Abstract
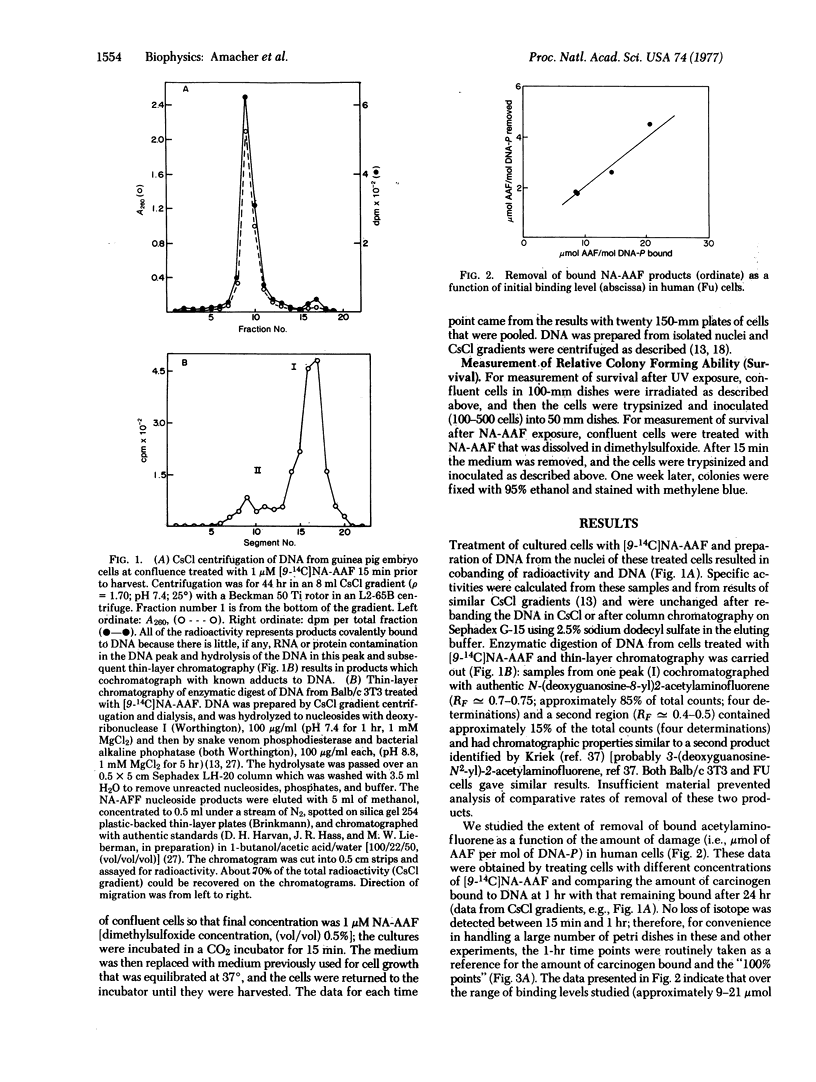
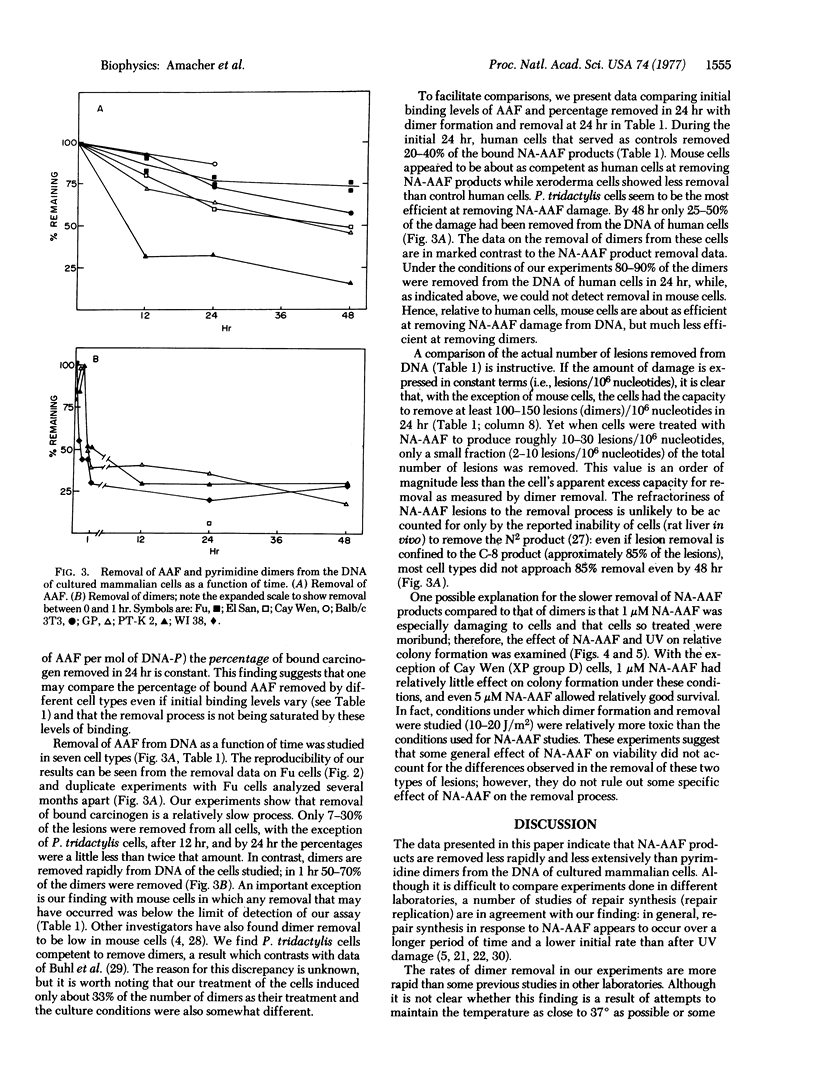
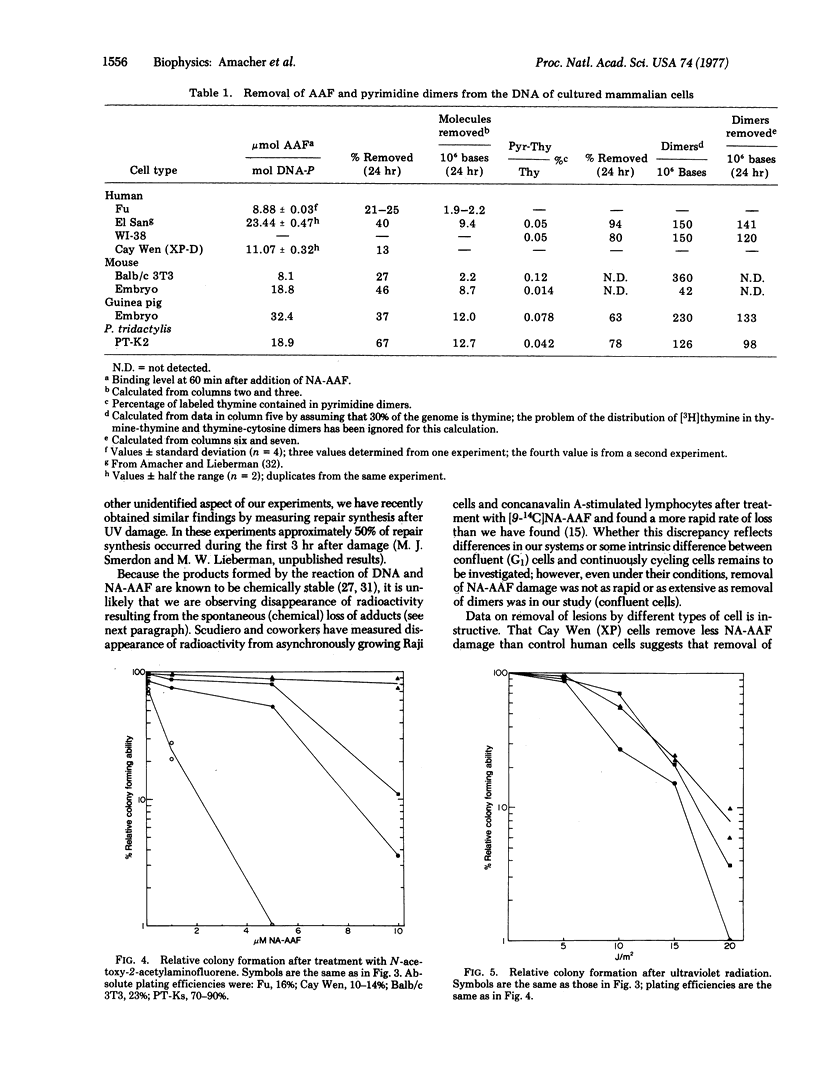
The rate and extent of disappearance of two DNA lesions (pyrimidine dimers and covalently bound acetylaminofluorene), both thought to be removed by the so-called wide-patch (approximately 100 nucleotides) repair process, were studied in a variety of cultured mammalian cells. With the exception of mouse cells, dimers were removed more rapidly and extensively than covalently bound acetylaminofluorene. In human cells, for example, about 50% of the dimers were excised from DNA in 1 hr while only 25-50% of the chemically induced lesions were excised from DNA after 48 hr. Surprisingly mouse cells, which remove few dimers, were about as competent as control human fibroblasts at removing acetylaminofluorene lesions; however, xeroderma pigmentosum cells (group D) removed fewer N-acetoxy-2-acetylaminofluorene-induced lesions than control human cells. Our data raise the possibility of separate repair processes for these two types of lesions and suggest that their expression may be under similar genetic control in human cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amacher D. E., Lieberman M. W. Removal of acetylaminofluorene from the DNA of control and repair-deficient human fibroblasts. Biochem Biophys Res Commun. 1977 Jan 10;74(1):285–290. doi: 10.1016/0006-291x(77)91406-1. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. DNA repair in Potorous tridactylus. Biophys J. 1974 Oct;14(10):791–803. doi: 10.1016/S0006-3495(74)85949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair with purines and pyrimidines in radiation- and carcinogen-damaged normal and xeroderma pigmentosum human cells. Cancer Res. 1973 Feb;33(2):362–369. [PubMed] [Google Scholar]
- Cleaver J. E. Repair of alkylation damage in ultraviolet-sensitive (xeroderma pigmentosum) human cells. Mutat Res. 1971 Aug;12(4):453–462. doi: 10.1016/0027-5107(71)90095-9. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Trosko J. E., Lett J. T. Excision repair (dimer excision, strand breakage and repair replication) in primary cultures of eukaryotic (bovine) cells. Exp Cell Res. 1972 Sep;74(1):67–80. doi: 10.1016/0014-4827(72)90482-x. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Hanawalt P. C. The timecourse of DNA repair replication in ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):206–217. doi: 10.1016/0005-2787(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Goldmann K., Friedberg E. C. Measurement of thymine dimers in DNA by thin-layer chromatography. Anal Biochem. 1973 May;53(1):124–131. doi: 10.1016/0003-2697(73)90413-2. [DOI] [PubMed] [Google Scholar]
- Ikenaga M., Ishii Y., Tada M., Kakunaga T., Takebe H. Excision-repair of 4-nitroquinolin-1-oxide damage responsible for killing, mutation, and cancer. Basic Life Sci. 1975;5B:763–771. doi: 10.1007/978-1-4684-2898-8_54. [DOI] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- Kriek E. Persistent binding of a new reaction product of the carcinogen N-hydroxy-N-2-acetylaminofluorene with guanine in rat liver DNA in vivo. Cancer Res. 1972 Oct;32(10):2042–2048. [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R. Post-replication repair of DNA in ultraviolet-irradiated mammalian cells. No gaps in DNA synthesized late after ultraviolet irradiation. Eur J Biochem. 1972 Dec 18;31(3):438–445. doi: 10.1111/j.1432-1033.1972.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W. Approaches to the analysis of fidelity of DNA repair in mammalian cells. Int Rev Cytol. 1976;45:1–23. doi: 10.1016/s0074-7696(08)60076-5. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W., Baney R. N., Lee R. E., Sell S., Farber E. Studies on DNA repair in human lymphocytes treated with proximate carcinogens and alkylating agents. Cancer Res. 1971 Sep;31(9):1297–1306. [PubMed] [Google Scholar]
- Lieberman M. W., Dipple A. Removal of bound carcinogen during DNA repair in nondividing human lymphocytes. Cancer Res. 1972 Sep;32(9):1855–1860. [PubMed] [Google Scholar]
- Lieberman M. W., Poirier M. C. Deoxyribonucleoside incorporation during DNA repair of carcinogen-induced damage in human diploid fibroblasts. Cancer Res. 1973 Sep;33(9):2097–2103. [PubMed] [Google Scholar]
- Lieberman M. W., Poirier M. C. Distribution of deoxyribonucleic acid repair synthesis among repetitive and unique sequences in the human diploid genome. Biochemistry. 1974 Jul 16;13(15):3018–3023. doi: 10.1021/bi00712a003. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Birch N., Otto J. R., MacCormick J. J. Cytotoxicity of carcinogenic aromatic amides in normal and xeroderma pigmentosum fibroblasts with different DNA repair capabilities. J Natl Cancer Inst. 1975 Jun;54(6):1287–1294. doi: 10.1093/jnci/54.6.1287. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M., Smith B. A., Crathorn A. R. Quantitative aspects of the repair of alkylated DNA in cultured mammalian cells. II. Non-semiconservative DNA synthesis ('repair synthesis') in HeLa and Chinese hamster cells following treatment with alkylating agents. Chem Biol Interact. 1971 Feb;3(1):49–68. doi: 10.1016/0009-2797(71)90025-1. [DOI] [PubMed] [Google Scholar]
- Scudiero D., Norin A., Karran P., Strauss B. DNA excision-repair deficiency of human peripheral blood lymphocytes treated with chemical carcinogens. Cancer Res. 1976 Apr;36(4):1397–1403. [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D. Defective repair of N-acetoxy-2-acetylaminofluorene-induced lesions in the DNA of xeroderma pigmentosum cells. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1019–1024. doi: 10.1016/s0006-291x(72)80243-2. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slor H. Induction of unscheduled DNA synthesis by the carcinogen 7-bromomethylbenz(a)anthracene and its removal from the DNA of normal and xeroderma pigmentosum lymphocytes. Mutat Res. 1973 Aug;19(2):231–235. doi: 10.1016/0027-5107(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Park W. D., Stein J. L., Lieberman M. W. Synthesis of nuclear proteins during DNA repair synthesis in human diploid fibroblasts damaged with ultraviolet radiation of N-acetoxy-2-acetylaminofluroene. Proc Natl Acad Sci U S A. 1976 May;73(5):1466–1470. doi: 10.1073/pnas.73.5.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich H. F., San R. H., Kawazoe Y. Increased sensitivity of xeroderma pigmentosum cells to some chemical carcinogens and mutagens. Mutat Res. 1973 Jan;17(1):127–137. doi: 10.1016/0027-5107(73)90261-3. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Rice M., Wagner E. K. Xeroderma pigmentosum cells contain low levels of photoreactivating enzyme. Proc Natl Acad Sci U S A. 1975 Jan;72(1):103–107. doi: 10.1073/pnas.72.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe H., Nii S., Ishii M. I., Utsumi H. Comparative studies of host-cell reactivation, colony forming ability and excision repair after UV irradiation of xeroderma pigmentosum, normal human and some other mammalian cells. Mutat Res. 1974 Dec;25(3):383–390. doi: 10.1016/0027-5107(74)90067-0. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Yager J. D. A sensitive method to measure physical and chemical carcinogen-induced "unscheduled DNA synthesis" in rapidly dividing eukaryotic cells. Exp Cell Res. 1974 Sep;88(1):47–55. doi: 10.1016/0014-4827(74)90616-8. [DOI] [PubMed] [Google Scholar]
- Westra J. G., Kriek E., Hittenhausen H. Identification of the persistently bound form of the carcinogen N-acetyl-2-aminofluorene to rat liver DNA in vivo. Chem Biol Interact. 1976 Oct 2;15(2):149–164. doi: 10.1016/0009-2797(76)90160-5. [DOI] [PubMed] [Google Scholar]



