Abstract
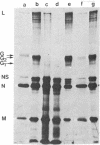
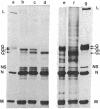
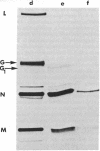
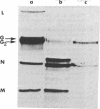
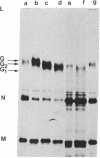
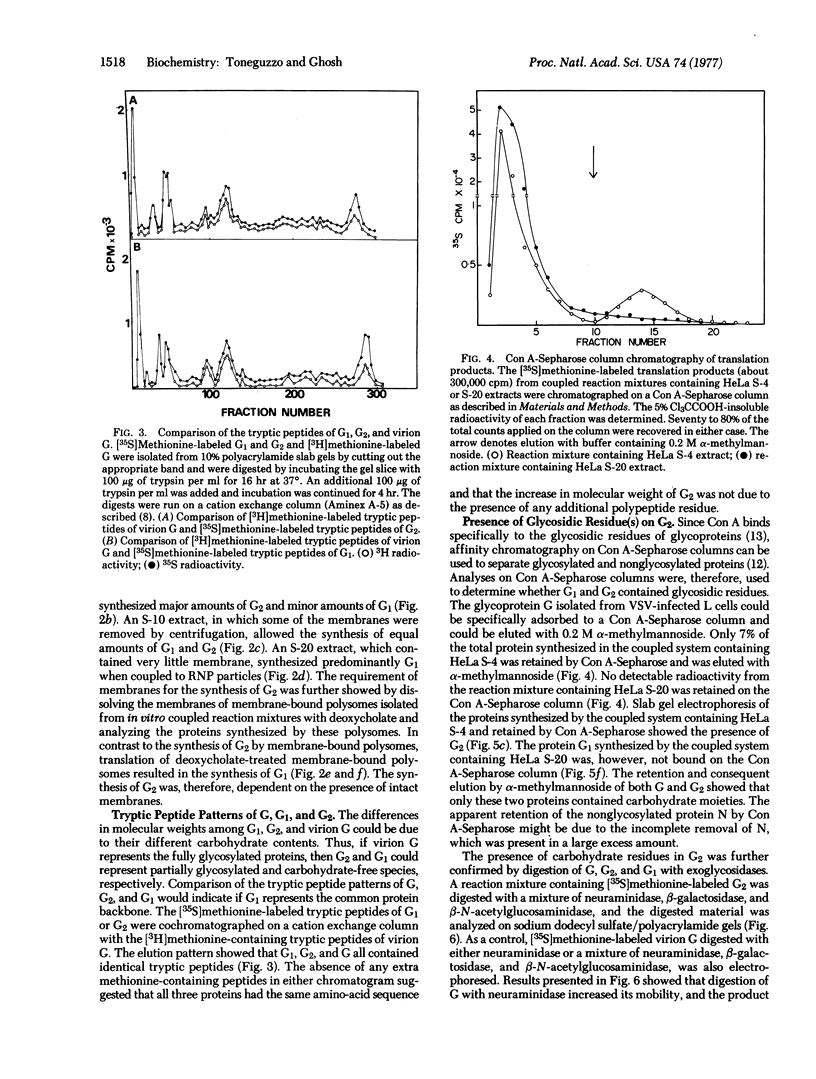
Coupling of ribonucleoprotein particles from L cells infected with vesicular stomatitis virus to a pre-incubated ribosomal system obtained from uninfected HeLa cells allowed synthesis of two proteins. G1 (molecular weight 63,000) and G2 (molecular weight 67,000), and all other proteins of vesicular stomatitis virus except the spike protein G (molecular weight 69,000). Analyses of the tryptic peptides showed that G1, G2, and G had identical peptide sequences. The synthesis of G2 required the presence of membranes; only G1 was synthesized in the absence of any membranes. G2 but not G1 was shown to be a glycoprotein by affinity chromatography on a concanavalin A-Sepharose column. Removal of sialic acid residues from G by neuraminidase resulted in a product having an identical mobility to G2. Digestion of G2 or G with a mixture of neuraminidase (EC 3.2.1.18), beta-galactosidase (EC 3.2.1.23), and beta-N-acetylglucosaminidase (EC 3.2.1.30), however, produced a protein of molecular weight 65,000. These data suggest that G2 is the desialated G and is formed by glycosylation of G1, which is the unglycosylated polypeptide backbone of G.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the viral mRNA species isolated from subcellular fractions of vesicular stomatitis virus-infected cells. J Virol. 1975 Apr;15(4):1012–1019. doi: 10.1128/jvi.15.4.1012-1019.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus grown in four mammalian cell lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4011–4014. doi: 10.1073/pnas.71.10.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P., Toneguzzo F., Wells S. Synthesis in vitro of vesicular stomatitis virus proteins in cytoplasmic extracts of L cells. Biochem Biophys Res Commun. 1973 Sep 5;54(1):228–233. doi: 10.1016/0006-291x(73)90912-1. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Ehrenfeld E., Summers D. F. In vitro synthesis of proteins by membrane-bound polyribosomes from vesicular stomatitis virus-infected HeLa cells. J Virol. 1974 Sep;14(3):560–571. doi: 10.1128/jvi.14.3.560-571.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Robertson J. S., Etchison J. R., Summers D. F. Glycosylation sites of vesicular stomatitis virus glycoprotein. J Virol. 1976 Sep;19(3):871–878. doi: 10.1128/jvi.19.3.871-878.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Eylar O. R., Wisseman C. L., Jr Isolation of the dengue virus envelope glycoprotein from membranes of infected cells by concanavalin A affinity chromatography. J Virol. 1976 Apr;18(1):132–140. doi: 10.1128/jvi.18.1.132-140.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Cell-free synthesis of vesicular stomatitis virus proteins: translation of membrane-bound polyribosomal mRNAs. FEBS Lett. 1975 Feb 15;50(3):369–373. doi: 10.1016/0014-5793(75)80530-8. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Characterization and translation of methylated and unmethylated vesicular stomatitis virus mRNA synthesized in vitro by ribonucleoprotein particles from vesicular stomatitis virus-infected L cells. J Virol. 1976 Feb;17(2):477–491. doi: 10.1128/jvi.17.2.477-491.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]