Abstract
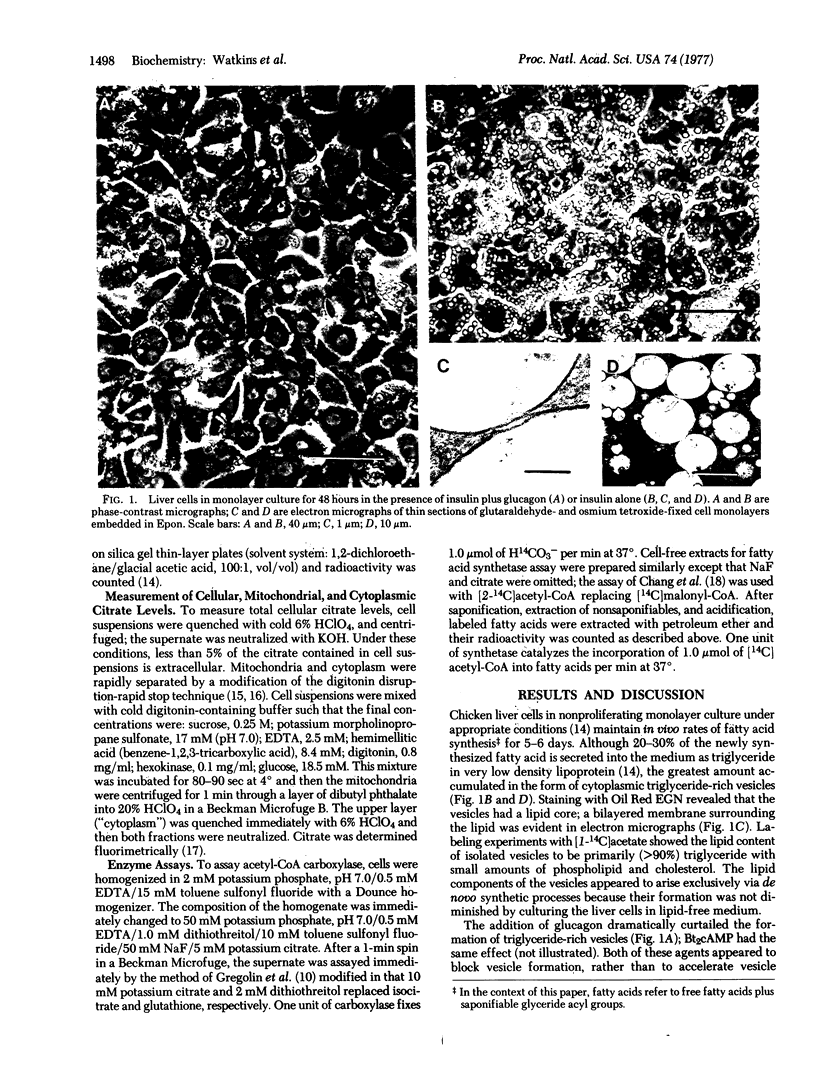
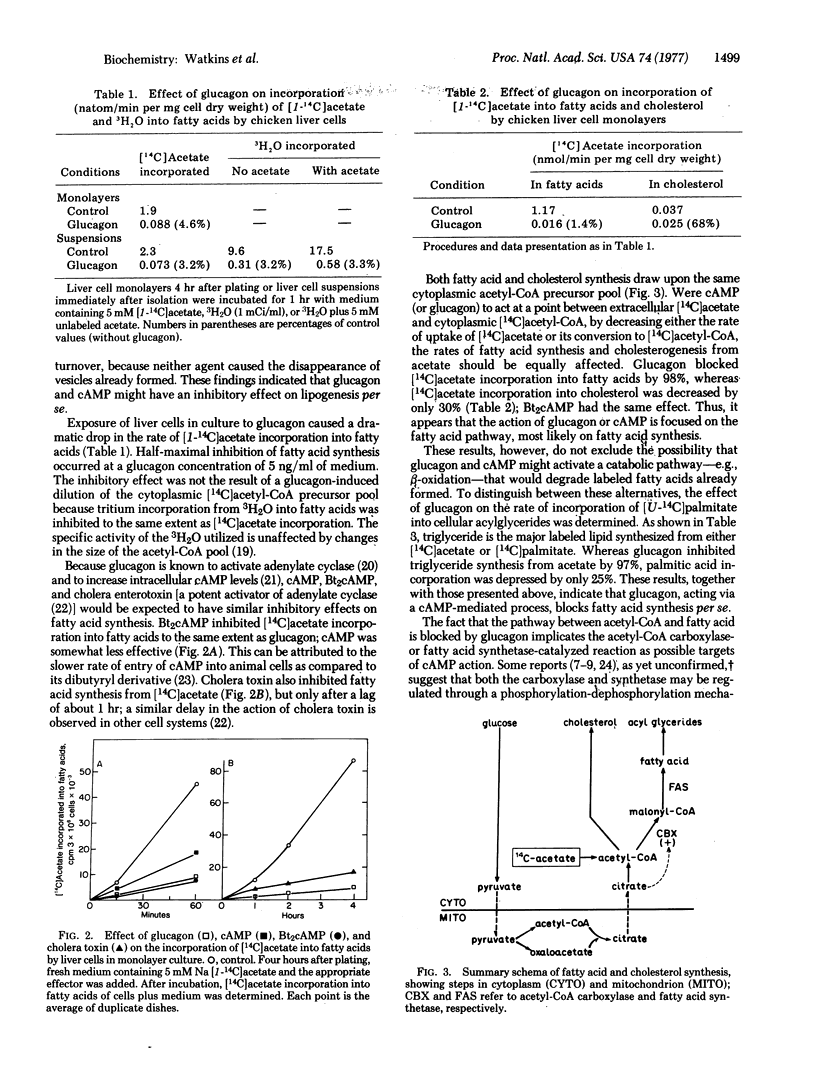
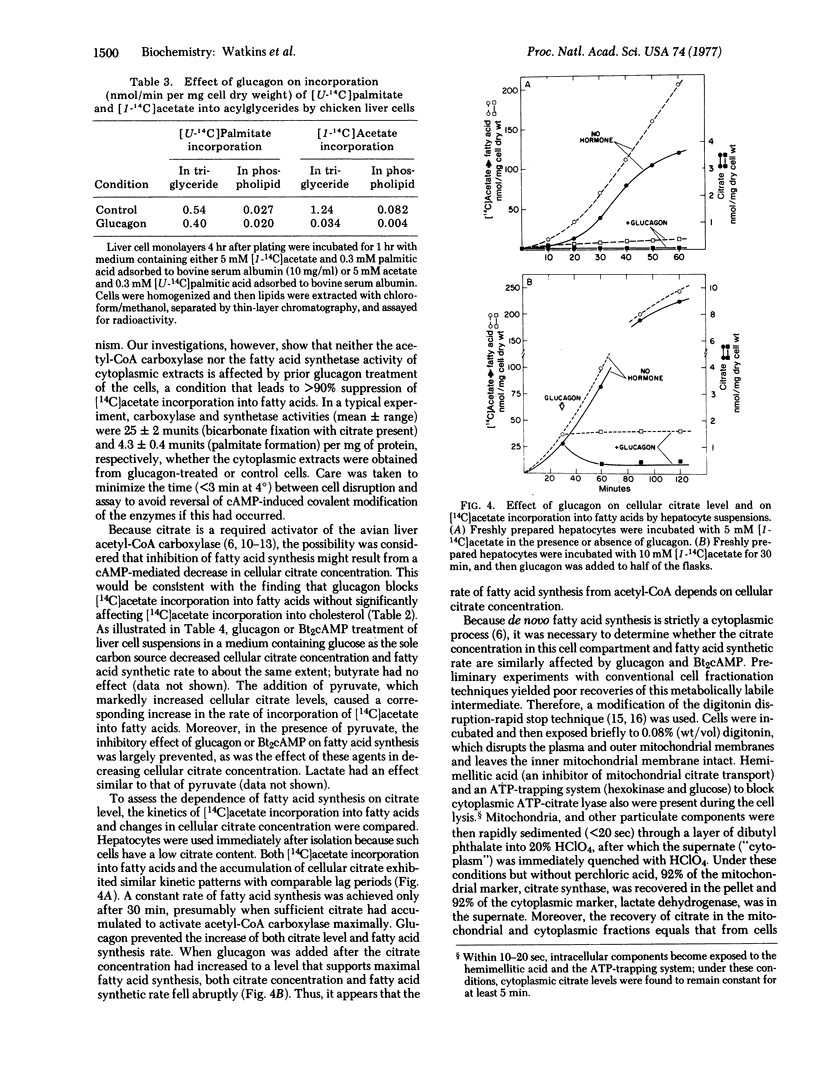
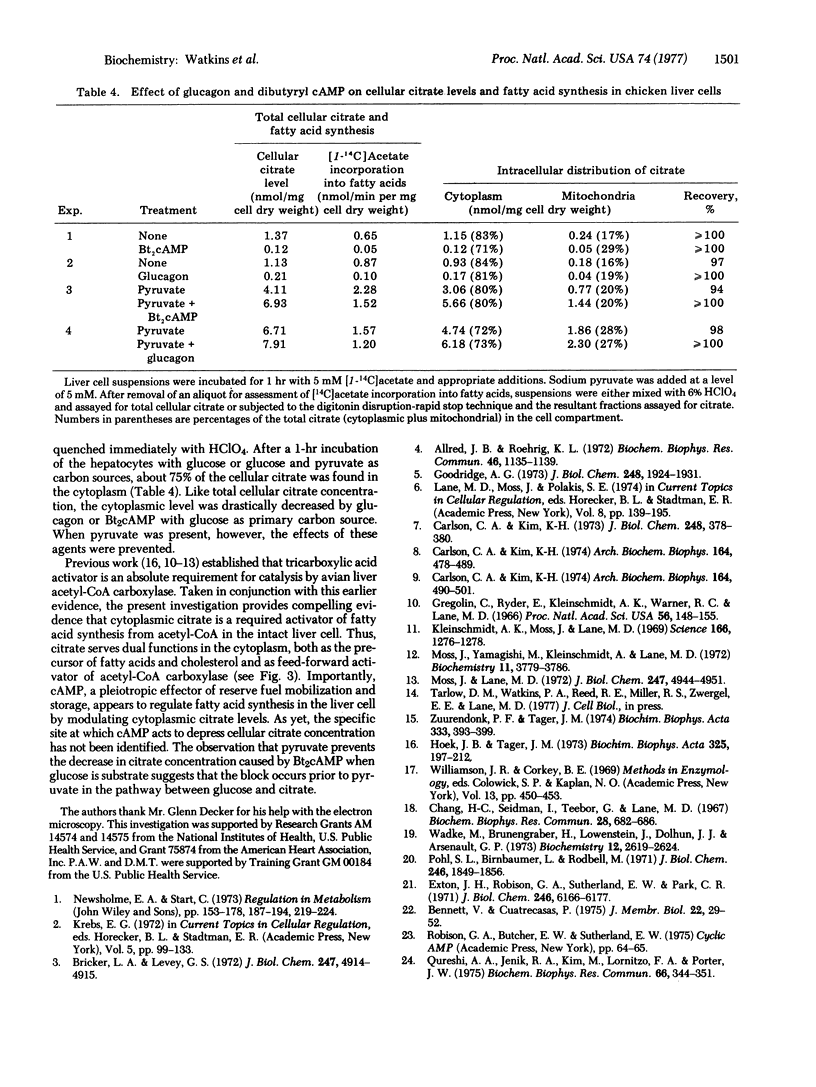
Labeling experiments with chicken liver cell monolayers and suspensions show that glucagon and N6, O2-dibutyryladenosine 3':5'-cyclic monophosphate (dibutyryl cyclic AMP) block fatty acid synthesis from acetate without appreciably affecting cholesterogenesis from acetate or acylglyceride synthesis from palmitate. Neither acetyl-CoA carboxylase [acetyl-CoA:carbon-dioxide ligase (ADP-forming), EC 6.4.1.2] activity assayed in the presence of citrate nor fatty acid synthetase activity is decreased in extracts of cells treated with glucagon. However, the cytoplasmic concentration of citrate, a required allosteric activator of acetyl-CoA carboxylase, is depressed more than 90% by glucagon or dibutyrl cyclic AMP. Pyruvate or lactate largely prevents the inhibitory action of these effectors on fatty acid synthesis by causing a large increase in cytoplasmic citrate level. Thus, it appears that glucagon, acting via cyclic AMP, inhibits fatty acid synthesis by blocking the formation of citrate, an essential activator of acetyl-CoA carboxylase.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred J. B., Roehrig K. L. Inhibition of hepatic lipogenesis by cyclic-3',5'-nucleotide monophosphates. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1135–1139. doi: 10.1016/s0006-291x(72)80092-5. [DOI] [PubMed] [Google Scholar]
- Bennett V., Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. J Membr Biol. 1975 Jun 3;22(1):29–52. doi: 10.1007/BF01868162. [DOI] [PubMed] [Google Scholar]
- Bricker L. A., Levey G. S. Evidence for regulatin of cholesterol and fatty acid synthesis in liver by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1972 Aug 10;247(15):4914–4915. [PubMed] [Google Scholar]
- Carlson C. A., Kim K. H. Differential effects of metabolites on the active and inactive forms of hepatic acetyl CoA carboxylase. Arch Biochem Biophys. 1974 Oct;164(2):490–501. doi: 10.1016/0003-9861(74)90059-9. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Kim K. H. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. Arch Biochem Biophys. 1974 Oct;164(2):478–489. doi: 10.1016/0003-9861(74)90058-7. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Kim K. H. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973 Jan 10;248(1):378–380. [PubMed] [Google Scholar]
- Chang H. C., Seidman I., Teebor G., Lane M. D. Liver acetyl CoA carboxylase and fatty acid synthetase: relative activities in the normal state and in hereditary obesity. Biochem Biophys Res Commun. 1967 Sep 7;28(5):682–686. doi: 10.1016/0006-291x(67)90369-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Robison G. A., Sutherland E. W., Park C. R. Studies on the role of adenosine 3',5'-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem. 1971 Oct 25;246(20):6166–6177. [PubMed] [Google Scholar]
- Goodridge A. G. Regulation of fatty acid synthesis in isolated hepatocytes prepared from the livers of neonatal chicks. J Biol Chem. 1973 Mar 25;248(6):1924–1931. [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Kleinschmidt A. K., Warner R. C., Lane M. D. Molecular characteristics of liver acetyl CoA carboxylase. Proc Natl Acad Sci U S A. 1966 Jul;56(1):148–155. doi: 10.1073/pnas.56.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J. B., Tager J. M. The oxidoreduction state of free NAD(P) and mass-action ratio of total nicotinamide nucleotides in isolated rat-liver mitochondria. Biochim Biophys Acta. 1973 Nov 22;325(2):197–212. doi: 10.1016/0005-2728(73)90096-0. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A. K., Moss J., Lane D. M. Acetyl coenzyme A carboxylase: filamentous nature of the animal enzymes. Science. 1969 Dec 5;166(3910):1276–1278. doi: 10.1126/science.166.3910.1276. [DOI] [PubMed] [Google Scholar]
- Moss J., Lane M. D. Acetyl coenzyme A carboxylase. 3. Further studies on the relation of catalytic activity to polymeric state. J Biol Chem. 1972 Aug 25;247(16):4944–4951. [PubMed] [Google Scholar]
- Moss J., Yamagishi M., Kleinschmidt A. K., Lane M. D. Acetyl coenzyme A carboxylase. Purification and properties of the bovine adipose tissue enzyme. Biochemistry. 1972 Sep 26;11(20):3779–3786. doi: 10.1021/bi00770a017. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Qureshi A. A., Jenik R. A., Kim M., Lornitzo F. A., Porter J. W. Separation of two active forms (holo-a and holo-b) of pigeon liver fatty acid synthetase and their interconversion by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1975 Sep 2;66(1):344–351. doi: 10.1016/s0006-291x(75)80334-2. [DOI] [PubMed] [Google Scholar]
- Wadke M., Brunengraber H., Lowenstein J. M., Dolhun J. J., Arsenault G. P. Fatty acid synthesis by liver perfused with deuterated and tritiated water. Biochemistry. 1973 Jul 3;12(14):2619–2624. doi: 10.1021/bi00738a011. [DOI] [PubMed] [Google Scholar]



