Abstract
Identifying the epileptogenic zone (EZ) in patients with refractory nonlesional frontal lobe epilepsy is frequently challenging. Intracranial EEG (icEEG) recordings are often required to better delineate the EZ, but the presence of an extensive network of connections allowing rapid ictal spread may result in bilateral homologous regional (or extremely diffuse) electrical ictal patterns. Here, we report a case where callosotomy performed after a first nonlateralizing icEEG study allowed for adequate identification of the EZ. The patient, an 18-year-old left-handed woman with daily atonic spells, had synchronous interictal and ictal epileptic activity from both supplementary motor areas (SMAs) during icEEG. Anterior partial callosotomy localized the EZ to the right SMA, as seizures were no longer associated with mirror-image ictal activity over the left SMA. Right SMA resection led to seizure freedom (follow-up of 23 months). This case exemplifies how a partial callosotomy followed by further icEEG recordings may adequately localize the EZ when initial icEEG recordings reveal bilateral synchronous focal or regional ictal activities.
Keywords: Anterior corpus callosotomy, Focal atonic seizures, Multistep invasive monitoring, Epilepsy surgery, Mirror focus
1. Introduction
Identifying the epileptogenic zone (EZ) is often challenging [1], [2] in patients with refractory nonlesional frontal lobe epilepsy. Reasons include the varied nonspecific clinical manifestations due to rapid spread of the epileptic activity to other regions through conduction pathways and the low spatiotemporal resolution of currently available noninvasive localization techniques [3], [4]. Intracranial EEG (icEEG) recordings are frequently required to better delineate the EZ [5], but the presence of an extensive network of connections allowing rapid ictal spread may result in bilateral homologous regional (or extremely diffuse) electrical ictal patterns. A slow continuous spread of ictal activity is associated with significantly better surgical outcomes than a fast continuous spread [6]. Here, we report a case where callosotomy performed after a first nonlateralizing icEEG study allowed for the adequate identification of the EZ.
2. Case report
The patient is an 18-year-old left-handed woman of normal intellect who began experiencing seizures at 12 years of age. The spells were characterized by sudden flaccidity of the lower extremities, a sense of a loss of balance, and feeling as if a heavy weight was falling on her head followed by a sudden fall and the inability to stand up for 10 to 15 s. At this point, the patient claimed to be unable to move her extremities. If supine, she would report a sensation of continuously falling into the bed for 10–15 s. Video-EEG recording also revealed episodes of awakening with or without minimal motor movements or extension of upper or lower extremities. The episodes would recur an average of 10 times per day with a peak frequency of more than 100 per day. On two occasions since the episodes began, these spells evolved into generalized tonic–clonic convulsions. After 8 antiepileptic drugs failed to improve her seizures, a presurgical evaluation was performed.
Her high-resolution brain 3-T MRI was normal. Despite a relatively early injection (15 s), the ictal SPECT showed multiple activation sites (bilateral temporal, orbitofrontal, and inferior frontal gyri). A second ictal SPECT showed a right mesial temporal activation. 18F-fluorodeoxyglucose positron emission tomography scan was nonlocalizing. Electroencephalography-correlated functional magnetic resonance imaging revealed maximal bold activations in the right cingulate and right superior frontal gyri. Functional magnetic resonance imaging for language showed bilateral activation without clear lateralization. Magnetoencephalography supported a mesial frontal focus with medial and lateral sources in front of the central sulcus bilaterally. Neuropsychological testing showed no significant deficits. More than 30 seizures, mostly nocturnal, were captured during video-EEG associated with arousal from sleep and minimal motor movements of the trunk and extremities. The ictal EEG consisted of repetitive central spikes followed within 3 s by diffuse tonic low-voltage fast activity admixed with muscle artifacts.
We proceeded with an intracranial study that included the implantation of seven subdural strips on the left side and nine on the right side sampling the median frontal gyrus, cingulate gyrus, superior frontal gyrus, and supplementary motor area (SMA) bilaterally (Fig. 1).
Fig. 1.
3D representation of the intracranial EEG study: a total of 16 subdural strip electrodes (90 contacts) were inserted to sample the medial, orbital, and dorsolateral aspects of the frontal lobe on both sides.
3. Results
Numerous seizures were recorded during the invasive EEG study associated with an early ictal pattern of low-voltage fast activity over both SMAs. Interictal and ictal activity from the right SMA mirrored that of the left SMA and vice versa. Careful scrutiny of ictal manifestations or spreading epileptic activity was unhelpful in lateralizing the EZ (Fig. 2). The decision was eventually made to perform an anterior partial callosotomy and continue with additional invasive icEEG recordings (Fig. 3). This course of action allowed for the proper identification of the EZ to the anterior portion of the right SMA as seizures were no longer associated with mirror-image ictal activity over the left SMA. We did not observe any independent seizures from the left SMA. The patient then returned a third time to the operating room for the removal of the electrodes and resection of the right SMA (Fig. 4). Postoperatively, the patient presented with a left lower limb weakness recovering to near full strength within a month with physical therapy. The patient has had no seizures since surgery (Engel 1; FU = 23 months).
Fig. 2.
Intracranial seizure recording (6 examples) before corpus callosotomy (left), with bilateral synchronous seizure onset in the bilateral interhemispheric electrodes: low-voltage fast activity over both supplementary motor areas (SMAs). Interictal and ictal activity from the right SMA mirrored that of the left SMA. Intracranial seizure recording after anterior partial corpus callosotomy (right), with unilateral seizure onset (low-voltage fast activity) in the right interhemispheric electrodes, allowing the localization of the seizure onset on the right anterior portion of the SMA.
Fig. 3.
Sagittal MR T1-weighted cut showing the anterior corpus callosotomy (arrow) and some of the interhemispheric subdural strip electrodes.
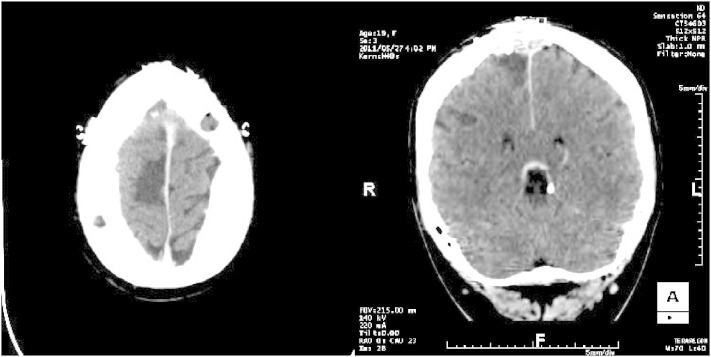
Fig. 4.
Axial and coronal views of the resected area (right supplementary motor area) on the postoperative CT.
4. Discussion
In frontal lobe epilepsy, an extensive network of connections allows for rapid ictal spread. This may result in bilateral homologous regional (or extremely diffuse) electrical ictal patterns, making it difficult to delineate the EZ [7], [8]. Clinically, this is reflected by a diversity of semiological features such as clonic activity, symmetric or asymmetric tonic posturing, complex gestural manifestations, or patterns of behavior [9]. We report here a patient with atonic seizures for whom callosotomy performed after a first nonlateralizing icEEG study allowed for the adequate identification of the EZ in the anterior portion of the right SMA. Although atonic seizures have traditionally been described as a clinical manifestation of generalized epilepsies [10], atonia has also been observed in patients with focal epilepsy [11]. The anterior portion of the supplementary motor area has been implicated in the pathophysiology of such focal atonic seizures as electrical stimulation of a ‘supplementary negative motor area’ can produce predominantly contralateral but also, to a lesser extent, ipsilateral atonia of predominantly limb muscles [12].
Few authors have previously noted that a partial anterior callosotomy followed by further icEEG recordings may be helpful in adequately localizing the EZ when icEEG recordings reveal bilateral synchronous focal or regional ictal activities. This was observed by Iwasaki et al. [13] with the lateralization of interictal spikes on scalp EEG after corpus callosotomy. Ono et al. reported using callosotomy in multistage epilepsy surgery [i.e., callosotomy (without prior icEEG) → icEEG → additional surgery)] for children with mostly cryptogenic or symptomatic refractory epilepsy [14]. Similarly, Lin et al. suggested that the addition of invasive monitoring for children undergoing corpus callosotomy for medically refractory symptomatic generalized or localization-related epilepsy could lead to the localization of surgically amenable seizure foci and targeted resections [15]. Finally, Silverberg et al. reported good outcomes in some patients who underwent a corpus callosotomy, followed at a later stage by focal resections after intracranial electroencephalography [16]. Our case differs slightly with intracranial monitoring as a first step, anterior callosotomy as a second step, further intracranial EEG recordings as a third step, and finally, a resection of the epileptogenic zone in the supplementary region in the fourth and final step, all during the same hospitalization.
In patients with cryptogenic or symptomatic generalized epilepsies, corpus callosotomy has been shown to help reduce the frequency of atonic seizures and drop attacks presumably by disrupting the rapid bilateral seizure spread that is responsible for sudden loss of consciousness or loss of posture [17], [18]. Tanriverdi et al. confirmed that drop attacks had a favorable outcome in 74.1% of patients, with 38.7% becoming free of this type of seizures [19]. Not unexpectedly, patients are rarely free of other seizure types [17]. Our patient attained seizure freedom after corpus callosotomy followed by resective surgery as only a single focal EZ was found.
We note with interest here that despite the fact that our patient had long-standing high-frequency seizures, a contralateral mirror focus was not observed [20], [21], [22]. Frank Morell first described the concept of secondary epileptogenesis in experimental animal models. A primary epileptogenic region generated epileptogenic potentials in a secondary epileptic region (histologically normal) via neuronal connections with the primary area [23], [24], [25], [26]. However, in human epilepsy, this concept remains controversial. We also note how well the patient recovered from the postoperative contralateral limb weakness as one could surmise that an anterior two-third callosotomy could impair recovery of motor function following SMA resection by limiting the recruitment of the contralateral healthy hemisphere [27], [28]. It is possible that the recruitment of areas in the vicinity of the resected area such as the lateral premotor cortex accounts for the excellent recovery [29].
5. Conclusion
Our case indicates that atonic seizures may arise from the anterior portion of the SMA and that anterior corpus callosotomy in a multistep invasive EEG monitoring followed by epilepsy surgery can be useful in selected patients with mesiofrontal epilepsy and nonlateralized icEEG findings.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
This work was not supported by a grant or otherwise. It was previously presented at the AES annual meeting, December 2011.
References
- 1.Cascino G.D. Surgical treatment for extratemporal epilepsy. Curr Treat Options Neurol. 2004;6(3):257–262. doi: 10.1007/s11940-004-0017-4. [DOI] [PubMed] [Google Scholar]
- 2.Jeha L.E., Najm I., Bingaman W., Dinner D., Widdess-Walsh P., Luders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130(Pt 2):574–584. doi: 10.1093/brain/awl364. [DOI] [PubMed] [Google Scholar]
- 3.Vulliemoz S., Vollmar C., Koepp M.J., Yogarajah M., O'Muircheartaigh J., Carmichael D.W. Connectivity of the supplementary motor area in juvenile myoclonic epilepsy and frontal lobe epilepsy. Epilepsia. 2011;52(3):507–514. doi: 10.1111/j.1528-1167.2010.02770.x. [DOI] [PubMed] [Google Scholar]
- 4.Widjaja E., Mahmoodabadi S.Z., Snead O.C., III, Almehdar A., Smith M.L. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia. 2011;52(9):1685–1691. doi: 10.1111/j.1528-1167.2011.03085.x. [DOI] [PubMed] [Google Scholar]
- 5.Au L., Leung H., Kwan P., Zhu X.L., Chan D.T., Wong H.T. Intracranial electroencephalogram to evaluate refractory temporal and frontal lobe epilepsy. Hong Kong Med J. 2011;17(6):453–459. [PubMed] [Google Scholar]
- 6.Kutsy R.L., Farrell D.F., Ojemann G.A. Ictal patterns of neocortical seizures monitored with intracranial electrodes: correlation with surgical outcome. Epilepsia. 1999;40(3):257–266. doi: 10.1111/j.1528-1157.1999.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 7.Falconer M.A., Kennedy W.A. Epilepsy due to small focal temporal lesions with bilateral independent spike-discharging foci. A study of seven cases relieved by operation. J Neurol Neurosurg Psychiatry. 1961;24:205–212. doi: 10.1136/jnnp.24.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jobst B.C., Siegel A.M., Thadani V.M., Roberts D.W., Rhodes H.C., Williamson P.D. Intractable seizures of frontal lobe origin: clinical characteristics, localizing signs, and results of surgery. Epilepsia. 2000;41(9):1139–1152. doi: 10.1111/j.1528-1157.2000.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 9.McGonigal A., Chauvel P. Frontal lobe epilepsy: seizure semiology and presurgical evaluation. Pract Neurol. 2004;4:260–273. [Google Scholar]
- 10.Widjaja E., Mahmoodabadi S.Z., Snead O.C., III, Almehdar A., Smith M.L. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia. 2011;52(9):1685–1691. doi: 10.1111/j.1528-1167.2011.03085.x. [DOI] [PubMed] [Google Scholar]
- 11.Kovac S., Diehl B. Atonic phenomena in focal seizures: nomenclature, clinical findings and pathophysiological concepts. Seizure. 2012;21:561–567. doi: 10.1016/j.seizure.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Lüders H.O., Dinner D.S., Morris H.H., Wyllie E., Comair Y.G. Cortical electrical stimulation in humans. The negative motor areas. Adv Neurol. 1995;67:115–129. [PubMed] [Google Scholar]
- 13.Iwasaki M., Nakasato N., Kakisaka Y., Kanno A., Uematsu M., Haginoya K. Lateralization of interictal spikes after corpus callosotomy. Clin Neurophysiol. 2011;122(11):2121–2127. doi: 10.1016/j.clinph.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Ono T., Baba H., Toda K., Ono K. Callosotomy and subsequent surgery for children with refractory epilepsy. Epilepsy Res. 2011;93(2–3):185–191. doi: 10.1016/j.eplepsyres.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Lin J.S., Lew S.M., Marcuccilli C.J., Mueller W.M., Matthews A.E., Koop J.I. Corpus callosotomy in multistage epilepsy surgery in the pediatric population. J Neurosurg Pediatr. 2011;7(2):189–200. doi: 10.3171/2010.11.PEDS10334. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg A., Parker-Menzer K., Devinsky O., Doyle W., Carlson C. Bilateral intracranial electroencephalographic monitoring immediately following corpus callosotomy. Epilepsia. 2010;51(10):2203–2206. doi: 10.1111/j.1528-1167.2010.02568.x. [DOI] [PubMed] [Google Scholar]
- 17.Abou-Khalil B.W. When should corpus callosotomy be offered as palliative therapy? Epilepsy Curr. 2010;10(1):9–10. doi: 10.1111/j.1535-7511.2009.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asadi-Pooya A.A., Sharan A., Nei M., Sperling M.R. Corpus callosotomy. Epilepsy Behav. Aug 2008;13(2):271–278. doi: 10.1016/j.yebeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Tanriverdi T., Olivier A., Poulin N., Andermann F., Dubeau F. Long-term seizure outcome after corpus callosotomy: a retrospective analysis of 95 patients. J Neurosurg. 2009;110(2):332–342. doi: 10.3171/2008.3.17570. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore R., Morris H., III, Van Ness P.C., Gilmore-Pollak W., Estes M. Mirror focus: function of seizure frequency and influence on outcome after surgery. Epilepsia. 1994;35(2):258–263. doi: 10.1111/j.1528-1157.1994.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 21.Morrell F., deToledo-Morrell L. From mirror focus to secondary epileptogenesis in man: an historical review. Adv Neurol. 1999;81:11–23. [PubMed] [Google Scholar]
- 22.Sampaio L., Yacubian E.M., Manreza M.L. The role of mirror focus in the surgical outcome of patients with indolent temporal lobe tumors. Arq Neuropsiquiatr. 2004;62(1):9–14. doi: 10.1590/s0004-282x2004000100002. [DOI] [PubMed] [Google Scholar]
- 23.Goddard G.V. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214(5092):1020–1021. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- 24.Morrell F. Secondary epileptogenic lesions. Epilepsia. 1960;1:538–560. doi: 10.1111/j.1528-1157.1959.tb04288.x. [DOI] [PubMed] [Google Scholar]
- 25.Morrell F. The role of secondary epileptogenesis in human epilepsy. Arch Neurol. 1991;48(12):1221–1224. doi: 10.1001/archneur.1991.00530240025011. [DOI] [PubMed] [Google Scholar]
- 26.Wilder B.J., King R.L., Schmidt R.P. Comparative study of secondary epileptogenesis. Epilepsia. 1968;9(4):275–289. doi: 10.1111/j.1528-1157.1968.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 27.Krainik A., Duffau H., Capelle L., Cornu P., Boch A.L., Mangin J.F. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62(8):1323–1332. doi: 10.1212/01.wnl.0000120547.83482.b1. [DOI] [PubMed] [Google Scholar]
- 28.Nair D.G., Hutchinson S., Fregni F., Alexander M., Pascual-Leone A., Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. NeuroImage. 2007;34(1):253–263. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg K., Nossek E., Liebling R., Fried I., Shapira-Lichter I., Hendler T. Prediction of neurological deficits and recovery after surgery in the supplementary motor area: a prospective study in 26 patients. J Neurosurg. 2010;113(6):1152–1163. doi: 10.3171/2010.6.JNS1090. [DOI] [PubMed] [Google Scholar]