Abstract
Schistosomiasis is the second most socioeconomically devastating parasitic disease worldwide, affecting over 240 million people in 77 countries on 5 continents and killing 300,000 people annually in sub-Saharan Africa alone. Neuroschistosomiasis is caused by granuloma formation around eggs that lodge in the CNS, with Schistosoma mansoni and Schistosoma haematobium usually affecting the spinal cord and Schistosoma japonicum causing most reported cerebral disease. We report a case of a previously healthy 25-year-old woman native to the United States who presented with a single generalized tonic–clonic seizure without other neurologic symptoms four years after spending a semester in Ghana where she went swimming once in a river. Brain MRI showed areas of signal abnormality and mottled nodular linear enhancement in the left temporal and right posterior temporal/parietal lobes and right cerebellum without mass effect. A biopsy of the left temporal lesion showed prominent granulomas with dense mixed inflammatory infiltrates composed of eosinophils, plasma cells, and lymphocytes surrounding refractile egg shells containing characteristic embryonal cells and von Lichtenberg's envelope and displaying the pathognomonic spine shape of S. mansoni. Serum ELISA and antibody immunoblots confirmed exposure to S. mansoni. In summary, we describe the atypical combination of cerebral schistosomiasis due to S. mansoni, after a prolonged interval of four years, from a single known exposure.
Keywords: Cerebral schistosomiasis, Neuroschistosomiasis, Schistosoma mansoni, Generalized tonic–clonic seizure, Granuloma
1. Introduction
Schistosomiasis has afflicted humans for thousands of years and is now the second most socioeconomically devastating parasitic disease worldwide, affecting over 240 million people in 77 countries on 5 continents and killing an estimated 300,000 people annually in sub-Saharan Africa alone [1], [2]. Nearly 800 million people are currently at risk of developing schistosomiasis worldwide [1]. The three main species of Schistosoma (digenetic blood trematode flatworms) infecting humans are Schistosoma haematobium, Schistosoma mansoni, and Schistosoma japonicum. Schistosoma mansoni and S. haematobium are endemic across much of Africa and the Middle East, often in overlapping geographic distributions. S. mansoni is also prevalent in areas of South America and the Caribbean. In contrast, S. japonicum is primarily found in the Far East. Two other species, Schistosoma mekongi and Schistosoma intercalatum, are more geographically restricted with S. mekongi found very focally in Southeast Asia and S. intercalatum found mainly in central West Africa [3], [4].
Infection by schistosomes requires contact with freshwater where mobile larval forms emerge from intermediate host snails and penetrate the skin, sometimes provoking an urticarial rash lasting several days. Adult worms migrate to the venous plexuses of specific host tissues, where they form mating pairs and copulate throughout their 3- to 5-year lifespan, laying eggs to be eliminated in either feces or urine (Fig. 1). The different species of Schistosoma show some correlation with specific tissue types, which in turn correspond to the mode of egg elimination. Schistosoma japonicum and S. mansoni show preference for the mesenteric venules of the bowel/rectum, while S. haematobium shows preference for the venous plexus of the bladder [5], [6], [7].
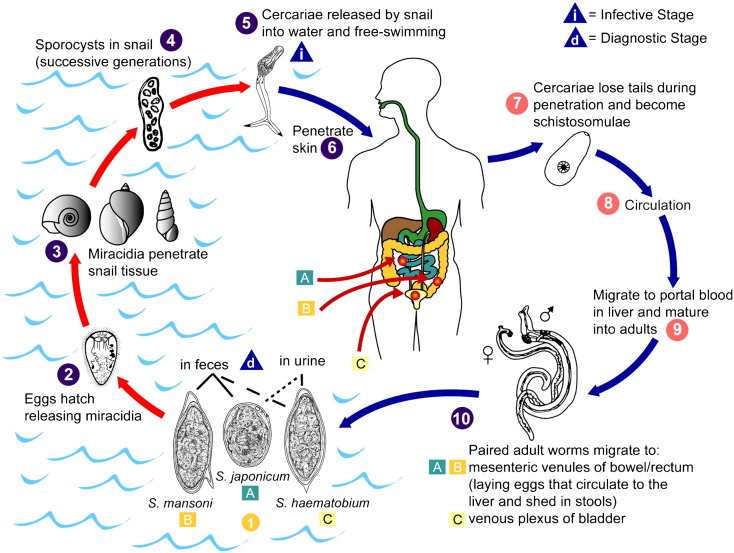
Fig. 1.
Schistosome life cycle. The stages of the schistosome life cycle (1–10) include (1) elimination from the host as eggs in feces or urine (diagnostic stage), (2) hatching of miracidia, (3) infection of species-specific aqueous snail intermediate hosts, (4) proliferation of sporocysts within snails, (5) release of cercariae into water (infective stage), (6) infection of host by skin penetration, (7) development into schistosomulae, (8) circulation, (9) maturation within portal vasculature, and (10) migration of paired adult worms to target organs. Elimination of schistosome eggs in either feces or urine depends on whether the adults reside in the mesenteric venules of the bowel/rectum (primarily (A) Schistosoma japonicum and (B) Schistosoma mansoni) or in the venous plexus of the bladder (primarily (C) Schistosoma haematobium), respectively.
Figure provided by A. J. da Silva and M. Moser for copyright-free dissemination through the Public Health Image Library of the Centers for Disease Control and Prevention [7].
Much of the pathology of schistosomiasis results from inflammatory reactions to the eggs and resulting damage of the surrounding organs. Hence, the tissue preferences of specific Schistosoma species lead in turn to some correlations between specific species and the incidence of particular organ injury. For instance, the pathology of S. mansoni and S. japonicum includes hepatic perisinusoidal egg granulomas/Symmers' pipe stem periportal fibrosis, portal hypertension, as well as Katayama fever. In contrast, the pathology of S. haematobium revolves around the urinary tract with frequent hematuria, bladder/lower ureter scarring, and calcification with resulting renal parenchymal damage and kidney failure as well as an association with squamous cell carcinoma of the bladder [3], [5], [8]. All three Schistosoma species can also affect the central nervous system as neuroschistosomiasis [4], [9].
Neuroschistosomiasis results when eggs, instead of being eliminated in feces or urine, spread to the central nervous system and induce the formation of granulomas. Involvement of the brain (cerebral/encephalic schistosomiasis or cerebral granulomatous disease) often leads to seizures, whereas involvement of the spinal cord (spinal cord schistosomiasis or schistosomal myeloradiculopathy) can cause cord compression resulting in paralysis. Similar to the organ distribution described above, different Schistosoma species have prevalence for affecting either the brain or spinal cord. Schistosoma japonicum primarily involves the brain, while S. mansoni and S. haematobium primarily affect the spinal cord. However, occasional cases of cerebral schistosomiasis due to S. mansoni continue to be reported, and it is possible that its frequency of cerebral involvement may be underreported [10], [11], [12], [13]. In areas where S. haematobium is endemic, spinal schistosomiasis is the leading cause of spinal cord damage. Involvement of the CNS is thought to be most frequently due to embolization of eggs, but some cases of adult schistosomes traveling to the CNS vasculature have been reported. Symptoms can present soon after infection in the case of acute schistosomal encephalopathy, but delayed reactions for months and sometimes for years have been reported in cerebral schistosomiasis [3], [4], [9].
2. Case presentation
The patient is a previously healthy 25-year-old female native of Massachusetts, U.S., who presented to the Brigham and Women's Hospital emergency department after the first seizure in her life. She had been feeling fatigued on the train that morning, and her symptoms worsened over the day. A coworker had her sit down, at which point she had difficulty answering questions. She then fell off a chair and had a witnessed generalized tonic–clonic seizure, which lasted for 3 min (without incontinence), followed by 30 min of more pronounced fatigue and confusion. In the emergency department, she was fully alert and oriented, followed all commands without difficulty, and had fluent speech. Concentration, attention, and memory were intact, as was her ability to read, name, and analyze a picture. The remainder of her neurologic exam was normal. A CT scan at that time also showed no abnormalities.
Her past medical history is notable only for headaches and eczema. Her headaches, which began in childhood, are infrequent and occur only once or twice per year. They are usually preceded by a scintillating scotoma and often resolve without medications. She also had a tick bite prior to her current course, though she had no subsequent rash and had a negative Lyme titer. In her family, she has a maternal uncle with epilepsy (unknown type), a maternal aunt with migraines, and a mother with cardiac arrhythmias. She is a vegetarian and does not smoke, drink, or use illicit drugs. She is HIV-negative. She has traveled to England twice and lived in Ghana for one semester of college, four years prior to the onset of symptoms. During that trip, she lived mainly in the university town of Legon, 12 km northeast of the capital city of Accra, and she reported swimming once during the trip in a freshwater river that runs parallel to the ocean, east of the capital city. She did not recall swimming in any lakes. She recalled no particular symptoms during her stay, apart from one episode of dysuria that lasted less than a day. In the last 4 years, she has been perfectly well until this episode.
She was subsequently followed in the clinic at weekly intervals and experienced an episode of altered consciousness (possibly a partial seizure) prior to the 4th visit. Peripheral blood showed no abnormal findings: WBC: 9.3, HGB: 12.9, HCT: 39.8, PLT: 226 (POLY: 58, BAND: 0, LYMPH: 31, MONO: 7, EOS: 2, and BASO: 0). Likewise, serum chemistries were within normal ranges: glucose: 91, BUN: 6, creatinine: 0.64, sodium: 140, potassium: 3.8, chloride: 100, total CO2: 26, alkaline phosphatase: 54, ALT: 11, AST: 17, total bilirubin: 0.2, total protein: 7.5, albumin: 4.6, calcium: 9.4, and magnesium: 1.9.
A lumbar puncture was performed, and CSF showed no abnormal findings: glucose: 55, protein: 19, WBC: 2 (91% lymphocytes), and RBC: 0. Likewise, rheumatologic labs were within normal ranges: CRP: 0.6, MPO: 0, PR3: 4, and ANA and ANCA: negative. She was started on the antiepileptic drug levetiracetam to prevent further seizures.
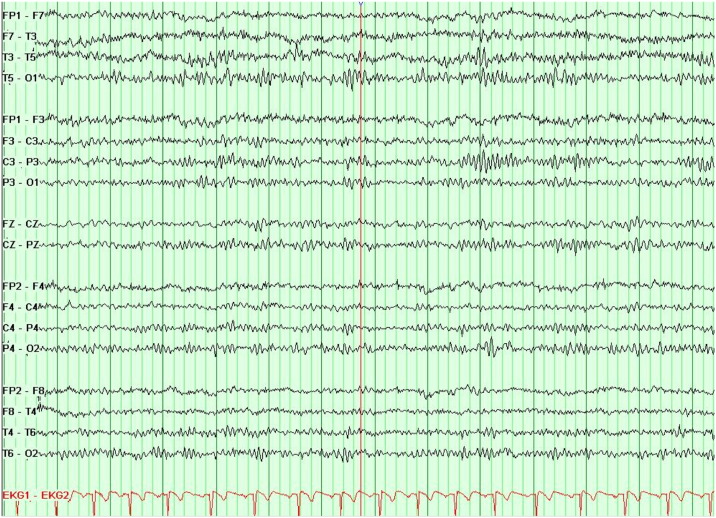
Electroencephalography (EEG) showed good organization at rest, but occasional abnormal left temporal theta slowing was noted, maximal at T3. There were no definite epileptiform abnormalities (Fig. 2). These findings are suggestive of mild left temporal dysfunction.
Fig. 2.
Electroencephalography shows left temporal dysfunction. This electroencephalogram (EEG), performed with anterior temporal and standard 10–20 electrodes during the waking, drowsy, and sleeping states, is abnormal because of occasional left temporal theta slowing. These findings are suggestive of mild left temporal dysfunction. There are no definite epileptiform abnormalities.
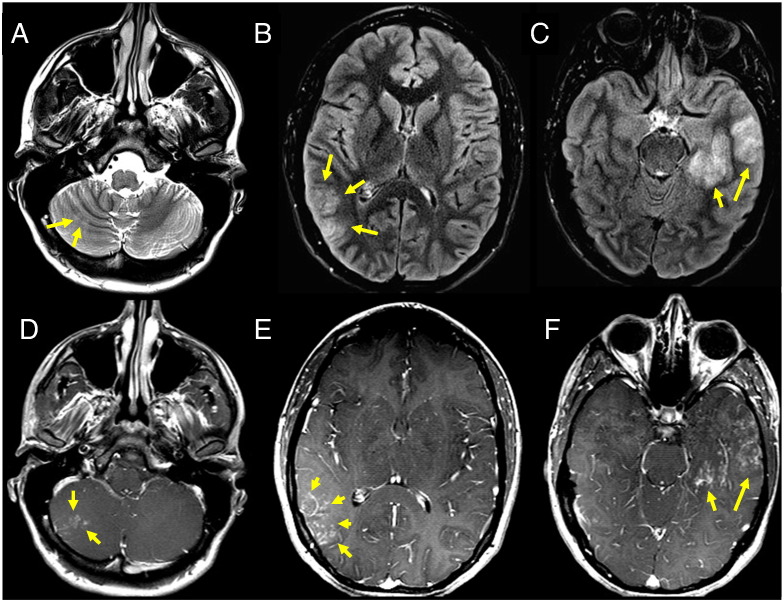
Brain MRI imaging showed areas of abnormal T2 and FLAIR signal at the medial and lateral aspects of the anterior left temporal and right posterior temporal parietal lobes (with the left more affected than the right), as well as a similar small area of abnormality of the right cerebellum (Figs. 3A–C). After contrast administration, all these areas showed mottled nodular linear enhancement without significant mass effect (Figs. 3D–F). No associated diffusion abnormality or mineralization or hemorrhage was seen. Likewise, there was no asymmetric hippocampal volume loss or gray matter heterotopia, and ventricles were normal in size. Possible etiologies of these imaging findings could include a spectrum of vasculitides, lymphomatoid granulomatosis, infection, and, less likely, granulomatous disease or tumor.
Fig. 3.
Lesions in the temporal/parietal cortex and cerebellum show nodular enhancement and edema. Axial T2 (A) and FLAIR (B, C) MRI images show increased signal at the right cerebellum (A, arrows) and right parietal (B, arrows) and left temporal (C, arrows) lobes consistent with edema. Axial post-gadolinium contrast images show corresponding clusters of small (1–3 mm) enhancing nodules at the right cerebellum (D, arrows) and right parietal (E, arrows) and left temporal (F, arrows) lobes. These findings likely represent granulomatous tissue surrounding the eggs. The differential diagnosis based on these radiologic findings would include a spectrum of vasculitides, lymphomatoid granulomatosis, infection, and, less likely, granulomatous disease or tumor.
Additional functional MRI (fMRI) brain mapping was performed for presurgical planning and showed findings consistent with left-sided language lateralization but also showed the functional language regions to be apart from the areas of left temporal lobe signal abnormality. An image-guided left temporal craniotomy for open biopsy of the area of signal abnormality was performed. Upon inspection, the surface of the brain was abnormal with some speckled appearance and some increased vascularity. A 0.5-cm biopsy was taken from the anterior/inferior left temporal lobe and appeared grossly abnormal with areas of possible necrosis. In addition, tissue was sent for microbiologic cultures.
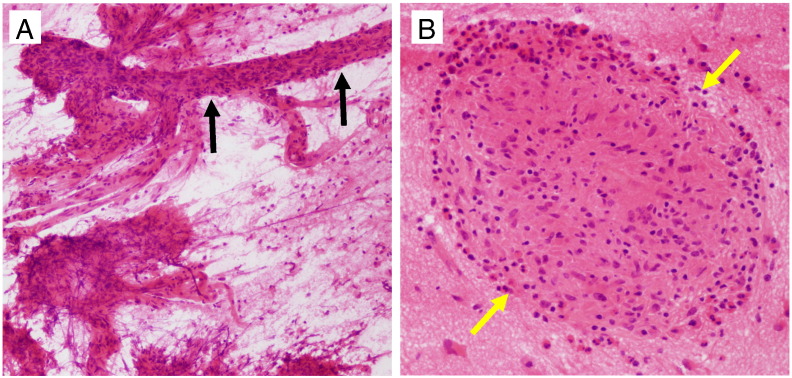
Initial intraoperative consultation with frozen section/smear evaluation showed collections of histiocytes concerning for granulomas, vascular involvement, and necrosis suspicious for an infectious process (Figs. 4A–B). Subsequent evaluation of paraffin-embedded permanent sections showed brain tissue with extensive granuloma formation and dense mixed inflammatory infiltrates comprised of eosinophils, plasma cells, histiocytes, and lymphocytes (Figs. 5A–B). Review of multiple tissue sections revealed refractile shell fragments at the center of each granuloma with occasional intact shells showing the pathognomonic acentric spine shape of S. mansoni (Fig. 6A) [14].
Fig. 4.
Tissue smear and frozen section reveal extensive inflammatory infiltrates and necrosis in the temporal cortex. (A) Smear preparation of left temporal cortex biopsy showing vessels with perivascular inflammation (black arrows) and areas of necrosis. (B) Frozen section of left temporal cortex biopsy showing prominent collections of mononuclear cells (macrophage/histiocyte clusters) and scattered eosinophils concerning for granulomas (yellow arrows). Pathologic differential of this appearance includes infection (TB vs. schistosomiasis), vasculitis, and lymphomatoid granulomatosis.
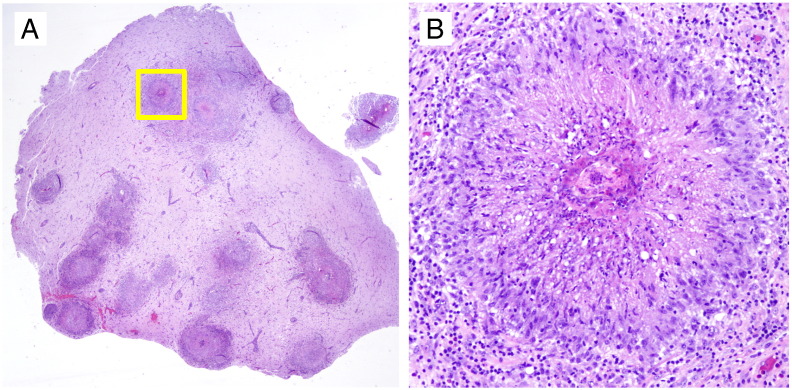
Fig. 5.
Extensive granulomas are present in the temporal cortex. (A) A paraffin section of the formalin-fixed temporal lobe biopsy shows reactive brain tissue extensively involved by prominent granuloma formation and reactive gliosis. (B) A magnified view of one granuloma pictured in the yellow box in (A) shows dense mixed inflammatory infiltrates comprised of eosinophils, plasma cells, and lymphocytes.
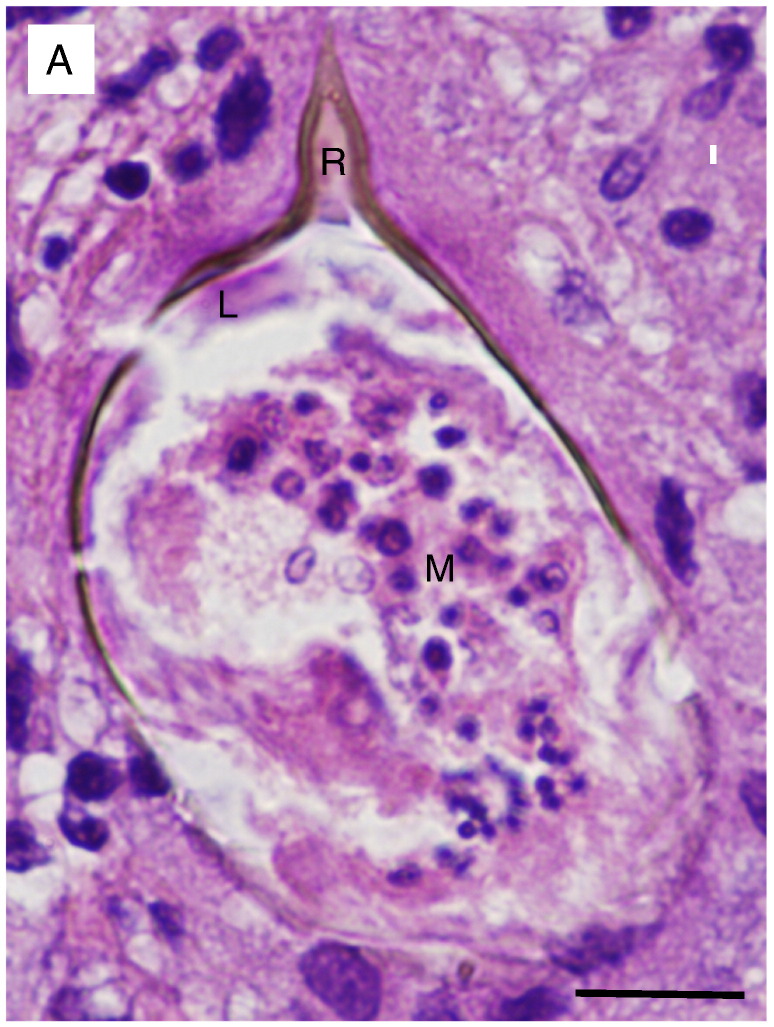
Fig. 6.
An infectious etiology: Schistosoma mansoni. (A) Magnification of the center of one of the granulomas reveals an egg with a refractile shell showing the pathognomonic acentric spine shape of Schistosoma mansoni. Reynolds' layer (R) has filled the spine but is not well defined with regard to the remaining egg shell. A single layer of squamous cells (von Lichtenberg's envelope) (L) is seen at the apex of the egg and is closely adherent to Reynold's layer. The envelope surrounds the developing miracidium (M) with multiple nuclei [14]. Neutrophils and histiocytes adjacent to the egg highlight the smaller size of the schistosome nuclei in comparison to those in human cells. (The image is taken at 40 × without a condenser in order to increase refraction and highlight the contour of the egg shell. Bar = 10 μm.).
Serum ELISA and antibody immunoblots performed at the Center for Disease Control and Prevention (CDC) confirmed exposure to S. mansoni and showed lack of exposure to S. haematobium, the other most prevalent schistosome species in Ghana. The S. mansoni AB FAST-ELISA produced a score of 100 (positive score considered > 10; 99% sensitivity for S. mansoni; 90% sensitivity for S. haematobium; 95% specificity for schistosomal infection). The S. mansoni AB immunoblot was positive, while the S. haematobium AB immunoblot was negative. Hence, the patient was diagnosed with cerebral schistosomiasis (neuroschistosomiasis) due to S. mansoni. Stool and urine were repeatedly negative for ova.
The patient was started on dexamethasone to control inflammation and received praziquantel (2 doses) immediately after the histologic diagnosis in order to kill any remaining adult schistosomes. The steroids were tapered over one month, at which point she received another course of praziquantel (2 doses). She was maintained on levetiracetam for seizures. She has had no additional symptoms in the subsequent twelve months. Subsequent serum analysis showed no eosinophilia, and stools continue to be negative for ova.
3. Discussion
Cerebral (encephalic) schistosomiasis was first described by Yamagiwa, a student of Rudolf Virchow, in 1889 [15]. It is most often associated with S. japonicum infection in East Asia, where that species is most prevalent, and has only occasionally been reported to be caused by S. mansoni or S. haematobium. In contrast, both S. mansoni and S. haematobium, but not S. japonicum, are endemic to Ghana, the likely source of infection for the current case [3]. Hence, this presentation of cerebral schistosomiasis due to S. mansoni four years after a single known exposure is an atypical presentation.
Cerebral schistosomiasis can present with variable clinical and imaging characteristics that can overlap with that of epilepsy, brain tumors, vasculitis, stroke, granulomatous disease, and other disorders [4]. Patients can present with a combination of partial or generalized tonic–clonic seizures, increased intracranial pressure, focal neurological deficits, and headache [16]. Electroencephalogram can help localize the disease in some cases but can also be normal in some patients [4], [17].
The lesions of neuroschistosomiasis can occur in both the cerebral hemispheres and cerebellum/brain stem. On postcontrast MRI, the lesions can appear as a large mass comprising multiple intensely enhancing nodules or as a central linear enhancement surrounded by multiple enhancing punctate nodules sometimes appearing “arborized.” Occasionally, they have also been reported to appear as nonenhancing irregular “finger-like” areas of abnormal signal consistent with increased edema [18], [19], [20]. However, the imaging characteristics of cerebral schistosomiasis due to S. mansoni, as in the current case, are not well described because of its comparable rarity. In the current case as well as in three other reported cases of cerebral schistosomiasis due to S. mansoni, the imaging does appear to show a similar pattern of enhancing punctate nodules clustered irregularly around some linear enhancement, with surrounding edema. Thus, this imaging pattern may be generally applicable to cerebral schistosomiasis due to both S. mansoni and S. japonicum [10], [11].
Direct pathologic evaluation of the involved tissue, as in the current case, is still the gold standard for diagnosis. Observation of the spine placement on the egg shell (lateral for S. mansoni, midline for S. haematobium, and absent for S. japonicum) is pathognomonic for each species. However, a combination of the serum indirect hemagglutination (IHA) test and enzyme-linked immunosorbent assay (ELISA) shows high sensitivity and specificity for S. mansoni, and both are often used in addition to histologic evaluation to confirm the species diagnosis, especially if intact eggs are not identified on histologic sections [16], [21]. Other tests such as evaluation of stool and urine for ova can be very useful if positive but are often negative. Overall, as in our case, a combination of these diagnostic approaches is required [4].
Schistosomiasis pathology is driven by host inflammatory response to the eggs and hence correlates with where the eggs are deposited. Schistosoma mansoni and S. japonicum can cause Katayama fever, hepatic perisinusoidal egg granulomas, and periportal fibrosis and portal hypertension, whereas S. haematobium is more frequently associated with hematuria and bladder wall scarring [3], [5]. Moreover, specific schistosome species are associated with the development of specific cancers, such as the correlation between S. haematobium infection and the development of squamous cell carcinoma of the bladder. In areas where S. haematobium is endemic, squamous cell carcinoma of the bladder is the leading form of cancer in the population. Other studies have likewise suggested links between S. japonicum and hepatocellular carcinoma and between S. mansoni and colorectal adenocarcinoma, although more evidence is needed [8].
Treatment of schistosomiasis consists of antischistosomal therapy (i.e., praziquantel) plus corticosteroids to control the inflammation [22]. Some cases involving the posterior fossa with resulting mass effect may also require treatment with surgical resection [20]. While the current patient showed involvement of both the cerebral cortex and cerebellum, there was no significant mass effect, and the patient was treated successfully with medical therapy after the diagnostic biopsy. Anticonvulsants are frequently needed for a period of time, but studies suggest that long-term antiepileptic therapy is not required in most cases of cerebral schistosomiasis [17]. Unfortunately, neuroschistosomiasis can sometimes result in significant postexposure chronic sequelae including medically refractory epilepsy that may require surgical treatment in the case of cerebral schistosomiasis and paralysis in the case of spinal cord schistosomiasis [4], [23]. The worst outcomes have been seen in spinal cord schistosomiasis where some studies have reported that only 15% of treated patients experience a complete recovery, while other studies have shown that early aggressive treatment can lead to full recovery in up to 65% of patients [4], [24].
Of note, other schistosome species are found indigenously in the U.S. but die after penetrating human skin, giving rise to a similar initial allergic condition called “swimmer's itch”, or cercarial dermatitis, but not progressing to the other manifestations of the disease. The primary hosts for these other species are instead water fowl (Austrobilharzia variglandis, Microbilharzia sp., Trichobilharzia ocellata, Trichobilharzia physella, Trichobilharzia stagnicolae) and rodents (Schistosomatium douthitti) [25]. Because the specific snail species required as intermediate hosts for S. japonicum, S. mansoni, and S. haematobium are not present in the United States, the transmission of these organisms in the United States waterways is currently unlikely [25].
4. Conclusions
In summary, we describe the atypical combination of cerebral schistosomiasis due to S. mansoni, after a prolonged four-year interval, from apparently a single exposure. The imaging characteristics of irregular nodular enhancement seen in this case mirror those reported in the much more frequent cerebral schistosomiasis due to S. japonicum and support previous suggestions that these imaging findings are applicable to cerebral schistosomiasis caused by both species. The clinical manifestations of cerebral schistosomiasis are variable and can overlap with that of epilepsy, brain tumors, vasculitis, and stroke. Hence, a combination of history, imaging data, laboratory findings, and pathologic analysis is critical for diagnosis.
Disclosures
All authors report no disclosures.
Acknowledgments
We thank the Longwood Neuropathology Training Program of Brigham and Women's Hospital, Boston Children's Hospital, Beth Israel Deaconess Medical Center, and Harvard Medical School. We also thank Marian Slaney and Sebastian Valentin of the Brigham and Women's Hospital clinical neuropathology laboratory for their technical expertise in histological preparation.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Matthew F. Rose, Email: mfrose@bics.bwh.harvard.edu.
Shakti H. Ramkissoon, Email: sramkissoon@partners.org.
References
- 1.Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Contis G., David A.R. The epidemiology of Bilharzia in ancient Egypt: 5000 years of schistosomiasis. Parasitol Today. 1996;12(7):253–255. [Google Scholar]
- 3.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368(9541):1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari T.C.A., Moreira P.R.R. Neuroschistosomiasis: clinical symptoms and pathogenesis. Lancet Neurol. 2011;10(9):853–864. doi: 10.1016/S1474-4422(11)70170-3. [DOI] [PubMed] [Google Scholar]
- 5.Kradin R.L. Saunders Elsevier; Philadelphia, PA: 2010. Diagnostic pathology of infectious disease. [Google Scholar]
- 6.Kumar V., Abbas A.K., Nelson F., Aster J.C. Eighth ed. Saunders/Elsevier; Philadelphia, PA: 2010. Robbins and Cotran pathologic basis of disease. [Google Scholar]
- 7.Da Silva A.J., Moser M. Public Health Image Library, Centers for Disease Control and Prevention; 2002. Illustration of the life cycle of the parasitic agents responsible for causing schistosomiasis [Internet] [Available from: http://phil.cdc.gov/phil/details.asp?pid=3417] [Google Scholar]
- 8.Palumbo E. Association between schistosomiasis and cancer: a review. Infect Dis Clin Pract. 2007;15(3):145–148. [Google Scholar]
- 9.Carod Artal F.J. Cerebral and spinal schistosomiasis. Curr Neurol Neurosci Rep. 2012;12(6):666–674. doi: 10.1007/s11910-012-0305-4. [DOI] [PubMed] [Google Scholar]
- 10.Ropper A.H., Stemmer-Rachamimov A. Case 21-2001. A 31-year-old man with an apparent seizure and a mass in the right parietal lobe. N Engl J Med. 2001;345(2):126–131. doi: 10.1056/NEJM200107123450208. [DOI] [PubMed] [Google Scholar]
- 11.Sanelli P.C., Lev M.H., Gonzalez R.G., Schaefer P.W. Unique linear and nodular MR enhancement pattern in schistosomiasis of the central nervous system: report of three patients. AJR Am J Roentgenol. 2001;177(6):1471–1474. doi: 10.2214/ajr.177.6.1771471. [DOI] [PubMed] [Google Scholar]
- 12.Pittella J.E. Neuroschistosomiasis. Brain Pathol Zurich Switz. 1997;7(1):649–662. doi: 10.1111/j.1750-3639.1997.tb01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scrimgeour E.M., Gajdusek D.C. Involvement of the central nervous system in Schistosoma mansoni and S. haematobium infection. A review. Brain J Neurol. 1985;108(Pt 4):1023–1038. doi: 10.1093/brain/108.4.1023. [DOI] [PubMed] [Google Scholar]
- 14.Neill P.J., Smith J.H., Doughty B.L., Kemp M. The ultrastructure of the Schistosoma mansoni egg. Am J Trop Med Hyg. 1988;39(1):52–65. doi: 10.4269/ajtmh.1988.39.52. [DOI] [PubMed] [Google Scholar]
- 15.Yamagiwa K. Beitrage zur aetiologie der jacksonchen epilepsie. Virchows Arch Pathol Anat. 1889;119:449–460. [Google Scholar]
- 16.Wu L., Wu M., Tian D., Chen S., Liu B., Chen Q. Clinical and imaging characteristics of cerebral schistosomiasis. Cell Biochem Biophys. 2012;62(2):289–295. doi: 10.1007/s12013-011-9294-1. [DOI] [PubMed] [Google Scholar]
- 17.Betting L.E., Pirani C., Jr., de Souza Queiroz L., Damasceno B.P., Cendes F. Seizures and cerebral schistosomiasis. Arch Neurol. 2005;62(6):1008–1010. doi: 10.1001/archneur.62.6.1008. [DOI] [PubMed] [Google Scholar]
- 18.Liu H.-Q., Feng X.-Y., Yao Z.-W., Sun H.-P. Characteristic magnetic resonance enhancement pattern in cerebral schistosomiasis. Chin Med Sci J Chung-Kuo Hsüeh Ko Hsüeh Tsa Chih Chin Acad Med Sci. 2006;21(4):223–227. [PubMed] [Google Scholar]
- 19.Liu H., Lim C.C.T., Feng X., Yao Z., Chen Y., Sun H. MRI in cerebral schistosomiasis: characteristic nodular enhancement in 33 patients. Am J Roentgenol. 2008;191(2):582–588. doi: 10.2214/AJR.07.3139. [DOI] [PubMed] [Google Scholar]
- 20.Shu K., Zhang S., Han L., Lei T. Surgical treatment of cerebellar schistosomiasis. Neurosurgery. 2009;64(5):941–943. doi: 10.1227/01.NEU.0000344000.79237.88. [discussion 943–944] [DOI] [PubMed] [Google Scholar]
- 21.Van Gool T., Vetter H., Vervoort T., Doenhoff M.J., Wetsteyn J., Overbosch D. Serodiagnosis of imported schistosomiasis by a combination of a commercial indirect hemagglutination test with Schistosoma mansoni adult worm antigens and an enzyme-linked immunosorbent assay with S. mansoni egg antigens. J Clin Microbiol. 2002;40(9):3432–3437. doi: 10.1128/JCM.40.9.3432-3437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross A.G.P., Bartley P.B., Sleigh A.C., Olds G.R., Li Y., Williams G.M. Schistosomiasis. N Engl J Med. 2002;346(16):1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 23.Carod-Artal F.J. Neurological complications of Schistosoma infection. Trans R Soc Trop Med Hyg. 2008;102(2):107–116. doi: 10.1016/j.trstmh.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Freitas A.R.R., Oliveira A.C.P., Silva L.J. Schistosomal myeloradiculopathy in a low-prevalence area: 27 cases (14 autochthonous) in Campinas, São Paulo, Brazil. Mem Inst Oswaldo Cruz. 2010;105(4):398–408. doi: 10.1590/s0074-02762010000400009. [DOI] [PubMed] [Google Scholar]
- 25.Brant S.V., Loker E.S. Schistosomes in the southwest United States and their potential for causing cercarial dermatitis or “swimmer's itch”. J Helminthol. 2009;83(2):191–198. doi: 10.1017/S0022149X09308020. [DOI] [PMC free article] [PubMed] [Google Scholar]