Abstract
We assessed the clinical characteristics and efficacy of neurotransmitters and levetiracetam in a patient with hyperphenylalaninemia due to dihydropteridine reductase (DHPR) deficiency who developed epileptic seizures. A boy with DHPR deficiency, who had been successfully treated with tetrahydrobiopterin (BH4), levodopa, and 5-hydroxytryptophan (5-HTP) since he was 2 months old, started having monthly episodes of blurred vision, loss of consciousness, and falls at the age of 12 years. He was taking BH4 510 mg/day, levodopa 670 mg/day, 5-HTP 670 mg/day, and entacapone 300 mg/day. We evaluated the seizure semiology, EEG findings, and efficacy of levodopa, 5-HTP, and levetiracetam (LEV). His seizures were comprised of an abrupt loss of awareness and eye deviation to the right. Interictal EEG showed slightly slow posterior-dominant rhythm in 7–8 Hz; intermittent, irregular slowing in the bilateral parieto-occipital region; and multiregional independent spikes in bilateral hemispheres. Ictal EEG showed a seizure pattern starting at the left temporal region. Brain MRI showed diffuse signal increase of deep white matter on T2-weighted and FLAIR images. Dosage increase of levodopa to 1340 mg/day, of 5-HTP to 1500 mg/day, or of both did not suppress seizures. Levetiracetam 2000 mg/day markedly reduced seizures without any adverse events. Patients with DHPR deficiency can develop epileptic seizures of partial onset which can be successfully and safely treated with LEV.
Keywords: Dihydropteridine reductase deficiency, Epileptic seizures, Levodopa, 5-Hydroxytryptophan, Levetiracetam
1. Introduction
Dihydropteridine reductase (DHPR) deficiency is a rare inherited disorder which affects the metabolism of tetrahydrobiopterin (BH4), causing hyperphenylalaninemia and neurotransmitter deficiency [1]. In Japan, about 1 per 2 million newborns is diagnosed with BH4 deficiency, and about 10% of them have DHPR deficiency [2]. In an international survey, DHPR deficiency is the second most common form of BH4 deficiency (34.7%) [3]. Dihydropteridine reductase deficiency in patients is highly associated with epileptic seizures that are frequently severe [3], [4]. However, the clinical characteristics of their seizures, EEG findings, and treatment strategies have not been fully established.
Here, we assessed the clinical characteristics and efficacy of neurotransmitters and levetiracetam (LEV) to epileptic seizures in a patient with DHPR deficiency who had been successfully treated with BH4, levodopa, and 5-hydroxytryptophan (5-HTP). Part of this manuscript was presented at the 30th International Epilepsy Congress, 2013, and appeared in an abstract form [5].
2. Case presentation
A boy, the second child of nonconsanguineous parents, was born at 38 weeks of gestation with a weight of 3.1 kg. At 4 days, phototherapy was done for jaundice. He was found to have hyperphenylalaninemia on a routine Guthrie test for newborn screening. His older brother is healthy. His serum phenylalanine (Phe) level ranged between 492 and 756 μmol/l (normal range: 38–91 μmol/l [4]). He was diagnosed as having DHPR deficiency based on a BH4 loading test; the measurement of urinary, serum, and CSF pterins; the DHPR activity in dried blood spots; and the presence of neurotransmitter metabolites in the urine and CSF. Details of these diagnostic tests were described in our previous paper [6]. Gene analysis revealed a compound heterozygous mutation of the QDPR gene (G18C/S59X, both are new mutations). At 2 months, treatment was started with BH4 15–20 mg/kg/day, levodopa 15 mg/kg/day, and 5-HTP 15 mg/kg/day, as previously described [4]. The dosage of BH4 was adjusted to keep his serum Phe concentration less than 480 μmol/l without dietary restriction of Phe [6]. Entacapone 300 mg/day was added at the age of 10 years. With this therapy, his physical development is within normal limits. Folic acid was not administered because its level in his serum – 3.4–5.9 ng/ml (normal range: 3.6–12.9 ng/ml) – is of sufficient amount. He attended an ordinary elementary school, though he showed mild mental retardation (IQ of around 60).
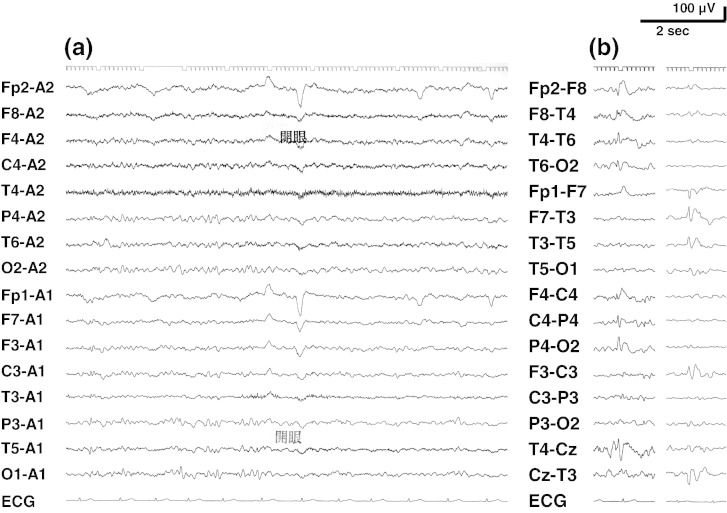
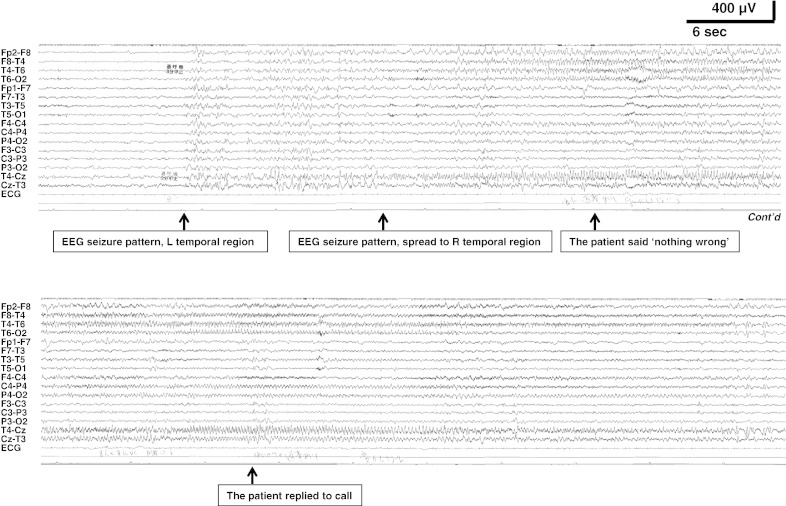
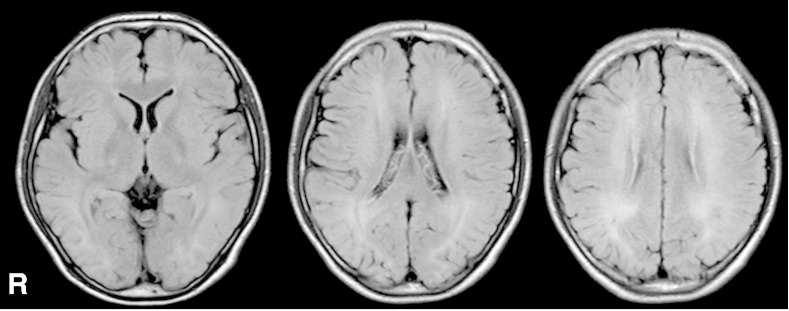
However, he started having monthly episodes of blurred vision, loss of consciousness, and falls at the age of 12 years. At that time, he was taking BH4 510 mg/day, levodopa 670 mg/day, 5-HTP 670 mg/day, and entacapone 300 mg/day. Interictal EEG showed slightly slow posterior-dominant rhythm in 7–8 Hz; intermittent irregular slow waves in the bilateral parieto-occipital regions and multiregional independent spikes in bilateral hemispheres (Fig. 1). During EEG recordings, he had habitual seizures which comprised an abrupt loss of awareness and eye deviation to the right without convulsion. Ictal EEG showed an electrographic seizure pattern starting at the left temporal region and then a burst of fast activities spreading to the other side (Fig. 2). Brain MRI showed diffuse signal increase of deep white matter on T2-weighted and FLAIR images (Fig. 3). Dosage increase of levodopa to 1340 mg/day, of 5-HTP to 1500 mg/day, or of both did not suppress his seizures. Levetiracetam 2000 mg/day markedly reduced seizures without any adverse events.
Fig. 1.
Interictal EEG. (a) Slightly slow posterior dominant rhythm in 7–8 Hz and intermittent irregular slow waves in the bilateral parieto-occipital regions. (b) Multiregional independent spikes in bilateral hemispheres.
Fig. 2.
Ictal EEG. An electroencephalographic seizure pattern starting at the left temporal region and then a rhythmic burst of fast activities starting at the right temporal region. The patient was conscious in this particular event, but the same pattern of EEG was observed while he exhibited consciousness disturbance in other complex partial seizures.
Fig. 3.
Brain MRI, FLAIR images at the age of 12 years. Note diffuse signal increase of deep white matter.
3. Discussion
The present male patient with DHPR deficiency, who had been treated with supplementation of neurotransmitters since he was 2 months old, developed epileptic seizures at the age of 12 years. By EEG evaluation of ictal and interictal epochs, we could document that his epileptic seizures were complex partial seizures originating from the left temporal region, and he also has diffuse and multiregional abnormalities. We also demonstrated that antiepileptic drugs such as LEV should be introduced in order to suppress his seizures; on the other hand, dosage increase of neurotransmitter supplementation was not effective.
A previous paper described that 6 out of 10 patients with DHPR deficiency, whose onset of neurotransmitter treatment was at 6 months of age or older, developed severe epileptic seizures [4]. The present case, where the treatment was started when the patient was 2 months old, suggests that neurotransmitter supplementation should be started earlier than 2 months to prevent the development of seizures. In literature describing severe seizures or convulsions in patients with DHPR deficiency, seizure types are myoclonic seizures [7] and seemingly generalized tonic–clonic seizures, considering that their EEG showed generalized hypsarrhythmic activity or diffuse sharp wave activity [4], [8], [9]. In contrast, our patient showed complex partial seizures. This may also suggest that the timing of treatment administration may affect the clinical seizure types.
Phenobarbital, clonazepam, and sodium valproate (VPA) have been used to treat severe seizures in patients with DHPR deficiency [4], [8], [9]. However, antiepileptic drugs can lead to adverse events; VPA, in particular, may lead to extrapyramidal tract signs that can worsen the symptoms in patients with DHPR deficiency and in patients with other metabolic disorders with dopamine depletion. Our experience with this patient suggests that LEV can be used in DHPR deficiency safely.
4. Conclusion
Patients with DHPR deficiency can develop epileptic seizures of partial onset, which can be successfully and safely treated with LEV. Further studies are needed to clarify the mechanism of epileptogenesis and the therapeutic strategies in patients with DHPR deficiency.
Conflict of interest
The authors declare that they have no conflict of interets.
Acknowledgment
This study was supported in part by Health and Labour Sciences Research Grants for Research on Rare and Intractable Diseases.
References
- 1.Shintaku H. Disorders of tetrahydrobiopterin metabolism and their treatment. Curr Drug Metab. 2002;3:123–131. doi: 10.2174/1389200024605145. [DOI] [PubMed] [Google Scholar]
- 2.Shintaku H., Ohwada M. Long-term follow-up of tetrahydrobiopterin therapy in patients with tetrahydrobiopterin deficiency in Japan. Brain Dev. 2013;35:406–410. doi: 10.1016/j.braindev.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Opladen T., Hoffmann G.F., Blau N. An international survey of patients with tetrahydrobiopterin deficiencies presenting with hyperphenylalaninaemia. J Inherit Metab Dis. 2012;35:963–973. doi: 10.1007/s10545-012-9506-x. [DOI] [PubMed] [Google Scholar]
- 4.Jäggi L., Zurflüh M.R., Schuler A., Ponzone A., Porta F., Fiori L. Outcome and long-term follow-up of 36 patients with tetrahydrobiopterin deficiency. Mol Genet Metab. 2008;93:295–305. doi: 10.1016/j.ymgme.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Furujo M., Kinoshita M., Shintaku H., Kubo T. Clinical characteristics of epileptic seizures in a case of dihydropteridine reductase deficiency. Epilepsia. 2013;54(s3):234. doi: 10.1016/j.ebcr.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furujo M., Ichiba Y., Sintaku H., Asada M. A case of dihydropteridine reductase deficiency. Pteridines. 2000;11:126–128. [Google Scholar]
- 7.Cotton R.G., Jennings I., Bracco G., Ponzone A., Guardamagna O. Tetrahydrobiopterin non-responsiveness in dihydropteridine reductase deficiency is associated with the presence of mutant protein. J Inherit Metab Dis. 1986;9:239–243. doi: 10.1007/BF01799654. [DOI] [PubMed] [Google Scholar]
- 8.Longhi R., Valsasina R., Buttè C., Paccanelli S., Riva E., Giovannini M. Cranial computerized tomography in dihydropteridine reductase deficiency. J Inherit Metab Dis. 1985;8:109–112. doi: 10.1007/BF01819291. [DOI] [PubMed] [Google Scholar]
- 9.Longhi R., Riva E., Valsasina R., Paccanelli S., Giovannini M. Phenylketonuria due to dihydropteridine reductase deficiency: presentation of two cases. J Inherit Metab Dis. 1985;8(s2):97–98. doi: 10.1007/BF01811476. [DOI] [PubMed] [Google Scholar]