Abstract
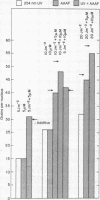
In normal human cells the amount of excision of ultraviolet damage to DNA saturates at high doses. In these cells some chemicals mimic ultraviolet damage as far as their biological and repair characteristics are concerned. One of these chemicals is N-acetoxy-2-acetylaminofluorene. We determined whether the limited repair capacity for ultraviolet damage was affected by treatment with N-acetoxy-2-acetylaminofluorene. To measure repair we determined unscheduled DNA synthesis and the number of sites sensitive to an ultraviolet endonuclease in an assay using an extract of Micrococcus luteus. The nuclease does not act on DNA treated with the chemical. The amount of unscheduled DNA synthesis due to a combined chemical and ultraviolet treatment was the sum of those observed from the separate treatments, even at saturation doses. The combined treatment did not affect the removal of nuclease-sensitive sites. We conclude that there are different rate-limiting steps in excision repair of the ultraviolet and the chemical damage and suggest a model involving a complex of enzymes to explain the data.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amacher D. E., Elliott J. A., Lieberman M. W. Differences in removal of acetylaminofluorene and pyrimidine dimers from the DNA of cultured mammalian cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1553–1557. doi: 10.1073/pnas.74.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Setlow R. B. Correlations between host-cell reactivation, ultraviolet reactivation and pyrimidine dimer excision in the DNA of bacteriophage lambda. J Mol Biol. 1970 Jul 14;51(1):131–144. doi: 10.1016/0022-2836(70)90275-5. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair with purines and pyrimidines in radiation- and carcinogen-damaged normal and xeroderma pigmentosum human cells. Cancer Res. 1973 Feb;33(2):362–369. [PubMed] [Google Scholar]
- Cleaver J. E., Trosko J. E. Absence of excision of ultraviolet-induced cyclobutane dimers in xeroderma pigmentosum. Photochem Photobiol. 1970 Jun;11(6):547–550. doi: 10.1111/j.1751-1097.1970.tb06025.x. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Enhancement of postreplication repair in Chinese hamster cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2396–2400. doi: 10.1073/pnas.73.7.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Kohn K. W., Kann H. E., Jr DNA single-strand breaks during repair of UV damage in human fibroblasts and abnormalities of repair in xeroderma pigmentosum. Proc Natl Acad Sci U S A. 1976 Jan;73(1):39–43. doi: 10.1073/pnas.73.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Kleijer W. J., Hoeksema J. L., Sluyter M. L., Bootsma D. Effects of inhibitors on repair of DNA in normal human and xeroderma pigmentosum cells after exposure to x-rays and ultraviolet irradiation. Mutat Res. 1973 Mar;17(3):385–394. doi: 10.1016/0027-5107(73)90010-9. [DOI] [PubMed] [Google Scholar]
- Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976 Jan 1;259(5538):64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- Miller J. A. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970 Mar;30(3):559–576. [PubMed] [Google Scholar]
- Paterson M. C., Lohman P. H., Sluyter M. L. Use of UV endonuclease from Micrococcus luteus to monitor the progress of DNA repair in UV-irradiated human cells. Mutat Res. 1973 Aug;19(2):245–256. doi: 10.1016/0027-5107(73)90083-3. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Setlow R. B., Faulcon F. M., Regan J. D. Defective repair of gamma-ray induced DNA damage in xeroderma pigmentosum cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1976 Feb;29(2):125–136. doi: 10.1080/09553007614550141. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Setlow J. K. Effects of radiation on polynucleotides. Annu Rev Biophys Bioeng. 1972;1:293–346. doi: 10.1146/annurev.bb.01.060172.001453. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Rice M., Wagner E. K. Xeroderma pigmentosum cells contain low levels of photoreactivating enzyme. Proc Natl Acad Sci U S A. 1975 Jan;72(1):103–107. doi: 10.1073/pnas.72.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Sekiguchi M., Okada Y. Restoration of ultraviolet-induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus). Proc Natl Acad Sci U S A. 1975 Oct;72(10):4071–4075. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosko J. E., Yager J. D. A sensitive method to measure physical and chemical carcinogen-induced "unscheduled DNA synthesis" in rapidly dividing eukaryotic cells. Exp Cell Res. 1974 Sep;88(1):47–55. doi: 10.1016/0014-4827(74)90616-8. [DOI] [PubMed] [Google Scholar]