. 205)
1. Introduction
The contrast between the essence of the Kraeplinian and Bleulerian views of schizophrenia is a difference that still drives research paradigms after nearly a century (McCarley et al., 1993). While at times seemingly ambivalent on the subject, Kraepelin saw the illness he termed dementia praecox as often having a deteriorating course; according to his view, cognitive impairments develop within a few years of positive symptoms. Bleuler’s concept of schizophrenia was more optimistic, assuming little, if any, progressive deterioration associated with the illness; this view is probably the one shared by most clinicians today. Nevertheless, DSM-III (American Psychiatric Association, 1987) and DSM-IV (American Psychiatric Association, 1994) criteria require a loss from a previous or expected level of functioning or achievement for a diagnosis of schizophrenia to be made; this view seems more in accordance with that of Kraepelin than Blueler.
Much recent research indicates that many of the deficit symptoms found in patients with schizophrenia occur during the pre-psychotic period, or within a few years of the first appearance of psychosis (Carone et al., 1991; Carpenter and Strauss, 1991; McGlashan, 1988). Excluding patients whose onset of illness is very abrupt, and where deficits could thus only occur after the onset of psychosis, it is unclear how to apportion deficits between the pre-psychotic and the first few years following the first psychotic episode. Grasping this aspect of the course of schizophrenia not only has implications for understanding the patho-etiology of the illness, but may also be important in attempting to decrease some of the long-term morbidity associated with schizophrenia. Furthermore, if any of the deficits associated with schizophrenia can be prevented, it would mean that some of the costs associated with the illness could also be averted.
Here, we propose a model that takes into account the interaction between the primary cause or causes of schizophrenia—their relation to changes in the brain— and secondary effects produced by a reaction to those primary events. An analogy might come from our knowledge of inflammation. Inflammation is a body’s localized, protective response to injury. It is often this reaction rather than the primary injury that causes loss of function, at times leaving physical scars. Inflammation can occur because of a physical, chemical, or biologic agent, and includes (i) the local reactions and resulting morphologic changes, (ii) the destruction or removal of the injurious material, and (iii) the response that leads to repair and healing. The signs of inflammation include redness, heat, swelling, pain, and inhibited or lost function, though no one of them is necessarily always present. Chronic inflammation can begin with rapid onset or slow, insidious onset and has vague or indefinite termination. It results when the injuring agent (or products resulting from its presence) persists in a lesion. We propose that psychosis is the mind’s inflammation—the psychological redness, swelling, heat, and pain resulting from an underlying irritant. This model thus accounts for a residual “scar” as the repair and healing (the inflammation) that takes place after psychosis (the injury) diminishes. Individuals who “scar” following psychosis would also be expected to have some deterioration or loss of function.
When one considers deterioration of the central nervous system (CNS), it is often in the context of degeneration, with a progression usually leading to death. Thus, while patients do not usually die directly from diseases such as Alzheimer’s or Huntington’s diseases, death from secondary causes such as infection is common. In addition to this morbid outcome, these diseases are clearly associated with pathological progression. Schizophrenia is different. There is little or no evidence of the extensive pathological progression so characteristic of Alzheimer’s and Huntington’s diseases; premature death from schizophrenia is most often related to violence (usually suicide) (Black and Fisher, 1992; Mortensen and Juel, 1993); and the neuropsychological changes that do accompany the disorder are, for the most part, thought to occur relatively early in the course of the illness and remain static thereafter.
One important issue that arises from considering differences between the classical degenerative CNS diseases and schizophrenia involves treatments for the latter. More specifically, if treatments for schizophrenia were unavailable, might some forms of the illness have similarities to these classical CNS degenerative diseases? Would the illness progress more rapidly, or would deterioration be more profound than what is found today? Some of the epidemiological and mirror-image studies discussed below indicate that schizophrenia has become a more benign illness than it was 60 years ago. Given that the neuropathological changes found in schizophrenia do not resemble those of Alzheimer’s or Huntington’s diseases, it might seem reasonable to conclude that there is no resemblance, and that our treatments only serve to suppress immediate symptoms. Yet there are many ways the brain could change without evidence of the massive loss of neurons and glial reaction typical of Alzheimer’s and Huntington’s diseases, including alterations to synaptic pruning or synaptic formation (Feinberg, 1982); a loss of synapses (Stevens, 1992; Wyatt, 1988); changes in function including sensitization (Lieberman, 1996); and loss of a limited number of cells through apoptosis, or programmed cell death (Coyle, 1996; Masserano et al., in press)
Here, we explore the evidence that treatments for schizophrenia, which consist primarily of antipsychotic medications, change the long-term morbidity of the illness. We also address the implications of these data for both clinicians and researchers.
2. Evidence supporting early intervention
Four types of studies provide evidence that early intervention may decrease the long-term morbidity of schizophrenia: epidemiological, mirror-image, delayed intervention, and contemporaneous control. Each is associated with difficulties in interpretation, some of which are discussed below. Results from a fifth type of study, discontinuation studies are ambiguous. Taken as a group, however, the studies appear to indicate that early intervention decreases the long-term morbidity of schizophrenia.
2.1. Epidemiological evidence (historical perspective of the incidence and severity of illness)
Several investigators (de Alarcon et al., 1990; Der et al., 1990; Eagles et al., 1988; Eagles and Whalley, 1985; Joyce, 1987; Munk-Jorgensen, 1987; Munk-Jorgensen and Jorgensen, 1986; Parker et al., 1985; Waddington and Youssef, 1994) have pointed to a substantial body of evidence that the incidence of schizophrenia has decreased over the last 50 to 60 years, while others (Bamrah et al., 1991; Castle et al., 1991; Häfner and Gattaz, 1991; Harrison et al., 1991; Kendell et al., 1993) have emphasized the many potential difficulties in making such estimates. One such difficulty is that there have been many changes in the way schizophrenia has been diagnosed over the last 50 to 60 years (Hegarty et al., 1994).
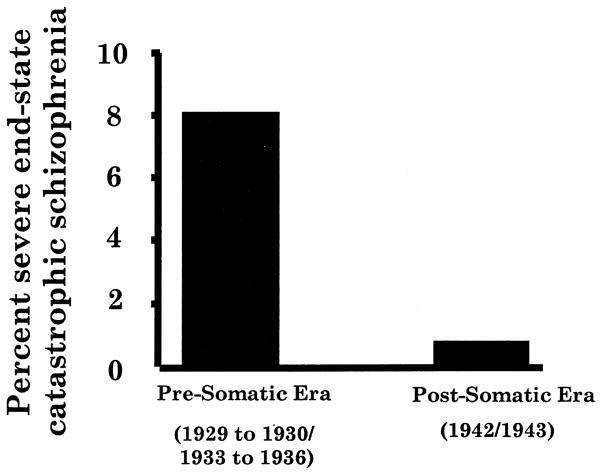
There appears to be less disagreement about the observation that the severity of schizophrenia has decreased during this time (Achté, 1961; Astrup and Noreik, 1966; Ey et al., 1957; Hare, 1983). The decrease in what was then called catastrophic schizophrenia (schizophrenia with a sudden onset and severe deterioration) appeared to take place between 1930 and the early 1940s (Figure 1); Manfred Blueler attributed this decrease to an improvement in psychosocial care (Bleuler, 1978) [1972]. It is less likely that changes in severity could be attributed to changes in diagnosis. For example, although patients with severe nutritional deficiencies and viral encephalitis might at times have been confused with schizophrenic patients, there is no reason to suspect that good clinicians such as Blueler, Ødegård, Astrup, Ey and Achté would often have been confused. There is also no reason to believe that these clinicians would have mistaken schizophrenia for an affective disorder, a misidentification that was common in North America from World War II until the early 1980s (American Psychiatric Association, 1987; Gurland et al., 1969). Furthermore, confusing schizophrenia with the affective disorders would probably have made the illness seem less, rather than more, severe. The decrease in severity could also be attributed to the use of convulsive therapies, which were introduced in the early 1940s. In fact, in a well-controlled study carried out in the late 1950s and early 1960s, May and colleagues demonstrated that ECT can decrease the long-term morbidity of first-episode schizophrenia (May et al., 1976b).
Fig. 1.
Decrease in incidence of catastrophic schizophrenia between the late 1920s to mid-1930s and early 1940s. (Data from Bleuler, 1978 [1972]).
2.2. Mirror-image studies
Mirror-image studies compare the outcomes of two similar patient populations—one from the pre-antipsychotic medication era, and one from the antipsychotic medication era. Nine mirror-image studies have been identified (Wyatt, 1995a, 1991), and all but two indicate that there was improvement in the long-term course of schizophrenia after the introduction of antipsychotic medications (Table 1). One study by Astrup and Noreik (1966), shown in Table 2, found that first-episode patients admitted to Oslo’s Gaustad Hospital were more likely to do worse if they were admitted in the pre-antipsychotic medication era (1938 to 1950) than if they were admitted during the antipsychotic medication era (1951 to 1957). Only patients receiving antipsychotic medications were included in the 1951 to 1957 group.
Table 1.
Results of the author’s analysis and/or reanalyzed (Wyatt, 1991) mirror-image studies comparing the long-term outcome of first-episode schizophrenic patients before and after the introduction of antipsychotic medications
| Study | Improvement between pre-, and post-antipsychotic periods |
|---|---|
| McWalter et al. (1961) | p=0.003 |
| Ødegård (1964) | p<0.06 |
| Peterson & Olson (1964) | p<0.001 |
| Astrup & Noreik (1966) | p=0.003 |
| Pritchard (1967a, b) | Non-significant |
| Shimazono (1973) | p=0.03 |
| Murakami (1971) | p=0.22 |
| Huber et al. (1979, 1980) | p<0.001 |
| Watt et al. (1983) | Non-significant |
Table 2.
Comparison of severe deterioration at least five years after first admission of schizophrenic patients to Norway’s Gaustad hospital from 1938 to 1950 (pre-antipsychotic medication era) and from 1951 to 1957 (antipsychotic medication era). Only patients receiving antipsychotic medications were included in the 1951 to 1957 group
| Treatment | n | % (n) patients with severe deterioration |
|---|---|---|
| Preneuroleptic | 1102 | 16 (176) |
| Neuroleptic | 237 | 9 (21) |
| Two-sample proportional test | p=0.003 |
Data adapted from Astrup & Noreik (1966) and Wyatt (1991).
The validity of these studies can be called into question, however, because patient matching was probably inexact, and because, even when only a few years separated the two patient groups, other variables in addition to the use of antipsychotic medications during the first hospital admission may have influenced patient outcome. For instance, one issue that has been raised is the effect of treatment with maintenance antipsychotic medication, now well known to reduce relapse rate. Subjects in the mirror-image studies who received antipsychotic medications were hospitalized before the importance of maintenance antipsychotic medications was known; therefore, it is unlikely that use of maintenance antipsychotic medications was a major factor in the improved long-term outcome of patients in these studies. Other factors, however, are more difficult to dismiss. For instance, when antipsychotic medications were first introduced in the mid-1950s, steps were already underway to improve living conditions in the large, impersonal, psychiatric hospitals of the period (Issac and Armat, 1990). These changes, in turn, made the experience less dehumanizing and desocializing, which undoubtedly made it easier for some patients to return to their previous living arrangements.
2.3. Delayed intervention studies
Delayed intervention studies compare the outcome of schizophrenic patients who were treated in the early phases of their illness with those for whom treatment was delayed. There are seven fairly well-controlled delayed intervention studies (Loebel et al., 1992; McEvoy et al., 1991; Szymanski et al., 1995; Waddington et al., 1995; Wyatt, 1991), and all but one shows that delaying intervention with antipsychotic medications leads to a poorer long-term outcome (Table 3). The Astrup and Noreik (1966) study, mentioned above, looked at patients who had their first episode of schizophrenia between 1938 and 1950 (Astrup et al., 1962) and between 1950 and 1958 (Astrup and Noreik, 1966). After a 5-year follow-up period, patients treated within 6 months of the onset of psychosis were found to be doing much better than patients for whom treatment began 6 months to 1 year after onset. Those patients treated between 6 months and 1 year after onset of psychosis also did much better than those patients for whom treatment was delayed by 2 or more years. Patients treated in the first (1938 to 1950) cohort, and some treated in the second (1950 to 1958) cohort, received some form of convulsive therapy; some patients treated in second cohort also received antipsychotic medications.
Table 3.
Results of the author’s analysis or reanalyzed (Wyatt, 1991) studies of early and delayed intervention with antipsychotic medications in first-episode schizophrenic patients
| Study | Improvement of outcome between early versus delayed intervention |
|---|---|
| Astrup & Noreik (1966) | p<0.0001 |
| Aritome (1978) | Inconclusive |
| Crow et al. (1986) | p=0.03 to 0.003 |
| Anzai et al. (1988) | p=0.03 |
| Loebel et al. (1992) | p=0.01 to 0.0001 |
| Waddington et al. (1995)* | p=0.02 |
| Szymanski et al. (1996) | p=0.0001 |
Patients were chronically ill but the difference appears to be related to prolonged delay in treatment.
Caution is needed to interpret these findings because numerous studies have demonstrated that patients with an acute onset have a better prognosis than patients whose onset of illness is more gradual (Stephens, 1970). Therefore, it is possible that individuals treated early in the course of their illness have a different form of schizophrenia than those for whom treatment is delayed. Because of the florid nature of acute symptoms, it is likely that patients with an acute onset would receive treatment more quickly than patients whose onset is gradual. One study by Loebel and colleagues (1992) attempted to control for the type of onset—gradual versus sudden—and found that those patients who had been ill longer, as measured by either the time elapsed between their first prodromal or first psychotic symptoms and their first treatment, had a worse outcome. The time elapsed between the onset of prodromal and the onset of psychotic symptoms (gradual versus sudden onset) was not related to outcome. Other prospective studies are now needed to confirm these findings.
2.4. Contemporaneous control studies
Contemporaneous control studies compare the outcome of two patient groups: those assigned to treatment with antipsychotic medications, and those assigned to treatment with a non-somatic therapy (Table 4). While these studies should provide the most solid information about the role of early intervention on the long-term morbidity of schizophrenia, they are associated with their own set of confounding issues. Most of these studies were undertaken because the experimenters felt that their type of psychosocial treatment had an advantage over standard treatment with antipsychotic medications. Often, considerable attention was paid to the patient receiving the psychosocial treatment, while the patients receiving antipsychotic medications were given “usual care”, which was at times wholly inadequate. Follow-up treatment was usually not equivalent for the two groups; those patients assigned to the psychosocial treatment group received much greater continuity of care, and perhaps caring. Patients treated with antipsychotic medications were often discharged sooner and to a less adequate treatment facility than the patients who received the psychosocial treatment. It is also possible that some of the psychosocial treatments themselves might have proffered benefits similar to those of antipsychotic medications; while there are no consistent data suggesting that the psychosocial treatments, when used by themselves, are efficacious, available data suggest that they may provide benefits for some patients.
Table 4.
Results of the author’s analysis or reanalyzed (Wyatt, 1991, 1995a) contemporaneous control group studies examining the effects of early intervention on the long-term outcome of schizophrenia
| Study | Improved outcome between early and normal intervention with antipsychotic medication |
|---|---|
| Wirt & Simon (1959) | p<0.01 |
| Pritchard (1967a, b) | p=0.1 |
| Schooler et al. (1967) | Inconclusive |
| May et al. (1976a, b) | p<0.05 |
| Wyatt et al. (1997) | (p<0.01) |
| Carpenter et al. (1977) | Inconclusive |
| Rappaport et al. (1978) | Inconclusive |
| Matthews et al. (1979) | Inconclusive |
| Karon & Vandenbos (1981) | Inconclusive |
From the point of view of the question addressed here, however, the most important and rigorous of the contemporaneous control studies was carried out by May and colleagues (May et al., 1976a, b, 1981). That study found that antipsychotic medications given to first-episode schizophrenic patients improved the long-term outcome of such patients when compared with those patients initially treated by milieu—or psychotherapy alone. We (Wyatt et al., 1997) re-examined the results of the study by May and colleagues, predicting that first-episode patients who were well enough to be discharged from the hospital within 6 months without receiving antipsychotic medication or ECT might be considered spontaneous responders and have a good long-term prognosis. The original study took place in the late 1950s and early 1960s, a period when lengthy hospitalizations were common, yet 25 of 89 patients receiving either milieu or psychotherapy left the hospital within 6 months, and 71 of 92 patients receiving antipsychotic medications, with or without psychotherapy, were also discharged within that time period. In the second year following discharge, adjusting for an uneven use of antipsychotic medications by the two groups in the first post-discharge year, the patients initially not treated with antipsychotic medications required more days of re-hospitalization. Thus, as a group, the patients who were not treated with antipsychotic medications during their first hospital admission did not fare as well as those patients who did receive them.
3. Evidence from discontinuation studies
Discontinuation studies examine the effects of stopping antipsychotic medications on the long-term morbidity of schizophrenia. There are four controlled studies examining the effects of medication discontinuation on the long-term course of schizophrenia. This is probably too few to draw even a tentative conclusion, especially since two of the studies looked at chronically ill, rather than first-episode, patients (Curson et al., 1985; Johnson et al., 1983).
Curson et al. (1985) traced a group of patients with chronic schizophrenia who had been in a study 7 years earlier. Results of that previous study showed that use of long-acting depot antipsychotic medications was associated with fewer relapses than placebo. In the follow-up study, the authors did not find any differences between the two groups after 7 years; however, patients who had had more relapses had worse social adjustment. It thus appears that, while one relapse from placebo substitution in already chronically ill patients did not affect patient outcome 7 years later, subsequent relapses did. What cannot be determined from this study is whether the patients relapsed because they were “breaking through” their medications or because they had discontinued them. Curson and colleagues finally concluded that social performance improves slowly after a relapse and that the improvement may continue long after the resolution of florid symptoms (Curson et al., 1985).
Another study, by Johnson et al. (1983), also found slow recovery of social performance following relapse due to withdrawal of antipsychotic medications. Stabilized chronic schizophrenic patients whose antipsychotic medications were discontinued had an 80% relapse rate compared with 23% of patients maintained on antipsychotic medications (Johnson et al., 1983). Significantly, even after a minimum of 6 months following the reinstitution of antipsychotic medications, the patients who had relapsed had not recovered the social adjustment they would have achieved had they not been taken off antipsychotic medications (Wyatt, 1991). Taken together, the studies by Curson and colleagues and Johnson and colleagues suggest that, while a brief placebo period and a relapse that occurs because of it may not have a large long-term effect on a group of already chronically ill schizophrenic patients, the apparently slow recovery period is an issue that must be considered. If slowed recovery is related to the number or extent of relapses for some patients, it is plausible that delayed recovery might, in time, appear to be less recovery. Might slow responsiveness eventually lead to treatment non-responsiveness or intractability? Another related study, by Szymanski and colleagues (1995), published preliminary results of an important prospective study of first-episode schizophrenic patients (Szymanski et al., 1995). With each relapse following medication discontinuation, the patients took longer to respond when antipsychotic medications were reinstituted. It is important to note, however, that increased time to clinical response was only present in some patients. These observations may be similar to the step-like changes found following each relapse. which have been described in a number of longitudinal studies (Bleuler, 1978, [1972]; Ciompi, 1980; Kraepelin, 1913; Snezhnevsky, 1966; Watt et al., 1983).
The results of another study, by Johnstone et al. (1990), provides evidence that discontinuing antipsychotic medications in first-episode patients can be valuable. First-episode patients were, after a period of stabilization on antipsychotic medications, assigned to continue treatment with either antipsychotic medications or placebo (Crow et al., 1986). In both groups, patients who had been ill longer than 1 year at the time of their first hospital admission were more likely to relapse than patients who had been ill for less than a year; patients who received placebo substitution were more likely to relapse than patients who continued to receive antipsychotic medications. Yet when the patients were followed-up after 2 years, it was found that those patients who had been ill for less than 1 year at the onset of treatment, but who had received placebo instead of active medication, had a higher level of occupational functioning than patients who remained on antipsychotic medications. One explanation for this counterintuitive finding is that, in patients who were treated early, treatment or some other aspect of their illness lessened the need for antipsychotic medications in subsequent years and, in fact, the continued use of antipsychotic medications produced cognitive blunting that decreased their normal level of functioning (Carpenter et al., 1988). The results of the study by Johnstone and colleagues emphasize the point, however, that it is extremely important to determine which patients do and do not need to be maintained on antipsychotic medications.
4. Considerations for clinical practice
Today, clinicians might delay the treatment of first-episode patients with schizophrenia for a few days (usually for diagnostic purposes), or for longer periods of time if they remain unsure of the diagnosis and do not want to unduly subject the patient to the risks of antipsychotic medications. There are no data even hinting that such minor delays in beginning treatment increase long-term morbidity. Unless such data become available, it would seem prudent to balance the patient’s immediate discomfort and risks against delaying institution of treatment for diagnostic clarity.
Once patients have been placed on antipsychotic medications, however, few will want to stay on them indefinitely. Most patients find the side effects of antipsychotic medications unpleasant; furthermore, the longer a patient remains on them, the greater the risk of tardive dyskensia. Even if these considerations were not a factor, many studies from other branches of medicine have noted that, once acute symptoms have subsided, patients begin to forget to take their medications. One might therefore expect that patients whose cognitive and motivational abilities may be impaired by their illness, such as individuals with schizophrenia, would be less likely than most patients to continue taking medications unless they are helped to do so or unless they perceive a risk in not doing so.
Studies have found that a percentage, perhaps as high as 20–30% of first-episode schizophrenic patients, do not need antipsychotic medications after they have recovered from their initial episode (Eaton et al., 1992; Johnstone et al., 1990; Ram et al., 1992). Thus, it is integral that the clinician have a rational approach to offer after a first-episode patient has stabilized at his or her best level of function. There is no experimentally set standard for how long to wait, but there is some consensus that, after a patient has been stable for a year or two, there should be a gradual reduction in the dose of antipsychotic medication (Kissling, 1991). The clinician must also learn, from experience, how fast to taper the medication. Our personal experience suggests that the dose is best reduced very slowly, perhaps over a period of a year or more (Wyatt, 1995b, in press). During this time, the patient, their family and their physician need to watch for prodromal symptoms and, if these occur, the dose of antipsychotic medication should be immediately augmented, usually by doubling it.
5. Considerations for clinical research
Clinical researchers, in contrast to practicing clinicians, will fairly commonly discontinue antipsychotic medications to answer a number of research questions. One could ask whether this is ever justified, and there are a number of reasons why it might be. First, there is at least as much evidence that taking patients off antipsychotic medications produces no long-term morbidity as there is evidence suggesting that it may be associated with long-term morbidity. If this were the extent of the dilemma, it might be prudent to follow the dictum, “first, do no harm” and never discontinue antipsychotic medications for research purposes; however, several factors complicate this issue.
Foremost among these complicating factors is the problem of first-episode patients. Since we do not know which patients will need to continue taking antipsychotic medications for many years, it is important to determine which patients will not need them. Clinical practice dictates that patients be given the opportunity to determine whether they will need to take antipsychotic medications indefinitely. Such determinations might well be done in research settings where the patient’s environment is usually better-controlled than in a clinical setting, and where there is the opportunity to learn (generalize) from the experience. Such control is important because, as medication is withdrawn, predetermined thresholds can be drawn for where “rescue” medication will be used if it is needed. Thus, instead of increasing the risk to patients, the research setting may actually decrease the risk.
If one accepts the observation that schizophrenic patients treated early in the course of their illness with antipsychotic medications (and perhaps other treatments) have better outcomes than those for whom treatment is delayed, there are several potential implications for research involving the discontinuation of antipsychotic medications.
First, on the positive side, there might be a subgroup of patients for whom further treatment with antipsychotic medications has become unnecessary. The course of the illness (perhaps together with environmental changes) could be greatly altered or the window of “vulnerability” might close so that the individual’s risk of further illness becomes markedly decreased. Studies of first-episode patients, even prior to the introduction of antipsychotic medications, have repeatedly demonstrated that there is a small subgroup of patients who do not need further treatment (Eaton et al., 1992; Ram et al., 1992). Does decreasing the amount of initial psychosis increase the likelihood that the individual will be in this subgroup, or even enlarge the subgroup?
Second, and on the negative side, there maybe a sub-group of patients for whom some of the long-term morbidity associated with schizophrenia was decreased by early intervention, but who nevertheless remain susceptible to further relapses. Such patients would be at greater risk for relapse when taken off antipsychotic medications than if they remained on their medications. In this hypothetical situation, the early treatment that prevented some of the long-term morbidity would leave patients vulnerable to further long-term morbidity associated with subsequent relapses. It would not, however, increase that vulnerability beyond what they would have experienced had they not been treated early.
Unfortunately, while there are a great number of studies in which antipsychotic medications have been discontinued, few have attempted to document the consequences of medication discontinuation other than to repeatedly note that most patients for whom anti-psychotic medications are discontinued will eventually relapse.
6. Conclusions
Substantial research efforts have been directed towards the possible relationship between early intervention and long-term outcome in schizophrenia for only a few years, although the idea has been present for at least half a century (Cameron, 1938). Evidence is beginning to accumulate that early treatment and, for some patients, perhaps sustained treatment, is of long-term value. Assuming that this proves correct, it will be important to design studies to determine how early and for which patients such treatment makes a difference. The problem for the future may be learning how to neither over- nor under-treat patients—a problem that must be addressed by well-conceived research efforts.
References
- Achté KA. Der Verlauf der Schizophrenien und der schizophreniformen Psychosen. Acta Psychiatrica Scandinavica. 1961;(Suppl 55) [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, D.C: The Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders-IV. Washington, D.C: The American Psychiatric Association; 1994. [Google Scholar]
- Anzai N, Okazaki Y, Miyauchi M, et al. Early neuroleptic medication within one year after onset can reduce risk of later relapses in schizophrenic patients? Annual Report, Pharmacopsychiatric Research Foundation. 1988;19:258–265. [Google Scholar]
- Aritome T. A study on the long-term prognosis of schizophrenia under psychotropic drug medication. Jikeikai Medical Journal. 1978;25:269–286. [Google Scholar]
- Astrup C, Fossum A, Holmboe R. Prognosis in functional psychoses. Springfield, IL: Charles C. Thomas; 1962. [Google Scholar]
- Astrup C, Noreik K. Functional psychoses: Diagnostic and prognostic models. Springfield, IL: Charles C. Thomas; 1966. [Google Scholar]
- Bamrah J, Freeman H, Goldberg D. Epidemiology of schizophrenia in Salford, 1974–84. British Journal of Psychiatry. 1991;159:802–810. doi: 10.1192/bjp.159.6.802. [DOI] [PubMed] [Google Scholar]
- Black DW, Fisher R. Mortality in DSM-IIIR schizophrenia. Schizophrenia Research. 1992;7:109–116. doi: 10.1016/0920-9964(92)90040-c. [DOI] [PubMed] [Google Scholar]
- Bleuler M. In: The schizophrenic disorders: Long-term patient and family studies. Clemens SM, editor. New Haven: Yale University Press; 1978[1972]. [Google Scholar]
- Cameron DE. Early schizophrenia. American Journal of Psychiatry. 1938;95:567–578. [Google Scholar]
- Carone BJ, Harrow M, Westermeyer JF. Posthospital course and outcome in schizophrenia. Archives of General Psychiatry. 1991;48:247–258. doi: 10.1001/archpsyc.1991.01810270059008. [DOI] [PubMed] [Google Scholar]
- Carpenter WJ, Heinrichs D, Wagman A. Deficit and nondeficit forms of schizophrenia: the concept. American Journal of Psychiatry. 1988;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Carpenter WTJ, Strauss JS. The prediction of outcome in schizophrenia. IV: Eleven-year follow-up of the Washington IPSS cohort. Journal of Nervous and Mental Disease. 1991;179:517–525. doi: 10.1097/00005053-199109000-00001. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, McGlashan TH, Strauss JS. The treatment of acute schizophrenia without drugs: An investigation of some current assumptions. American Journal of Psychiatry. 1977;134:14–20. doi: 10.1176/ajp.134.1.14. [DOI] [PubMed] [Google Scholar]
- Castle D, Wessely S, Der G. The incidence of operationally defined schizophrenia in Cambrwell, 1965–84. British Journal of Psychiatry. 1991;159:790–794. doi: 10.1192/bjp.159.6.790. [DOI] [PubMed] [Google Scholar]
- Ciompi L. The natural history of schizophrenia in the long term. British Journal of Psychiatry. 1980;136:420–423. doi: 10.1192/bjp.136.5.413. [DOI] [PubMed] [Google Scholar]
- Coyle J. The glutamate dysfunction hypothesis for schizophrenia. Harvard Review of Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Crow TJ, MacMillan JF, Johnson AL, Johnstone EC. A randomized controlled trial of prophylactic neuroleptic treatment. British Journal of Psychiatry. 1986;148:120–127. doi: 10.1192/bjp.148.2.120. [DOI] [PubMed] [Google Scholar]
- Curson DA, Barnes TRE, Bamber RW, Platt SD, Hirsch SR, Duffy JC. Long-term depot maintenance of chronic schizophrenic out-patients: The seven year follow-up of the medical research council flupenazine/placebo trial. British Journal of Psychiatry. 1985;146:464–480. doi: 10.1192/bjp.146.5.464. [DOI] [PubMed] [Google Scholar]
- de Alarcon J, Seagroatt V, Godacre M. Trends in schizophrenia. Lancet. 1990;335:852–853. [Google Scholar]
- Der G, Gupta S, Murray RM. Is schizophrenia disappearing? Lancet. 1990;335:513–516. doi: 10.1016/0140-6736(90)90745-q. [DOI] [PubMed] [Google Scholar]
- Eagles J, Hunter D, McCance C. Decline in the diagnosis of schizophrenia among first contacts with psychiatric services in North East Scotland, 1969–1984. British Journal of Psychiatry. 1988;152:793–798. doi: 10.1192/bjp.152.6.793. [DOI] [PubMed] [Google Scholar]
- Eagles JM, Whalley LJ. Decline in the diagnosis of schizophrenia among first admissions to Scottish mental hospitals from, 1969–78. British Journal of Psychiatry. 1985;146:151–154. doi: 10.1192/bjp.146.2.151. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Bilker W, Haro JM, Herrman H, Mortensen PB, Freeman H, Burgess P. Long-term course of hospitalization for schizophrenia: Part II. Change with passage of time. Schizophrenia Bulletin. 1992;18:229–241. doi: 10.1093/schbul/18.2.229. [DOI] [PubMed] [Google Scholar]
- Ey H, Igert C, Rappard P. Psychoses aigues et evolutions schizophreniqes dans un service de 1930 a 1956. Annales Medipsychologiques. 1957;115:231–240. [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of Psychiatric Research. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Gurland BJ, Fleiss JL, Cooper JE, Kendell RE, Simon R. Cross-national study of diagnosis of the mental disorders: some comparisons of diagnostic criteria from the first investigation. American Journal of Psychiatry. 1969;125:30–39. doi: 10.1176/ajp.125.10s.30. [DOI] [PubMed] [Google Scholar]
- Häfner H, Gattaz W. Is schizophrenia disappearing. European Archives of Psychiatry and Clinical Neuroscience. 1991;240:374–376. doi: 10.1007/BF02279770. [DOI] [PubMed] [Google Scholar]
- Hare EH. Was insanity on the increase? British Journal of Psychiatry. 1983;142:439–455. doi: 10.1192/bjp.142.5.439. [DOI] [PubMed] [Google Scholar]
- Harrison G, Copper J, Gancarczyk R. Changes in the administrative incidence of schizophrenia. British Journal of Psychiatry. 1991;159:811–816. doi: 10.1192/bjp.159.6.811. [DOI] [PubMed] [Google Scholar]
- Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C, Oepen G. One hundred years of schizophrenia: A meta-analysis of the outcome literature. American Journal of Psychiatry. 1994;151:1409–1416. doi: 10.1176/ajp.151.10.1409. [DOI] [PubMed] [Google Scholar]
- Huber G, Gross G, Schuttler R. Monographien aus dem Gesamptgebiete der Psychiatrie. Berlin: Springer-Verlag; 1979. Schizophrenie, Verlaufsund sozialpsychiatrische Langzeituntersuchungen an den 1945 bis 1959 in Bonn hospitalisierten schizophrenen Kranken. [PubMed] [Google Scholar]
- Huber G, Gross G, Schuttler R. Longitudinal studies of schizophrenic patients. In: Clemens SM, translator. Schizophrenia Bulletin. Vol. 6. 1980. pp. 592–605. [DOI] [PubMed] [Google Scholar]
- Issac R, Armat V. Madness in the streets: How psychiatry and the law abandoned the mentally ill. New York: The Free Press; 1990. [Google Scholar]
- Johnson DAW, Pasterski G, Ludlow JM, Street K, Taylor RDW. The discontinuance of maintenance neuroleptic therapy in chronic schizophrenic patients: Drug and social consequences. Acta Psychiatrica Scandinavica. 1983;67:339–352. doi: 10.1111/j.1600-0447.1983.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Macmillan JF, Frith CD, Benn DK, Crow TJ. Further investigation of the predictors of outcome following first schizophrenic episodes. British Journal of Psychiatry. 1990;157:182–189. doi: 10.1192/bjp.157.2.182. [DOI] [PubMed] [Google Scholar]
- Joyce P. Changing trends in first admissions and readmissions for mania and schizophrenia in New Zealand, 1974 to 1984. Australian and New Zealand Journal of Psychiatry. 1987;21:82–86. doi: 10.3109/00048678709160903. [DOI] [PubMed] [Google Scholar]
- Karon BP, Vandenbos GR. Psychotherapy of Schizophrenia: The Treatment of Choice. Northvale, NJ: Jason Aronson; 1981. [Google Scholar]
- Kendell RE, Malcolm DE, Adams W. The problem of detecting changes in the incidence of schizophrenia. British Journal of Psychiatry. 1993;162:212–218. doi: 10.1192/bjp.162.2.212. [DOI] [PubMed] [Google Scholar]
- Kissling W, editor. Guidelines for Neuroleptic Relapse Prevention in Schizophrenia. New York: Springer-Verlag; 1991. [Google Scholar]
- Kraepelin E. Psychiatrie. Leipzig: Aufl; 1913. [Google Scholar]
- Kraepelin E. In: Dementia praecox and paraphrenia [1919] Barclay RM, translator. Huntington, New York: Robert E. Krieger; 1971. [Google Scholar]
- Lieberman JA. Evidence for sensitization in the early stage of schizophrenia. European Neuropsychopharmacology. 1996;6(3):155. [Google Scholar]
- Loebel AD, Lieberman JA, Alvir JM, Mayerhoff DI, Geisler SH, Szymanski SR. Duration of psychosis and outcome in first-episode schizophrenia. American Journal of Psychiatry. 1992;149:1183–1188. doi: 10.1176/ajp.149.9.1183. [DOI] [PubMed] [Google Scholar]
- Masserano JM, Gong L, Kulaga H, Baker I, Wyatt RJ. Dopamine induced apoptosis in a catecholaminergic cell line is associated with a decrease in bcl-2. Molecular Neuropharmacology in press. [Google Scholar]
- Matthews SM, Roper MT, Mosher LR, et al. A non-neuroleptic treatment for schizophrenia: analysis of the two-year post-discharge risk of relapse. Schizophrenia Bulletin. 1979;5:322–333. doi: 10.1093/schbul/5.2.322. [DOI] [PubMed] [Google Scholar]
- May PRA, Tuma AH, Dixon WJ. Schizophrenia: A follow-up study of results of treatment: I. Design and other problems. Archives of General Psychiatry. 1976;33:474–478. doi: 10.1001/archpsyc.1976.01770040042008. [DOI] [PubMed] [Google Scholar]
- May PRA, Tuma AH, Dixon WJ. Schizophrenia: A follow-up study of results of five forms of treatment. Archives of General Psychiatry. 1981;38:776–784. doi: 10.1001/archpsyc.1981.01780320056006. [DOI] [PubMed] [Google Scholar]
- May PRA, Tuma AH, Yale C, Potepam P, Dixon WJ. Schizophrenia: A follow-up study of results of treatment: II. Hospital stay over two to five years. Archives of General Psychiatry. 1976;33:481–486. doi: 10.1001/archpsyc.1976.01770040047009. [DOI] [PubMed] [Google Scholar]
- McCarley R, Shenton M, O’Donnell B, Nestor P. Uniting Kraepelin and Bleuler: The psychology of schizophrenia and the biology of the temporal lobe abnormalities. Harvard Reviews in Psychiatry. 1993;1:36–56. doi: 10.3109/10673229309017055. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Schooler NR, Wilson WH. Predictors of therapeutic response to haloperidol in acute schizophrenia. Psychopharmacology Bulletin. 1991;27:97–101. [PubMed] [Google Scholar]
- McGlashan TH. A selective review of recent North American long-term followup studies of schizophrenia. Schizophrenia Bulletin. 1988;14:515–542. doi: 10.1093/schbul/14.4.515. [DOI] [PubMed] [Google Scholar]
- McWalter HS, Mercer R, Sutherland MM, et al. Outcomes of treatment of schizophrenia in a North-East Scottish mental hospital. American Journal of Psychiatry. 1961;118:529–533. [Google Scholar]
- Mortensen PB, Juel K. Mortality and causes of death in first admitted schizophrenic patients. British Journal of Psychiatry. 1993;163:183–189. doi: 10.1192/bjp.163.2.183. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P. Why has the incidence of schizophrenia in Danish psychiatric institutions decreased since, 1970? Acta Psychiatrica Scandinavia. 1987;75:62–68. doi: 10.1111/j.1600-0447.1987.tb02752.x. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P, Jorgensen P. Decreasing rates of first-admission diagnoses of schizophrenia among females in Denmark, 1970–84. Acta Psychiatrica Scandinavica. 1986;74:379–383. doi: 10.1111/j.1600-0447.1986.tb06257.x. [DOI] [PubMed] [Google Scholar]
- Murakami K. Changes in clinical course and symptomatology of schizophrenia following introduction of pharmacotherapy (in Japanese) Psychiatrie Neurologia Japonica. 1971;73:635–649. [PubMed] [Google Scholar]
- Ødegård O. Pattern of discharge from Norwegian psychiatric hospitals before and after the introduction of psychotropic drugs. American Journal of Psychiatry. 1964;120:772–778. doi: 10.1176/ajp.120.8.772. [DOI] [PubMed] [Google Scholar]
- Parker G, O’Donnell M, Walter S. Changes in the diagnoses of the function psychoses associated with the introduction of lithium. British Journal of Psychiatry. 1985;146:377–382. doi: 10.1192/bjp.146.4.377. [DOI] [PubMed] [Google Scholar]
- Peterson DB, Olson GW. First admitted schizophrenics in drug era. Archives of General Psychiatry. 1964;11:137–144. doi: 10.1001/archpsyc.1964.01720260031004. [DOI] [PubMed] [Google Scholar]
- Pritchard DM. Prognosis of schizophrenia before and after pharmacotherapy: I. Short term outcome. British Journal of Psychiatry. 1967a;113:1345–1352. doi: 10.1192/bjp.113.505.1345. [DOI] [PubMed] [Google Scholar]
- Pritchard DM. Prognosis of schizophrenia before and after pharmacotherapy: II. Three-year follow-up. British Journal of Psychiatry. 1967b;113:1353–1359. doi: 10.1192/bjp.113.505.1353. [DOI] [PubMed] [Google Scholar]
- Ram R, Bromet EJ, Eaton WW, Pato C, Schwartz JE. The natural course of schizophrenia: a review of first-admission studies. Schizophrenia Bulletin. 1992;18:185–207. doi: 10.1093/schbul/18.2.185. [DOI] [PubMed] [Google Scholar]
- Rappaport M, Hopkins HK, Hall K, et al. Are there schizophrenics for whom drugs may be unnecessary or contra-indicated? International Pharmacopsychiatry. 1978;13:100–111. doi: 10.1159/000468327. [DOI] [PubMed] [Google Scholar]
- Schooler NR, Goldberg SC, Boothe H, et al. One year after discharge: Community adjustment of schizophrenic patients. American Journal of Psychiatry. 1967;123:986–995. doi: 10.1176/ajp.123.8.986. [DOI] [PubMed] [Google Scholar]
- Shimazono Y. Comment and Discussion. In: Mitsuda H, Fukada T, editors. Biological Mechanisms of Schizophrenia and Schizophrenia-like Psychosis. Tokyo: Igaku Shin Ltd; 1973. pp. 126–128. [Google Scholar]
- Snezhnevsky A. The prognosis of schizophrenia. International Journal of Psychiatry. 1966;2:635–638. [PubMed] [Google Scholar]
- Stephens JH. Long-term course and prognosis in schizophrenia. Seminars in Psychiatry. 1970;2:464–485. [PubMed] [Google Scholar]
- Stevens JR. Abnormal reinnervation as a basis for schizophrenia: a hypothesis [published erratum appears in Arch Gen Psychiatry, 1992 Sep:49(9):708] Archives of General Psychiatry. 1992;49:238–243. doi: 10.1001/archpsyc.1992.01820030070009. [DOI] [PubMed] [Google Scholar]
- Szymanski SR, Cannon TD, Gallacher F, Erwin RJ, Gur RE. Course of treatment response in first-episode and chronic schizophrenia. American Journal of Psychiatry. 1996;153:519–525. doi: 10.1176/ajp.153.4.519. [DOI] [PubMed] [Google Scholar]
- Szymanski S, Lieberman JA, Alvir JM, Mayerhoff D, Loebel A, Geisler S, Chakos M, Koreen A, Jody D, Kane J. Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. American Journal of Psychiatry. 1995;152:698–703. doi: 10.1176/ajp.152.5.698. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Youssef HA. Evidence for a gender-specific decline in the rate of schizophrenia in rural Ireland over a 50-year period. British Journal of Psychiatry. 1994;164:171–176. doi: 10.1192/bjp.164.2.171. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Youssef HA, Kinsella A. Sequential cross-sectional and ten year prospective study of severe negative symptoms in relation to duration of initially untreated psychosis in chronic schizophrenia. Psychological Medicine. 1995;25:663–670. doi: 10.1017/s0033291700035108. [DOI] [PubMed] [Google Scholar]
- Watt DC, Katz K, Shepherd M. The natural history of schizophrenia: A 5-year prospective follow-up of a representative sample of schizophrenics by means of standardized clinical and social assessment. Psychological Medicine. 1983;13:663–670. doi: 10.1017/s0033291700048091. [DOI] [PubMed] [Google Scholar]
- Wirt RD, Simon W. Differential Treatment and Prognosis in Schizophrenia. Springfield; Illinois: 1959. [Google Scholar]
- Wyatt RJ. The dopamine hypothesis: Variations on a theme (II) Psychopharmacology Bulletin. 1988;22:923–927. [PubMed] [Google Scholar]
- Wyatt RJ. Neuroleptics and the natural course of schizophrenia. Schizophrenia Bulletin. 1991;17:325–351. doi: 10.1093/schbul/17.2.325. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ. Antipsychotic medication and the long-term course of schizophrenia: Therapeutic and theoretical implications. In: Shriqui C, Nasrallah H, editors. Contemporary issues in the treatment of schizophrenia. Washington, D.C: American Psychiatric Press; 1995a. pp. 385–410. [Google Scholar]
- Wyatt RJ. The risks of discontinuing antipsychotic medications. Archives of General Psychiatry. 1995;52:205–208. doi: 10.1001/archpsyc.1995.03950150037007. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Green MF, Tuma AH. Long-term morbidity associated with delayed treatment of first admission schizophrenic patients: A re-analysis of the Camarillo State Hospital data. Psychological Medicine. 1997;27:261–268. doi: 10.1017/s0033291796004345. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Pina LM, Henter ID. First-episode schizophrenia: Early intervention and medication discontinuation in the context of course and treatment. British Journal of Psychiatry. in press. [PubMed] [Google Scholar]