Abstract
Introduction
Occipital lobe seizures are a recognized manifestation of diabetic nonketotic hyperglycemia, though not as common as focal motor seizures. Occipital lobe white matter T2 hypointensity may suggest this diagnosis.
Methods
We present a case of a 66-year-old man with hyperglycemia-related occipital lobe seizures who presented with confusion, intermittent visual hallucinations, and homonymous hemianopia.
Results
Magnetic resonance imaging showed subcortical T2 hypointensity within the left occipital lobe with adjacent leptomeningeal enhancement. These findings were transient with disappearance in a follow-up MRI. The EEG captured frequent seizures originating in the left occipital region. HbA1c level was 13.4% on presentation, and finger stick blood glucose level was 400 mg/dl.
Conclusion
Hyperglycemia should be considered in the etiology of differential diagnosis of patients with visual abnormalities suspicious for seizures, especially when the MRI shows focal subcortical T2 hypointensity with or without leptomeningeal enhancement.
Keywords: Occipital seizures, Hyperglycemia, Subcortical T2 hypointensity
1. Introduction
Visual phenomena related to hyperglycemia are not uncommon. These symptoms can vary from mild blurry vision to homonymous hemianopia without EEG findings or even prominent electroclinical occipital seizures. Prompt recognition of occipital seizures as a manifestation of nonketotic hyperglycemia is essential as it is easily reversible. Although hyperglycemia typically causes focal motor seizures, occipital seizures have also been associated with hyperglycemia. Our case highlights the key features of hyperglycemia-induced occipital lobe seizures and illustrates the need for clinical vigilance since the presenting symptoms may be nonspecific or mistaken for migraine, temporal arteritis, or ischemia. Prominent features of this case include elevated glycated hemoglobin (HbA1c), electrographic seizures, and reversible subcortical T2 hypointensity with leptomeningeal enhancement.
2. Materials and methods
We report the case of a 66-year-old man with subacute onset of visual symptoms and discuss his hospital course, diagnosis, treatment, and follow-up. We review the literature for similar case reports and present the diagnostic modalities utilized in those cases along with the treatment protocols implemented.
3. Case presentation
3.1. History
A 66-year-old right-handed man with history of non-insulin-dependent diabetes mellitus type II, hypertension, and hyperlipidemia was transferred to our institution from another hospital for fluctuating alteration of mental status, hyponatremia (Na level of 126 mEq/l), and visual disturbances, with finger stick blood glucose level of 400 mg/dl for further work-up.
Prior to arrival, he had 1 week of intermittent confusion with apraxia (not knowing what to do with his toothbrush, difficulty getting dressed). He reported visual hallucinations consisting of mathematical figures in his peripheral vision and people in his home. He did not have a history of seizures or migraines, nor did he have any known risk factors for epilepsy. On examination, he had a right homonymous hemianopia and deficits in attention, praxis, and calculations. During examination, he also had 4 episodes characterized by a head turn to the right, unresponsiveness, and right-beating nystagmus. Each episode lasted < 1 min. The patient was immediately responsive and conversant thereafter.
3.2. Evaluation
Laboratory abnormalities included a blood glucose level of 359 mg/dl, an HbA1c level of 13.4%, and a sodium level of 129 mEq/l. The remainder of his metabolic work-up was unremarkable. No ketoacidosis was found in serum or urine. Magnetic resonance imaging of the brain showed subcortical T2 hypointensity within the left occipital lobe (Fig. 1). Postcontrast images showed leptomeningeal enhancement along the left parieto-occipital region. These findings raised radiological concern for subacute infarction or infectious etiology such as encephalitis or paraneoplastic syndrome, but lumbar puncture revealed a normal opening pressure, while CSF analysis showed normal protein and cell counts and was notable only for elevated glucose.
Fig. 1.
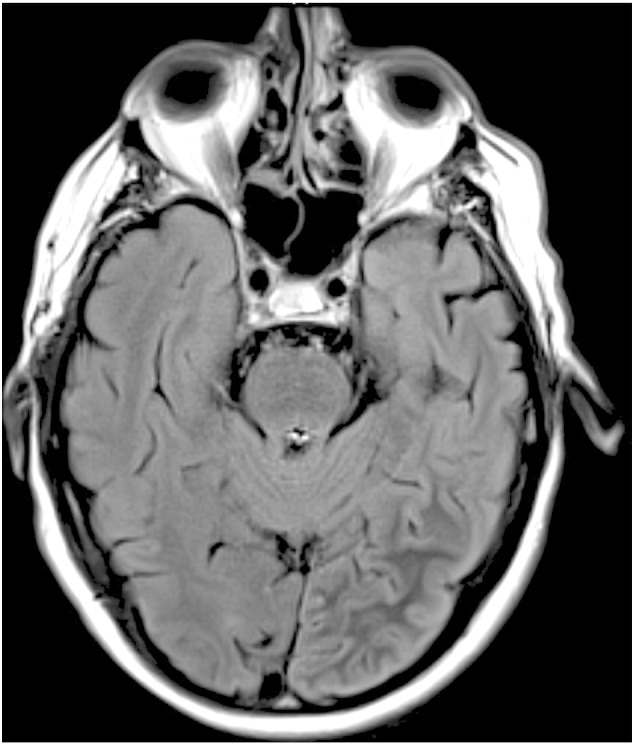
MRI of the brain demonstrating left parieto-occipital subcortical T2 hypointensity on fluid-attenuated inversion recovery.
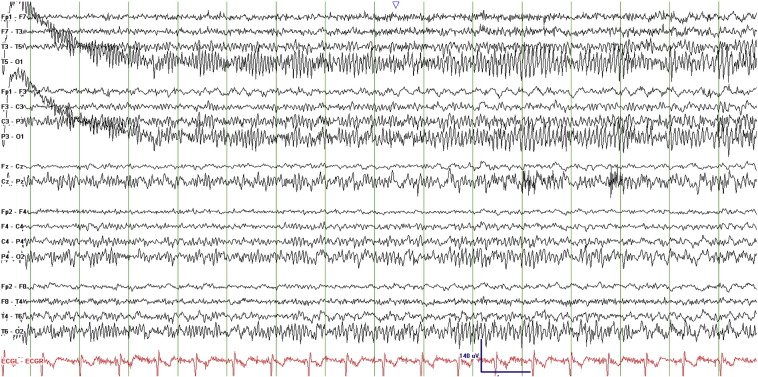
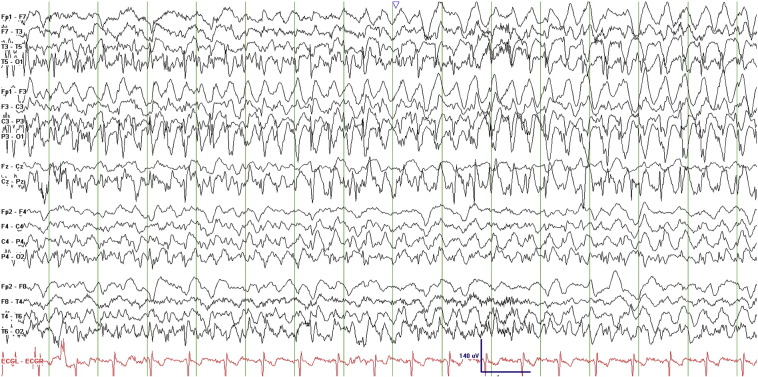
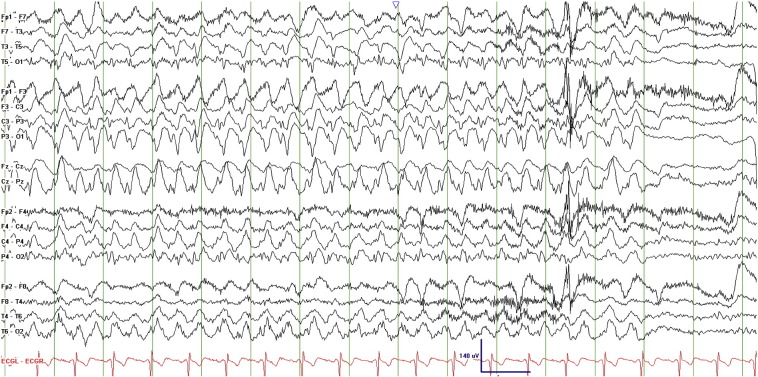
An initial 22-minute scalp EEG recording captured 7 seizures, each lasting 1–2 min, and characterized electrographically by left occipital polyspikes at a frequency of 20–25 Hz with spread to the right occipital region within about 3 s followed by diffuse bilateral involvement before abruptly terminating. Some of the seizures evolved to approximately 2-Hz spike-and-wave discharges with left occipitoparietal predominance. There was minimal postictal slowing (Figs. 2–4, Fig. 3, Fig. 4).
Figs. 2–4.
Ictal EEG recordings showing left occipital polyspikes with spread to the right occipital region followed by diffuse bilateral involvement.
Fig. 3.
Fig. 4.
3.3. Treatment and follow-up
Continuous EEG was initiated demonstrating continued seizures. Seizures stopped within 1 h following administration of phenytoin, lorazepam, insulin, and aggressive fluid hydration. Follow-up MRI 2 months later showed resolution of subcortical T2 hypointensity and leptomeningeal enhancement. The patient remained seizure-free since then with good glycemic control.
4. Discussion
Nonketotic hyperglycemia (NKH) as a cause of focal seizures is well-described [1]. In some cases, partial seizures are the presenting feature of undiagnosed diabetes mellitus. This was initially described by Maccario and colleagues in 1965 [2] and later by others [3], [4], [5]. The most commonly encountered seizure types in NKH are focal motor seizures and epilepsia partialis continua [6]. However, occipital lobe seizures have been reported in the setting of hyperglycemia. In the majority of cases, the seizures occurred in the setting of only moderate hyperglycemia and in the absence of significant hyperosmolality. Thus, this may be more related to long-standing hyperglycemia (as reflected by the elevated HbA1c) than to the degree of hyperglycemia in the acute setting [7]. Several of these cases have demonstrated subcortical T2 hypointensity in the involved occipital lobe [7], [8], [9], [10], [11], [12], [13].
In spite of the increasing recognition of this entity, pathophysiology still remains unclear. Intracellular dehydration from fluid shifts related to hyperosmolality gradient can also trigger seizures. It has been postulated that cellular dehydration caused by hyperosmolality inhibits the Krebs cycle. This may lead to a compensatory increase in metabolism of GABA to succinic acid and subsequent depletion of GABA which results in neuronal hyperexcitability [14], [15]. However, this explanation does not fully account for the evidence that seizures may occur with hyperglycemia in the absence of significant hyperosmolality. It is possible that hyperglycemia itself may have a proconvulsant effect [16].
Symptoms related to occipital seizures may not cause severe impairments, thus delaying diagnosis and treatment, but seizures must be promptly recognized as they are easily treatable. Symptoms may include visual phenomena such as colored circles or letters or other well-formed visual hallucinations, headache, confusion, blurry vision, or homonymous hemianopia. Migraine with aura, temporal arteritis, and posterior reversible encephalopathy syndrome (PRES) are some of the other diagnoses often under consideration. A high index of clinical suspicion may be needed to recognize acute symptomatic occipital seizures in the setting of hyperglycemia.
While EEG is the gold standard for diagnosing seizures, subtle MRI findings may suggest the diagnosis. Magnetic resonance imaging may show reversible diffusion abnormalities, FLAIR and T2 hyperintensities in the cortical gray matter, and/or hypointensities in the subcortical white matter [9]. Reversible bilateral striatal T2 hyperintensity in NKH has also been reported [11]. Subcortical hypointensity is becoming increasingly recognized in relation to nonketotic hyperglycemia and has been attributed to transient free radical accumulation during excitotoxic damage from seizures [10], [11]. Fludeoxyglucose - Positron emission tomography(FDG - PET) performed in a patient with hyperglycemia-related right occipital seizures and right occipital subcortical T2 hypointensity showed associated right occipital cortical hypermetabolism [17]. Magnetic Resonance spectroscopy performed in a similar patient showed a moderate decrement in NAA and lipid spikes, suggesting a component of laminar necrosis in the pathology despite the transient abnormalities on T2-weighted MRI [8].
Subcortical T2 hypointensity is rare but has also been described as an unusual manifestation of ischemia, multiple sclerosis, leptomeningeal metastasis, and meningitis or meningoencephalitis [18]. Leptomeningeal enhancement has also been reported with hyperglycemia-associated seizures though it may be less common [19]. These findings can raise concern for subacute infarct, encephalitis, malignancy, or a paraneoplastic process. These were excluded in our case by CSF studies and the resolution of these findings on the follow-up MRI. Aggressive fluid management and glycemic control should be the focus of therapy. While anticonvulsants are certainly warranted in the acute setting of status epilepticus, it is unclear whether long-term AED therapy is needed as the seizures are provoked by a reversible process. Our patient was treated with phenytoin and recovered completely, but phenytoin may not be the optimal AED choice given concerns that phenytoin may worsen hyperglycemia [20]. Gamma-Aminobutyric Acid(GABA)-enhancing medications are suggested as strategic alternatives, though not well studied in this condition.
5. Conclusion
We report a case of multiple electroclinical occipital seizures in the setting of hyperglycemia and elevated HbA1c with focal leptomeningeal enhancement and subcortical T2 hypointensity on MRI. Occipital lobe seizures should be considered in the differential diagnosis when patients with hyperglycemia present with visual changes, and occipital T2 hypointensity should raise suspicion for hyperglycemia-associated seizures.
References
- 1.Stahlman G.C., Auerbach P.S., Strickland W.G. Neurologic manifestations of non-ketotic hyperglycemia. J Tenn Med Assoc. 1988;81:77–80. [PubMed] [Google Scholar]
- 2.Maccario M., Messis C.P., Vastola E.F. Focal seizures as a manifestation of hyperglycemia without ketoacidosis. A report of seven cases with review of the literature. Neurology. 1965;15:195–206. doi: 10.1212/wnl.15.3.195. [DOI] [PubMed] [Google Scholar]
- 3.Martinez M.D., Megias S.M. Occipital seizures with electroencephalographic alterations as the initial manifestation of diabetes mellitus. Endocrinol Nutr. 2009;56:458–460. doi: 10.1016/S1575-0922(09)73314-5. [DOI] [PubMed] [Google Scholar]
- 4.Omar H.R., El-Khabiry E., Vaughan S. Seizure as the first presentation of diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2012;3:175. doi: 10.1177/2042018812459879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennis A., Corbin D., Fraser H. Focal seizures and non-ketotic hyperglycaemia. J Neurol Neurosurg Psychiatry. 1992;55:195–197. doi: 10.1136/jnnp.55.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moien-Afshari F., Tellez-Zenteno J.F. Occipital seizures induced by hyperglycemia: a case report and review of literature. Seizure. 2009;18:382–385. doi: 10.1016/j.seizure.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Hung W.L., Hsieh P.F., Lee Y.C., Chang M.H. Occipital lobe seizures related to marked elevation of hemoglobin A1C: report of two cases. Seizure. 2010;19:359–362. doi: 10.1016/j.seizure.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Donat A., Guilloton L., Bonnet C., Depreux G., Lamboley J.L., Drouet A. Partial visual seizures induced by non-ketosic hyperglycemia: magnetic resonance imaging and visual evoked potential descriptions. A study of two cases reports with radiologic and electrophysiologic abnormalities. Rev Neurol (Paris) 2013;169:154–161. doi: 10.1016/j.neurol.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Goto H., Kumagai T., Momozaki N. MRI findings of occipital seizures in non-ketotic hyperglycemia. Intern Med. 2011;50:367–368. doi: 10.2169/internalmedicine.50.4684. [DOI] [PubMed] [Google Scholar]
- 10.Hattori H., Matsuoka O., Ishida H., Hisatsune S., Yamano T. Magnetic resonance imaging in occipital lobe epilepsy with frequent seizures. Pediatr Neurol. 2003;28:216–218. doi: 10.1016/s0887-8994(02)00615-x. [DOI] [PubMed] [Google Scholar]
- 11.Raghavendra S., Ashalatha R., Thomas S.V., Kesavadas C. Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology. 2007;49:299–305. doi: 10.1007/s00234-006-0189-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang C.P., Hsieh P.F., Chen C.C., Lin W.Y., Hu W.H., Yang D.Y. Hyperglycemia with occipital seizures: images and visual evoked potentials. Epilepsia. 2005;46:1140–1144. doi: 10.1111/j.1528-1167.2005.56404.x. [DOI] [PubMed] [Google Scholar]
- 13.Lavin P.J. Hyperglycemic hemianopia: a reversible complication of non-ketotic hyperglycemia. Neurology. 2005;65:616–619. doi: 10.1212/01.wnl.0000173064.80826.b8. [DOI] [PubMed] [Google Scholar]
- 14.Oztas B., Camurcu S. Blood–brain barrier permeability after electrically induced seizure in normoglycemic, hypoglycemic, and hyperglycemic rats. Psychiatry Res. 1989;29:151–159. doi: 10.1016/0165-1781(89)90029-2. [DOI] [PubMed] [Google Scholar]
- 15.Harden C.L., Rosenbaum D.H., Daras M. Hyperglycemia presenting with occipital seizures. Epilepsia. 1991;32:215–220. doi: 10.1111/j.1528-1157.1991.tb05247.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwechter E.M., Velíšková J., Velíšek L. Correlation between extracellular glucose and seizure susceptibility in adult rats. Ann. Neurol. 2003;53:91–101. doi: 10.1002/ana.10415. [DOI] [PubMed] [Google Scholar]
- 17.Stayman A., Abou-Khalil B.W., Lavin P., Azar N.J. Homonymous hemianopia in nonketotic hyperglycemia is an ictal phenomenon. Neurol. Clin. Pract. 2013;3:392–397. doi: 10.1212/CPJ.0b013e3182a7bb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.H., Na D.G., Choi K.H., Kim K.J., Ryoo J.W., Lee S.Y. Subcortical low intensity on MR images of meningitis, viral encephalitis, and leptomeningeal metastasis. Am. J. Neuroradiol. 2002;23:535–542. [PMC free article] [PubMed] [Google Scholar]
- 19.Lin W-S, Kao H-W, Sung Y-F. Reversible magnetic resonance imaging abnormality in a case of diabetic hyperglycemia related epilepsia partialis continua.
- 20.Banner W., Jr., Johnson D.G., Walson P.D., Jung D. Effects of single large doses of phenytoin on glucose homeostasis—a preliminary report. J Clin Pharmacol. 1982;22:79–81. doi: 10.1002/j.1552-4604.1982.tb02652.x. [DOI] [PubMed] [Google Scholar]