Abstract
Burst suppression (BS) is an electroencephalogram (EEG) pattern that is characterized by brief bursts of spikes, sharp waves, or slow waves of relatively high amplitude alternating with periods of relatively flat EEG or isoelectric periods. The pattern is usually associated with coma, severe encephalopathy of various etiologies, or general anesthesia. We describe an unusual case of anoxic brain injury in which a BS pattern was seen during behaviorally defined sleep during a routine outpatient EEG study.
Keywords: Burst suppression, Sleep, Disconnection hypothesis
1. Case
A three-year-old male patient was referred to the outpatient EEG laboratory for a routine study. He was the product of normal pregnancy and delivery. At the age of one, he was accidentally administered a neuromuscular blocker instead of intended antibiotics. As a result, he suffered respiratory arrest without loss of cardiac output and was subsequently intubated, ventilated, and admitted to the intensive care unit (ICU). A few days later, a head computed tomography (CT) scan showed generalized edema. His EEG did not show evolving or organized patterns to suggest electrographic seizures.
Following extubation, he had intact brainstem reflexes, but his neurological exam was notable for unresponsiveness, inability to follow commands or to track, decorticate posturing, minimal spontaneous limb movement, diffuse hypertonia, hyperreflexia, and sustained clonus. By the time he left the hospital, he was able to feed, demonstrate spontaneous (albeit small and infrequent) movements, selectively smile in response to his parent's voice, and cease whining in response to being held. Behaviorally defined sleep–wake cycling was irregular, and he generally slept only a few hours each day. A magnetic resonance imaging (MRI) was consistent with confluent peripheral and central patterns of hypoxic–ischemic injury.
At the age of three, his parents became concerned about possible seizures. They reported staring spells and behavioral arrest lasting seconds at the frequency of six times per month. These episodes were described as a fixed gaze in whatever direction he was looking. There was no evidence of automatisms or limb jerking. An EEG was requested to evaluate for possible seizures.
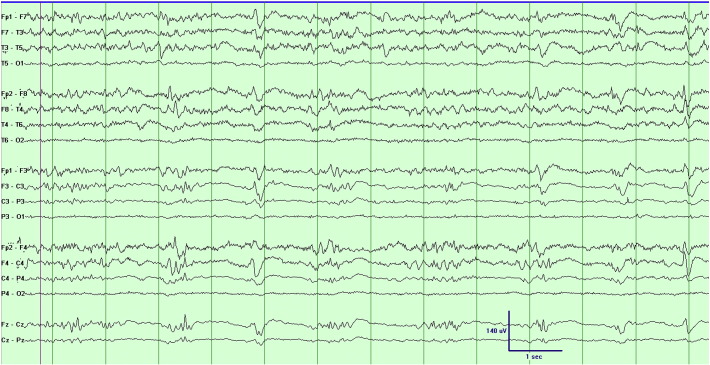
Fig. 1 (bipolar montage) and Fig. 2 (average reference montage of the same epoch) illustrate the EEG during the awake state with eyes open. The awake EEG shows continuous frontally-predominant background activity, with frequent multifocal epileptiform spikes, maximal alternately over the right or left frontal head regions and occasionally bifrontally. The EEG during behaviorally defined sleep (eyes closed, no movement) is shown in Fig. 3 (bipolar montage) and Fig. 4 (average reference montage of the same epoch), demonstrating a discontinuous background with a BS pattern consisting of alternating periods of diffuse background attenuation and bursts of fast activity with embedded spikes. No electrographic seizures were recorded.
Fig. 1.
EEG (bipolar montage) during the awake state with eyes open.
Fig. 2.
EEG (average reference montage of the same epoch in Fig. 1) during the awake state with eyes open.
Fig. 3.
The EEG (bipolar montage) during behaviorally defined sleep.
Fig. 4.
The EEG (average reference montage of the same epoch in Fig. 3) during behaviorally defined sleep.
2. Discussion
Burst suppression is an EEG pattern which consists of alternative periods of slow waves of high amplitude (the burst) and periods of flat EEG (the suppression) and was first described by Swank and Watson in 1949 [1], [2]. Burst suppression is usually observed in severe brain pathology [3] such as hypoxic–ischemic encephalopathies, coma of various causes, some forms of long-standing status epilepticus [4], and drug-related intoxication. It is also seen in hypothermia and general anesthesia. Burst suppression also occurs in the context of childhood encephalopathies such as early infantile myoclonic encephalopathy (EIME) and Ohtahara syndrome [5], [6]. A new lethal case of joint contractures, facial abnormalities, pachygyria plus early-onset encephalopathy with a BS pattern has also been described [7]. Burst suppression has also been reported in patients with dengue encephalopathy [8]. Burst suppression is usually associated with a poor prognosis, especially in the context of anoxic–ischemic encephalopathy [9].
Our current understanding of the physiological mechanisms responsible for generating burst-suppression EEG in humans is incomplete. According to the “disconnection hypothesis”, burst suppression arises as a spontaneous synchronized cortical rhythm whenever the cortex is diffusely disconnected from thalamic input. This idea is supported by experiments in which either pharmacological disconnection or surgical anatomical isolation of the cortex results in burst suppression [10], [11].
The case described herein is the first case in which we have observed a reversible burst-suppression EEG pattern consistently associated with behavioral sleep [12]. This case can be viewed within the framework of the disconnection hypothesis if we hypothesize that sleep is also a state of cortical disconnection. Thalamic reticular nucleus-driven hyperpolarization of thalamocortical neurons projecting to the cortex has been implicated in nonrapid-eye-movement sleep oscillations. We speculate that the wake-to-sleep state switch in this case was altered in a manner that resulted in reversible disconnection. Assessing the recuperative value of this pattern in the setting of severe neurological impairment is challenging. It is interesting to speculate in the context of recent work [13] attempting to “replace” sleep with time under anesthesia that, in this case, a BS pattern is subserving at least some role in sleep homeostasis.
Conflict of interest
None.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Amzica F. Basic physiology of burst-suppression. Epilepsia. 2009;50(s12):38–39. doi: 10.1111/j.1528-1167.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 2.Swank R., Watson C. Effects of barbiturates and ether on spontaneous electrical activity of dog brain. J Neurophysiol. 1949;12:137–160. doi: 10.1152/jn.1949.12.2.137. [DOI] [PubMed] [Google Scholar]
- 3.Niedermeyer E., Sherman D., Geocadin R., Hansen H., Hanley D. The burst-suppression electroencephalogram. Clin Electroencephalogr. 1999;30(3):99. doi: 10.1177/155005949903000305. [DOI] [PubMed] [Google Scholar]
- 4.Van Putten M., Van Putten M. Discovery of recurrent multiple brain states in non-convulsive status epilepticus. Clin Neurophysiol. 2007;118(12):2798–2804. doi: 10.1016/j.clinph.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Clarke M., Gill J., Noronha M., Mckinlay I. Early infantile epileptic encephalopathy with suppression burst: Ohtahara syndrome. Dev Med Child Neurol. 1987;29(4):520–528. doi: 10.1111/j.1469-8749.1987.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamatogi Y., Ohtahara S. Early-infantile epileptic encephalopathy with suppression-bursts, Ohtahara syndrome; its overview referring to our 16 cases. Brain Dev. 2002;24(1):13–23. doi: 10.1016/s0387-7604(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 7.Guerra M., Hrachovy R., Lugli L., Mizrahi E., Ferrari F. A new lethal case of joint contractures, facial abnormalities, pachygyria plus early-onset encephalopathy with a suppression-burst EEG pattern. Eur J Paediatr Neurol. 2007;11(5):318–321. doi: 10.1016/j.ejpn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Liou L., Lan S., Lai C. Electroencephalography burst suppression in a patient with dengue encephalopathy: a case report. Clin Neurophysiol. 2008;119(10):2205–2208. doi: 10.1016/j.clinph.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Smith S. EEG in neurological conditions other than epilepsy: when does it help, what does it add? J Neurol Neurosurg Psychiatry. 2005;76(Suppl. 2):8–12. doi: 10.1136/jnnp.2005.068486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steriade M., Amzica F., Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol. 1994;90(1):1–16. doi: 10.1016/0013-4694(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 11.Niedermeyer E. The burst-suppression electroencephalogram. Am J Electroneurodiagnostic Technol. 2009;49(4):333. [PubMed] [Google Scholar]
- 12.Brown E., Lydic R., Schiff N. General anesthesia, sleep, and coma. N Engl J Med. 2010;363(27):2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson A., Faraguna U., Tononi G., Cirelli C. Effects of anesthesia on the response to sleep deprivation. Sleep. 2010;33(12):1659. doi: 10.1093/sleep/33.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]