Abstract
While genetic modification of adenoviral vectors can produce vectors with modified tropism, incorporation of targeting peptides/proteins into the structural context of the virion can also result in destruction of ligand targeting or virion integrity. To combat this problem, we have developed a versatile targeting system using metabolically biotinylated adenoviral vectors bearing biotinylated fiber proteins. These vectors have been demonstrated to be useful as a platform for avidin-based ligand screening and vector targeting by conjugating biotinylated ligands to the virus using high-affinity tetrameric avidin (Kd = 10−15 M). The biotinylated vector could also be purified by biotin-reversible binding on monomeric avidin (Kd = 10−7 M). In this report, a second metabolically biotinylated adenovirus vector, Ad-IX-BAP, has been engineered by fusing a biotin acceptor peptide (BAP) to the C-terminus of the adenovirus pIX protein. This biotinylated vector displays twice as many biotins and was markedly superior for single-step affinity purification on monomeric avidin resin. However, unlike the fiber-biotinylated vector, Ad-IX-BAP failed to retarget to cells with biotinylated antibodies including anti-CD71 against the transferrin receptor. In contrast, Ad-IX-BAP was retargeted if transferrin, the cognate ligand for CD71, was used as a ligand rather than the anti-CD71. This work demonstrates the utility of metabolic biotinylation as a molecular screening tool to assess the utility of different viral capsid proteins for ligand display and the biology and compatibility of different ligands and receptors for vector targeting applications. These results also demonstrate the utility of the pIX-biotinylated vector as a platform for gentle single-step affinity purification of adenoviral vectors.
Introduction
Adenoviral (Ad) gene delivery vectors are attractive due to their high transduction efficiency across a wide variety of dividing and nondividing cell types, the relatively large insert size that they can accommodate, and the high titers that they can produce. The adenovirus serotype 5 (Ad5) capsid is an icosahedral particle, the bulk of which measures about 85 nm in diameter. The capsid comprises 240 hexon trimers, forming all 20 facets of the icosahedron. Present at each of the 12 vertices are penton complexes, each consisting of a homopentameric penton base and the trimeric fiber protein, extending roughly 31.5 nm outward [26,30]. Protein IX (pIX) is a small (14.3 kDa) polypeptide of unknown structure that acts as a capsid cement, stabilizing the interactions between certain hexon trimers on each facet of the virion [8]. Twelve molecules of pIX are thought to be associated with each facet of the icosahedron, with an overall stoichiometry of 240 pIX monomers per virion [32]. The C-terminus of pIX is known to be localized on the outer side of the icosahedral facets [1]. In addition to its role as a capsid cement, pIX is required for packaging of full-length viral genomes and acts as a transcriptional activator of various viral and cellular promoters [9,24].
In vitro work has shown that transduction of Ad5 is mediated by the initial binding of the C-terminal knob domain of the fiber protein to the cellular receptor CAR. Secondary interactions between exposed arginine-glycine-aspartate (RGD) motifs present in surface loops of the penton base and cell surface ανβ3 or ανβ5 integrins promotes vector internalization by endocytosis [4,35]. The mechanisms of in vivo Ad5 transduction are still poorly understood, but they are thought to involve interactions between the fiber shaft and cell-surface heparan sulfate glycosaminoglycans [28,29], in addition to CAR-mediated transduction.
The development of physically targeted adenoviral vectors will require the ablation of natural Ad5 tropism and introduction of new targeting ligands. Detailed structural data on the major capsid proteins have facilitated the genetic incorporation of foreign peptides and proteins into exposed regions of the Ad5 capsid. Short peptides and epitopes have been successfully engineered into the HI loop and C-terminus of the fiber protein [3,6,23], the RGD-containing the hypervariable loop of penton base [36], and hypervariable region 5 of the hexon [33] and onto the C-terminus of minor capsid protein IX [7]. Direct insertion of peptide ligands into the structural context of the Ad5 capsid has been shown to retarget vector tropism effectively; however, it is impossible to predict which ligands will be tolerated and which will disturb ligand or capsid function [37]. Direct genetic insertion of peptide and protein ligands within the Ad5 capsid also requires that a new vector be constructed for each specific targeting application.
As an alternate solution to the problems associated with the unpredictable nature of capsid–ligand compatibility, we have previously developed a versatile vector targeting system based on the fusion of a 70-amino-acid truncation of the Propionibacterium shermanii 1.3S trans-carboxylase domain (PSTCD) to the fiber C-terminus [17]. The PSTCD functions as a natural biotin acceptor peptide (BAP) and is enzymatically biotinylated by endogenous holocarboxylase synthetase (HCS) upon expression in mammalian cells [18,19]. Vectors with BAP-modified fibers were metabolically biotinylated during production in 293A cells and these vectors were shown to be useful platforms for avidin-based ligand screening and vector targeting by conjugating biotinylated ligands to the virus using high-affinity tetrameric avidin (Kd = 10−15 M). The biotinylated vector could also be purified by biotin-reversible binding on monomeric avidin (Kd = 10−7 M).
Given the utility of this prototype metabolically biotinylated vector, we have explored the development of alternate Ad5 vectors with increased numbers of BAPs per virion to determine if these would enhance either vector targeting or vector purification. The fiber protein is present in only 36 copies per virion, therefore the previously described Ad-Fiber-BAP vector can display a maximum of only 36 biotin molecules per virion, assuming that 100% of the Fiber-BAP molecules are biotinylated by cellular HCS. In contrast, fusion of the BAP to the C-terminus of pIX should result in a much higher maximum display of 240 biotins per virion. We present here the construction and characterization of a pIX-modified, metabolically biotinylated Ad5 vector. The addition of a 71-amino-acid PSTCD truncation (hereafter referred to as BAP) to the C-terminus of pIX through a 13-residue linker had no deleterious effects on vector morphology, thermostability, or infectivity. The biotinylated Ad-IX-BAP vector displayed more biotins than Ad-Fiber-BAP and these biotins were shown to be accessible to avidin binding. We present data on the vector targeting ability of Ad-IX-BAP and we found that the vector was surprisingly inefficient at redirecting vector tropism through biotinylated antibodies, unlike the fiber-modified vector. Biotinylated transferrin, on the other hand, was effective at redirecting the transduction of both metabolically biotinylated vectors. In addition, we show that these metabolically biotinylated vectors can be coupled to paramagnetic µbeads for the control of transduction through magnetic force. Finally, we demonstrate that single-step avidin-based purification of Ad-IX-BAP was substantially more efficient compared to Ad-Fiber-BAP and wild-type vectors. Results indicate that the locale of biotin display is an important factor for both avidin-based vector targeting and purification.
Results
Construction of a pIX-BAP Modified Vector
We generated wild-type and pIX-modified vectors expressing the dsRed2 red fluorescent protein with the Ad-Easy system [12]. The pIX gene is positioned very close to the E1 region of the Ad genome and is encoded in the right homology region of the Ad-Easy shuttle plasmid. Thus, any modifications to the pIX gene of the shuttle plasmid are carried over to the recombinant Ad genome during homologous recombination in BJ5183 cells. To modify the wild-type copy of the pIX gene on the Ad-Easy shuttle plasmid, we took advantage of a homologous recombination system in Escherichia coli based on the phage λ Red genes. This system, reviewed in [20], allows recombination based on as few as 36 bp of sequence homology. Thus, we were able to incorporate homologous sequences into our oligonucleotide design and efficiently recombine linear PCR products containing antibiotic resistance markers in a site-specific fashion (Fig. 1A). Recombinant plasmids were generated by simply cotransforming BW25113 cells with the linear PCR product and the target plasmid, followed by antibiotic selection. We constructed a pIX-BAP fusion under control of the CMV promoter and were able to show efficient expression and biotinylation of the fusion protein (data not shown). To replace the wild-type copy of pIX with the BAP fusion, we used a two-step recombination strategy outlined in Fig. 1A. Briefly, we designed oligos to insert a SpeI-flanked zeocin-resistance gene (ZeoR) immediately downstream of the pIX-BAP gene, before the SV40 poly(A) signal sequence on pCMV-IX-BAP. We then used the resulting pCMV-IX-BAP-ZeoR as template for a second PCR using oligos designed to replace the wild-type pIX gene present in pShuttle with the pIX-BAP-ZeoR cassette. We then excised the ZeoR cassette by SpeI digestion and religated to yield pIX-BAP-Shuttle, an Ad-Easy system shuttle plasmid with the pIX-BAP in place of the wild-type copy of pIX (Fig. 1A). The fusion protein consists of the entire 140 residues of wild-type pIX, a 13-amino-acid linker sequence, and a 71-residue truncation of the PSTCD BAP (Fig. 1B).
FIG. 1.
(A) Diagram of the Red recombination scheme used to construct the pIX-modified shuttle plasmid. (1) PCR of ZeoR cassette using primers with 39-bp overhangs homologous to sequences of pCMV-IX-BAP, for (2) site-specific recombination between the IX-BAP fusion and the SV40 poly(A) signal. (3) The resulting construct, pCMV-IX-BAP-ZeoR, was used as template for a second (4) PCR, again using primers for (5) site-specific recombination. (6) The resulting shuttle plasmid, pIX-BAP-Shuttle-ZeoR, was SpeI digested and self-ligated to remove the ZeoR cassette and used to construct Ad-IX-BAP with the Ad-Easy vector system. (B) Sequence of the modified protein IX fused to the 71-amino-acid BAP through a 13-residue linker peptide. Only a SpeI scar (underlined) remains between the TAA stop and the native pIX poly(A) signal sequence.
We then cloned the dsRed2 red fluorescent protein reporter gene into pIX-BAP-Shuttle and recombined this plasmid in recBC-deficient BJ5183 bacteria using the Ad-Easy system. We used the resulting vector to generate Ad-IX-BAP expressing the dsRed2 fluorescent reporter from 293A cells. We amplified and CsCl banded the vector, which yielded normal titers of virus.
Characterization of the pIX-Modified Vector
Previous work has demonstrated that fusion of the BAP to the C-terminus of the fiber results in a small but significant decrease in CAR-mediated transduction, presumably due to steric hindrance of the fiber knob–CAR interaction [17]. To determine if the fusion of 84 amino acids to the C-terminus of protein IX had similar detrimental effects, we transduced CAR-positive HeLa cells at various multiplicity of infection (m.o.i). In contrast to Ad-Fiber-BAP, the pIX-modified vector displayed no significant decrease in bioactivity compared to an unmodified Ad control vector (Fig. 2A). We assessed protein IX function of Ad-IX-BAP indirectly by heating the vector to 47.5°C for various lengths of time and measuring the remaining bioactivity by transduction of HeLa cells. Results demonstrate no significant difference between the thermostability profiles of wild-type and pIX-modified vectors (Fig. 2B). In contrast, the profile of Ad-ΔIX, a vector devoid of pIX, shows a substantial decrease in bioactivity upon heat treatment. These results indicate that the pIX-BAP fusion was encapsidated and maintained its normal function as a capsid cement protein [8]. Transmission electron microscopy of the pIX-modified virus demonstrated no obvious defects in virion morphology (Fig. 2C).
FIG. 2.
Characterization of Ad-IX-BAP. (A) CAR-mediated transduction. HeLa cells were transduced with BAP-modified or wild-type vectors at various multiplicities of infection (m.o.i.). (B) Capsid thermostability assay. Vectors were heated for various times and assayed for bioactivity on HeLa cells. Transduction efficiency was measured by flow cytometry 48 h posttransduction. Results are presented as means ± standard deviation of three experiments. (C) Virion morphology as revealed by negative-stain TEM. Scale bar represents 50 nm.
The original work on pIX engineering demonstrated that the addition of a ~ 25-lysine peptide to the C-terminus of pIX (through a Flag epitope spacer peptide) resulted in a vector with decreased thermostability and infectivity [7]. It was therefore surprising to find that the addition of the 71-amino-acid BAP through a 13-aminoacid linker peptide in our work had no deleterious effects on virion morphology, thermostability, or CAR-mediated cellular transduction. The results might be explained by the fact that the polylysine peptide introduces 25 positive charges per pIX and 6000 positive charges per virion. In contrast, the PSTCD BAP folds into a compact β-sandwich structure [22] that introduces only 1 net negative charge per pIX protein and 240 negative charges per virion.
BAP-Modified pIX Is Encapsidated and Displays Biotins on the Virion Surface
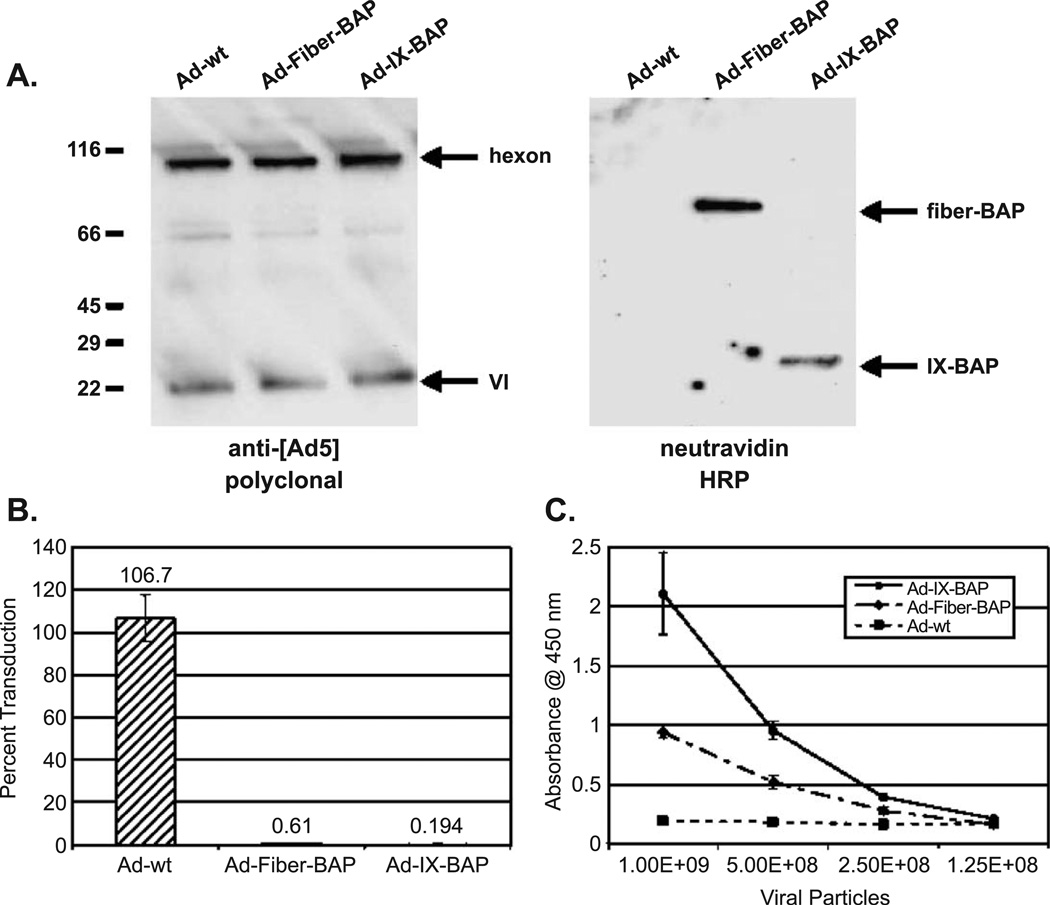
We examined biotinylation of the Ad-IX-BAP vector first by Western blot in comparison to Ad-Fiber-BAP, the fiber biotinylated virus [17]. We denatured CsCl-purified virions in SDS and ran them on a polyacrylamide gel. We blotted separated proteins onto PVDF membranes and detected them with either anti-[Ad5] polyclonal as a loading control or neutravidin–HRP to detect biotinylated species (Fig. 3A). Probing with neutravidin – HRP confirmed that the 23-kDa biotinylated pIX protein was properly incorporated into the virions during encapsidation. We first tested surface biotinylation of Ad-IX-BAP virions by capture of the virus on streptavidin-coated magnetic particles (Fig. 3B). We incubated streptavidin-coated magnetic particles with wild-type or biotinylated vector and then captured the particles on a MACS magnetic column. To determine if any virus was depleted from the solution, we added the flow through from the binding reaction to HeLa cells and tested them for transduction (Fig. 3B). Both biotinylated Ad-IX-BAP and Ad-Fiber-BAP displayed a marked drop in gene transfer efficiency when incubated with the magnetic particles compared to the unmodified Ad control vector. More than 99% of the bioactivity of both vectors appeared to be depleted from solution, indicating that the vast majority of the fiber- and pIX-modified virions display at least one biotin per virion. We also used direct ELISA to determine if the biotinylated fusion is displayed on the surface of the capsid and accessible to avidin binding (Fig. 3C). We immobilized serial dilutions of wild-type or pIX modified vectors on a microtiter plate, blocked it with milk, and probed it with neutravidin–HRP under native conditions. We quantitated bound HRP with a chromogenic reaction and detection at 450 nm. We included Ad-Fiber-BAP vector as a positive control for surface biotinylation. Results demonstrate that the biotin groups on Ad-IX-BAP are accessible to avidin binding on the surface of the capsids (Fig. 3C). Ad-IX-BAP consistently yielded higher absorbance values than the fiber-modified biotinylated vector, suggesting that the pIX-modified virus presented more biotin groups per virion. Comparison to a standard curve of biotinylated maltose-binding protein indicated that approximately 100% of the fibers were biotinylated for Ad-Fiber-BAP (data not shown). In contrast, only approximately 30% of the 240 pIX-BAP molecules were detected by avidin–HRP. This suggests that only 30% of pIX proteins are biotinylated or that only 30% are available for binding to avidin in this assay. Regardless, the pIX biotinylated virus appears to present approximately twice as many biotins per virion than the original vector with biotinylated fibers.
FIG. 3.
BAP-modified vectors are metabolically biotinylated and display biotins on their surface. (A) Western blot of vectors. 5 × 108 viral particles were boiled in Laemmli loading buffer and loaded onto a 7.5% polyacrylamide gel. Separated proteins were blotted onto PVDF membranes and probed with rabbit anti-[Ad5] polyclonal antibody (loading control) or neutravidin – HRP. (B) Magnetic µparticle capture assay. Vectors were incubated with streptavidin-coated paramagnetic µparticles and run through a MACS magnetic column as described under Materials and Methods. Samples were assayed for transduction on HeLa cells by flow cytometry and percentage transduction (output/input) was calculated for each vector. Results are presented as means ± standard deviation of three experiments. (C) Surface biotinylation ELISA. Serial dilutions of BAP-modified or wild-type vectors were adsorbed onto wells of an ELISA plate. Wells were blocked, washed, and probed with neutravidin – HRP. Results are presented as means ± standard deviation of three experiments.
Redirection of Ad-IX-BAP Transduction with Biotinylated Ligands
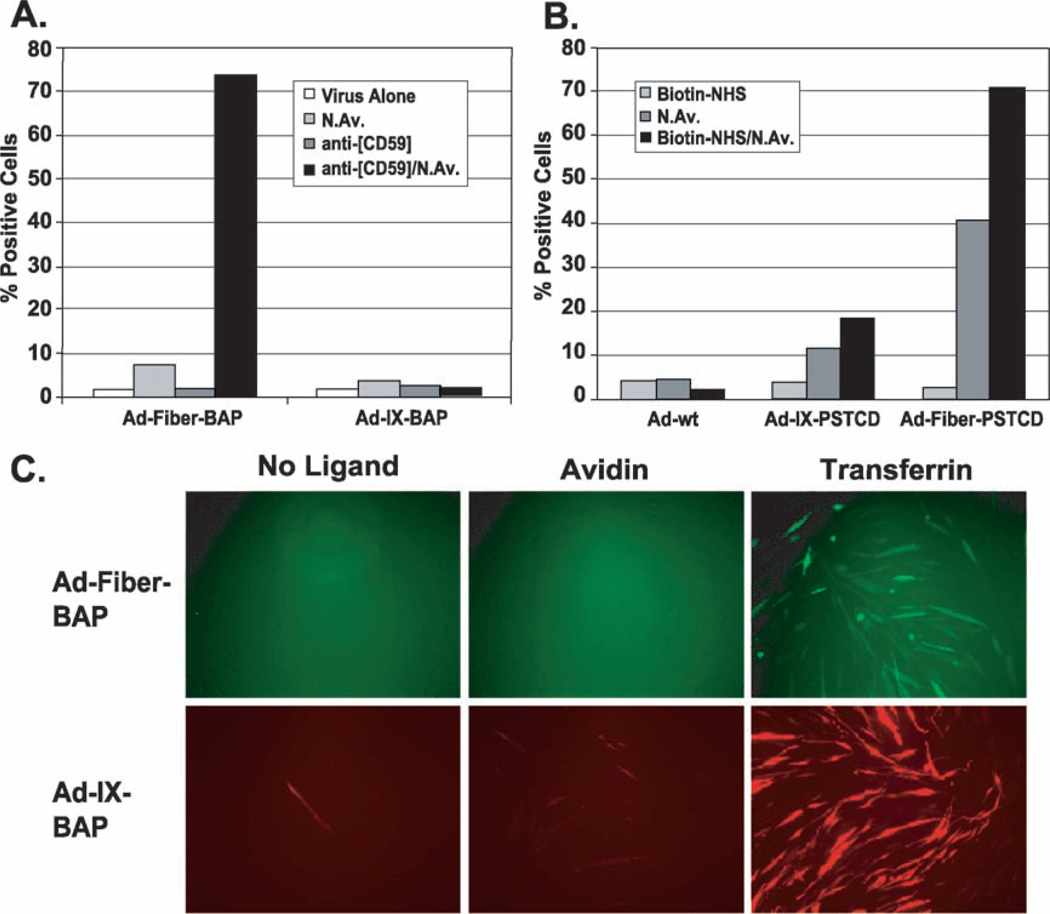
The biotinylated fiber vector, Ad-Fiber-BAP, can be retargeted to new receptors on cells using biotinylated antibodies, proteins, carbohydrates, lectins, and nucleic acids [2,17]. Given the differences between fiber and protein IX stoichiometry and location within the capsid, we tested whether the pIX-biotinylated vector could be similarly retargeted with biotinylated ligands. We incubated CD59-positive K562 erythroid leukemia cells, normally refractory to Ad5 infection, with biotinylated anti- [CD59] antibody followed by neutravidin and biotinylated vector. Consistent with previous work, Ad-Fiber-BAP was successfully retargeted (Fig. 4A). In contrast, Ad-pIX-BAP demonstrated no gain-of-function transduction, despite the fact that it displayed more biotins per virion than the fiber-modified vector (Fig. 2C).We obtained similar results using biotinylated anti-[CD71] as the targeting ligand (data not shown). Direct chemical biotinylation of cell surface proteins using amine-reactive NHS–biotin can also mediate avidin-dependent transduction of K562 cells by Ad-Fiber-BAP [17]. In this format, Ad-IX-BAP mediated detectable but low retargeting activity on chemically biotinylated K562 cells. However, direct comparison revealed that Ad-Fiber-BAP still outperformed Ad-IX-BAP (Fig. 4B).
FIG. 4.
Redirection of Ad-IX-BAP tropism with biotinylated ligands. (A) Transduction of CAR-negative K562 cells with biotinylated antibodies. Cells were incubated with biotinylated anti-[CD59] antibody, washed, saturated with neutravidin, washed, and incubated with viral vectors. Controls were incubated with antibody or avidin alone, followed by vector. Transduction efficiency was measured by flow cytometry 48 h posttransduction. (B) Transduction of chemically biotinylated K562 cells. Cell surface proteins of K562 cells were chemically biotinylated with NHS-LC-biotin. Cells were then saturated with neutravidin, washed, and incubated with vector. Controls include treatment with NHS-biotin or neutravidin alone, followed by vector. Transduction efficiency was measured by flow cytometry 96 h posttransduction. (C) Transduction of murine C2C12 myotubes with biotinylated transferrin. Cells were incubated with biotinylated transferrin, washed, bound with excess neutravidin, washed, and incubated with viral vectors. Controls were incubated with buffer or avidin alone, followed by vector. Cells were photographed under a fluorescence microscope 36 h posttransduction.
These data suggested that there was a fundamental difference in how Ad-IX-BAP displays cell-targeting ligands or how this vector enables functional ligand–receptor interactions by these ligands. Given that the large 150-kDa biotinylated antibody ligands failed to retarget Ad-pIX-BAP, we sought alternate small ligands to test the vector. One smaller ligand with demonstrated utility is transferrin [13,38]. Transferrin is an ~ 85-kDa protein that shuttles Fe3+ ions into cells through endocytosis of the transferrin receptor. Transferrin was of particular interest, since this is the cognate ligand the transferrin receptor CD71, the same receptor that previously failed for Ad-IX-BAP targeting with the biotinylated antibody against CD71. To test this cognate ligand, we pretreated murine C2C12 myotubes with either neutravidin alone or biotinylated transferrin in conjunction with neutravidin prior to transduction with Ad-IX-BAP or Ad-Fiber-BAP. Contrary to prior results with biotinylated antibodies, transferrin was able to increase significantly both Ad-IX-BAP and Ad-Fiber-BAP transduction of the murine myotubes (Fig. 4C). We have also observed similar results with SkBr3 breast cancer cells and K562 cells (data not shown), confirming the positive effect of transferrin. These data highlight not only the effects of different capsid proteins for ligand display (fiber vs pIX), but also the effects of ligand–receptor biology on functional vector targeting.
Magnetic Targeting with Biotinylated pIX Vector
Previous studies have demonstrated that chemically and metabolically biotinylated Ad5 vectors can be complexed with biotin-binding paramagnetic µbeads and that transduction of refractory cells can be enhanced by physically localizing the vector onto cells using magnetic force [2,16]. To test this application for the biotinylated pIX vector, we transduced refractory CAR-deficient Chinese hamster ovary (CHO) cells with biotinylated vectors in the absence or presence of 1-µm streptavidin-coated paramagnetic beads and in the absence or presence of a 5-mm magnet (Fig. 5A). Transduction was visualized 24 h later by fluorescence microscopy or by luciferase luminescence using a Night Owl imaging system. As expected, the wild-type vector and metabolically biotinylated vectors alone failed to transduce the CHO cells under all conditions (Fig. 5). In contrast, incubation of the biotinylated vectors with magnetic beads alone caused a dramatic increase in transduction of the metabolically biotinylated vectors, but not by wild-type Ad5 vector. In the absence of the magnet, transduction was increased by the biotinylated vectors because the heavy paramagnetic beads eventually settled to the bottom of the dishes bringing the vectors in contact with the CHO cell monolayer. In the presence of a magnetic field, transduction was markedly increased. Luciferase imaging using the Night Owl camera system demonstrated that this transduction was focused to the precise area where the magnet was placed (Fig. 5B).
FIG. 5.
Magnetofection with metabolically biotinylated vectors. (A) Transduction of normally refractory CHO cells. Vectors were combined with or without streptavidin-coated paramagnetic µbeads (1 µm diameter) and used to transduce CHO cells at an m.o.i. of 1000 in the presence or absence of a small 5-mmdiameter rare-earth magnet disk. Transduction was visualized by fluorescence microscopy (original magnification 10×) 24 h posttransduction. (B) Transduction was precisely localized to the cells directly above the magnet. 1 µg luciferin was added to each well and transduction of the Ad-Fiber-BAP vector was imaged with the Night Owl cooled CCD camera to visualize directly localization of EGFP – luciferase expression.
Avidin-Based Purification
Previous work demonstrated that the fiber-biotinylated Ad5 vector could be purified from crude cellular lysates by single-step affinity chromatography on monomeric avidin resin [17]. While fiber-biotinylated vectors could be purified, less than 30% of input activity from crude lysates was eluted from the resin. This inefficient recovery appeared to be due to copurification of free biotinylated fiber and penton base capsomers on the resin that may compete for avidin binding on the column. Alternatively, copurified fiber or penton base in the eluate may compete or block vector binding to cells when the transduction activity of the fractions was measured. Given these issues, we tested Ad-IX-BAP for vector purification, since this vector displayed more biotins per virion than Ad-Fiber-BAP. In addition, since pIX is not itself a cell-binding capsomer (unlike fiber and penton base), copurification of biotinylated pIX should not inhibit transduction by competing with virions for cell binding.
To test Ad-IX-BAP for purification, we infected 293A cells with Ad-wt, Ad-IX-BAP, or Ad-Fiber-BAP vectors at low m.o.i. and harvested the cells 72 h later. We washed the cell pellets gently and lysed them by freeze –thaw. We then incubated crude lysates with monomeric avidin resin, washed them, and eluted them with buffer containing 5 mM biotin. We analyzed all collected fractions for the presence of vector by measuring transduction on HeLa cells. Unmodified Ad vector exhibited nonspecific binding to the resin and was gradually released during the various washes. Ad-Fiber-BAP bound the avidin resin specifically, but only approximately 30% of the transduction activity of the input fraction was observed upon elution, consistent with earlier findings [17]. In contrast to the wild-type and fiber-modified vectors, Ad-IX-BAP tightly bound the resin and the majority of the bioactivity specifically eluted with free biotin (Figs. 6A and 6B). In fact, slightly higher levels of transduction were observed in the elution fraction, suggesting the biotinylated capsids were purified away from all the free fibers present in the crude lysate input fraction. Indeed, gel analysis shows that the elution fraction of Ad-IX-BAP lacks the excess fibers present in the elution of Ad- Fiber-BAP (Fig. 6C). These data demonstrate that Ad- IX-BAP is an effective vector for single-step affinity purification of adenovirus using monomeric avidin columns.
FIG. 6.
Avidin-based purification of metabolically biotinylated vectors. 293A cells were grown to ~95% confluence in 150-mm dishes and infected with wildtype or metabolically biotinylated vectors at an m.o.i. of 20. 72 h later the cells were collected, lysed by 3× freeze – thaw, and pelleted. Supernatants were mixed with monomeric avidin resin, washed 4×, and eluted with 5 mM biotin. Fractions were collected and assayed for (A) transduction of HeLa cells by fluorescence microscopy. Note. High background fluorescence in all the input and early fractions was due to the free fluorescent protein (dsRed or EGFP – luciferase) present in the 293A cell lysate. (B) Quantification of the Ad-IXBAP fractions by flow cytometry. (C) Silver-stained gel analysis of total protein content of the purification fractions for biotinylated vectors reveals the abundance of free biotinylated fiber present with Ad-Fiber-BAP.
Discussion
Previous work with a fiber-biotinylated adenovirus demonstrated that these biotin-tagged vectors can be targeted to new receptors by conjugation to biotinylated ligands using tetrameric avidin (Kd = 10−15 M) and can also be purified by biotin-reversible binding on monomeric avidin (Kd = 10−7 M). In this work, we have engineered a metabolically biotinylated adenoviral vector in which covalent biotinylation is targeted to the 240 copies of the pIX cement protein of the virus rather than the 36 copies of the fiber.
We demonstrate here that the modified protein IX is biotinylated and efficiently encapsidated into virus particles. Despite the fact that the pIX biotinylated vector Ad-IX-BAP displayed approximately 70 biotins per virion, it was surprisingly ineffective at redirecting vector tropism through biotinylated antibodies. In contrast, the fiber-biotinylated vector Ad-Fiber-BAP could readily transduce refractory K562 cells in an avidin-dependent manner when transduction was rerouted through biotinylated antibodies against cell surface CD59/CD71 or when the K562 cells were chemically biotinylated with NHS–biotin. Ad-IX-BAP lacked the ability to transduce K562 cells through the use of biotinylated antibodies and displayed only marginal ability to redirect transduction with the chemical biotinylation approach.
In contrast to the monoclonal antibody ligands, transduction of Ad-IX-BAP was effectively redirected when transferrin, the cognate ligand for CD71, was used rather than the antibody against CD71. This puzzling observation is most likely explained by key differences in the biology of the fiber and pIX proteins as well as the biology of ligand–receptor interactions during endosomal uptake and escape. Receptor binding is a critical step for the process of infection, but release from the receptor after viral uptake is equally important. Adenovirus accomplishes this by sequentially shedding its coat proteins during the processes of binding, uptake, endosomal escape, and cytosolic trafficking. Normally, initial binding is mediated between cell surface CAR and the fiber protein. Interactions between cell surface integrins and the penton base RGD motifs promote endocytosis of the vector and concurrent release of the fibers from the penton base [15]. It has been estimated that about 10 min after the initial binding event, 85% of the bound virions are internalized in coated vesicles and 90% of the fibers have been dissociated from the capsids [11]. In contrast, protein IX is thought to dissociate sometime between 30 and 60 min postbinding, well after endosomolysis and cytosolic escape [10]. Given this, retargeting Ad-Fiber-BAP through monoclonal antibodies against cell surface CD59 and CD71 or transferrin should result in normal uptake, disassembly, and trafficking, as the fiber protein is naturally released in the early stages of transduction. On the other hand, retargeting of Ad-IX-BAP through alternate receptors might interfere with the normal dismantling program of the capsid and thereby interfere with transduction. Ad-IX-BAP virions bound to cell surface receptors through high-affinity antibody interactions may actually remain trapped with the receptors during uptake and endosomal acidification. Ad-IX-BAP therefore either may be recycled to the cell surface or may not escape the lysed endosomal membrane debris after endosome lysis and be unable to proceed to the nucleus. Unlike the high-affinity antibody–receptor interactions, which most likely remain intact during the drop in endosomal pH, transferrin responds to the acidification by undergoing a conformational change, thereby releasing its bound Fe3+ ions [21]. Upon neutralization of pH this apotransferrin releases from CD71 as part of its natural role as an iron scavenger and transporter [21]. Normally the pH-dependent release of apotransferrin occurs at the cell surface upon recycling of the endosomal vesicle, but the presence of an adenoviral virion in the endosome should cause endosomal lysis in response to the drop in pH [14]. This pH of the lysed endosome should rapidly equilibrate to neutral cytosolic pH, thereby triggering release of the bound apotransferrin from CD71, thereby releasing Ad-IX-BAP from the receptor to allow it to traffic effectively to the nucleus. In this case, Ad-IX-BAP should then be able to continue with normal cytosolic trafficking and transduction only when targeted through transferrin. This explanation seems plausible, but future experimentation and analysis of initial binding, uptake, and trafficking is necessary to determine why Ad-IX-BAP transduction can be redirected only through transferrin and not an antibody against the transferrin receptor, while Ad-Fiber-BAP can be targeted through either the natural ligand or the antibody.
While targeted transduction of the pIX-biotinylated vector gave variable results with biotinylated ligands, the vector could be effectively retargeted with magnetic force. Through the use of streptavidin-coated paramagnetic µbeads and small rare-earth magnets, we demonstrated precise control of both Ad-IX-BAP and Ad-Fiber- BAP transduction, on cells normally refractory to Ad5 infection. This magnetofection technology [25] could be useful for a wide variety of applications, including tissue engineering, in which cells on only a certain area of the scaffold need to be transduced, or cell biology, in which a precise population of cells in a monolayer could express a secreted signal and the effects of a local concentration gradient on neighboring cells could be studied.
Finally, we show that the covalently attached biotin molecules on Ad-IX-BAP can be used as an affinity tag for vector purification on monomeric avidin resin. Previous data with the Ad-Fiber-BAP vector suggested some potential for avidin-based purification of metabolically biotinylated vectors [17]; however, in this work we show that the Ad-IX-BAP clearly outperforms the previously characterized Ad-Fiber-BAP in this application. Fiber proteins are produced in excess during adenoviral infection, and the majority of the fibers are not incorporated into mature virions [31,34]. Monomeric avidin purification of Ad-Fiber-BAP from crude 293A lysate resulted in copurification of this unencapsidated excess biotinylated fiber in addition to the biotinylated vector. We speculate that these fibers or fiber–penton base capsomers act as inhibitors of transduction by competing with vector for CAR and integrin binding. As a result of the free competitor, only ~30% of vector bioactivity could be recovered when Ad-Fiber-BAP was eluted from monomeric avidin columns. In contrast to fiber, protein IX is not involved in virion–cell interactions. Therefore, if biotinylated free pIX is copurified, it should not block cell binding by the eluted virus and should not perturb measurements of the vector activity after purification. This is consistent with the observation that nearly 100% of the bioactivity of Ad-IX-BAP was recovered upon biotin elution from the monomeric avidin columns. These results indicate that covalent attachment of biotin to the fiber protein results in suboptimal avidin-based purification, and modification of a capsid protein with no involvement in the process of vector binding can greatly enhance the effectiveness of avidin-based purification by avoiding problems associated with copurification of free fibers. Metabolically biotinylated pIX-modified vectors are convenient for easy purification of Ad vectors from small-scale viral preps, which cannot be effectively banded by CsCl density gradient ultracentrifugation. These modified vectors might also be of great use for the selective removal of Ad5 helpers from AAV preparations.
In conclusion, this work demonstrates the utility of metabolically biotinylated Ad-IX-BAP for avidin-based magnetic and ligand targeting as well as affinity purification. Unlike chemical biotinylation [27], which results in random biotinylation of the virion surface, the position and magnitude of metabolic biotinylation are controlled genetically, allowing proper comparison of the effects of modification of two separate capsid proteins. Ligand targeting experiments show potential for pIX-mediated targeting but also raise many questions about the nature of targeted transduction and redirection of tropism. These data highlight the importance of understanding the biology of vector binding, entry, and trafficking as well as receptor/ligand biology for the successful design of targeted vectors. These data again demonstrate the utility of metabolically biotinylated vectors to screen for optimal ligand–receptor interaction. This application enables the use of the biotinylated vectors themselves either for vector targeting or as a platform for prescreening ligands prior to going through the effort of genetically engineering these ligands into the capsids of viral vectors. Finally, these data demonstrate potential for the use of the biotinylated vectors to begin unraveling the complex biology of vector–ligand–receptor interactions to develop better functional vectors for cell targeting efforts.
Materials and Methods
Materials
Plasmid DNA purification columns were purchased from Qiagen (Chatsworth, CA, USA). The Ad-Easy vector system was purchased from Quantum Biotechnologies (Montreal, Canada). The pEM7-Zeo vector were purchased from Invitrogen (Carlsbad, CA, USA). The pDsRed2-N1 vector was purchased from Clontech (Palo Alto, CA, USA). Restriction endonucleases, T4 DNA ligase, and streptavidin-coated superparamagnetic µbeads (1 µm diameter) were purchased from New England Biolabs (Beverly, MA, USA). The PinPoint-Xa2 plasmid and SoftLink monomeric avidin resin were purchased from Promega (Madison, WI, USA). Neutravidin – HRP conjugate and biotinylated transferrin were purchased from Molecular Probes (Eugene, OR, USA). Streptavidin-coated paramagnetic µparticles (50 nm diameter) and magnetic µcolumns were purchased from Miltenyi Biotech (Auburn, CA, USA). Biotinylated anti-[CD59] and anti-[CD71] antibodies were purchased from Pharmingen (San Diego, CA, USA). Anti-[Ad5] polyclonal antibody was purchased from Abcam (Cambridgeshire, UK). Sulfo-NHS-LC-biotin, neutravidin, Super-Signal Dura chemiluminescence substrate, and donkey anti-[rabbit] –HRP secondary were purchased from Pierce Biotechnologies (Rockford, IL, USA). Neodymium rare-earth magnets (disk, 5 mm diameter × 2.5 mm height) were purchased from Indigo Instruments (Waterloo, Ontario, Canada). High-glucose DMEM with l-arginine, antibiotic – antimycotic, and Lipofectamine were purchased from Gibco BRL (Gaithersburg, MD, USA). All other supplies and reagents were purchased from Sigma (St. Louis, MO, USA), Fisher Scientific (Pittsburgh, PA, USA), or VWR (Houston, TX, USA).
Bacterial Strains
XL-1 blue cells were purchased from Stratagene and were used for all clonings and DNA preps unless indicated otherwise. Strain BW25113, genotype lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBA-DAH33 ΔrhaBADLD78, was obtained from the E. coli Genetic Stock Center http://cgsc.biology.yale.edu/). BW25113 harbors a temperature-sensitive plasmid, pKD46, encoding the λ Red recombination genes under control of the l-arabinose-inducible ParaB promoter [5]. Strain BJ5183, genotype endA sbcBC recBC galK met thi-1 bioT hsdR(Strr), was purchased from Quantum Biotechnologies.
Plasmid Constructs
A protein IX –GFP fusion was originally cloned between the BglII and XhoI sites of pShuttle-CMV (Quantum Biotechnologies). The resulting pCMV-IX-GFP was digested with AgeI and XhoI to excise the GFP gene, and a 71-amino-acid truncation of the PSTCD BAP was PCR amplified from the PinPoint-Xa2 vector (Promega) using oligonucleotides PSTCD5′A (ATCTTACGACCGGT GGGCTCCGGCTCCGGAGAGGGCGAGATTCCCGCTCCG) and PSTCD3′S (AGCTTGTCGAC TTACCCGATCTTGATGAGACCCTG). The product was gel purified, digested with AgeI and SalI, and cloned into the AgeI- and XhoI-digested pCMV-IX backbone, producing pCMV-IX-BAP. This plasmid contains a protein IX–BAP fusion under CMV control.
Replacement of the genomic pIX gene within the right homology region of pShuttle-CMV with the modified fusion was accomplished in a two-step recombination procedure mediated by the Red genes of phage λ (Fig. 1). First, a SpeI-flanked zeocin-resistance cassette was PCR amplified from pEM7-Zeo using oligos PSTCDSpeIZeo5′ (GCGGTGCAGGGCGGTCAGGGTCTCATCAAGATCGGGTAAACTAGTCGCGATATCGCTAGCTCGAGC) and PST CD SpeIZeo3′ (TTGCATTCATTTTATGTTTCAGGTTCAGGGGGAGGTGTGACTAGTCCTGCAGGTCGACTCTAGAGG). Sequences in italics are 39-bp regions of homology for insertion of the zeocin-resistance gene into the pCMV-IX-BAP, SpeI sites are underlined. The resulting product was DpnI-digested and gel-purified, and 0.5 µg of DNA was cotransformed along with 1 µg of pCMV-IX-BAP into electrocompetent BW25113 cells. Cells were transformed in a 1-cm-gap cuvette in an Eppendorf 2510 electroporator at 2500 V. Transformed cells were rescued with 1 ml of cold SOC supplemented with 0.1% l-arabinose and incubated overnight at 30°C to induce expression of the Red genes and allow homologous recombination. The transformation was then plated on LB agar with kanamycin and zeocin to select for recombinants. Proper recombination resulted in the insertion of the SpeI-flanked zeocin-resistance cassette in between the 3′ end of the IX –BAP coding region and the SV40 polyadenylation signal sequence. Zeocin-resistant clones were grown and screened by restriction digestion, positive clones were transformed into XL-1 Blue cells. The resulting plasmid, pCMV-IX-BAP-ZeoR, was then used as template for PCR with oligos IX5′ (CCGCTTGCAAGCAGTGCAGCTTCCCGTTCATCCGCCCGCGATGAC) and IX3′UTR (GATCCAAATCCAAACAGAGTCTGGTTTTTTATTTATGTTACTAGTCCTGCAGGTCGACTC). The italicized sequence of IX3′UTR corresponds to the 3′ untranslated region of the wild-type protein IX gene within the context of the Ad5 genome. The 958-bp product consisted of a portion of the pIX gene, the C-terminal BAP fusion, and the SpeI-flanked zeocin-resistance cassette (see Fig. 1). This product was then DpnI-digested, purified, and cotransformed along with pShuttle-CMV into BW25113, and kanamycin/zeocin-resistant clones were selected as before. Homologous recombination resulted in the creation of pIX-BAPZeoR-Shuttle, which encodes a pIX –BAP fusion in place of the wild-type copy of the pIX gene in the right arm homology region of the adenoviral genomic sequence on the shuttle plasmid. The zeocin-resistance gene was then removed by SpeI digestion and self-ligation to create pIX-BAP-Shuttle. The dsRed2 gene was cut from pDsRed2-N1 with BglII and NotI and cloned into the MCS of pIX-BAP-Shuttle and pShuttle-CMV digested with BglII and NotI to create pIX-BAP-Shuttle-dsRed and pShuttle-dsRed. These shuttle plasmids were then linearized with PmeI, gel purified, and recombined with pAd-Easy1 by cotransformation into BJ5183 cells and selection on kanamycin as previously described. The resulting pAd-IX-BAP contains the CMV-dsRed expression cassette in the E1A region and a pIX –BAP fusion in place of the wild-type pIX gene (see Fig. 1). pAd-wt contains the wt pIX gene and also expresses the dsRed gene from the CMV promoter.
pAd-ΔIX, a vector deleted for the pIX gene, was created by λ Red recombination as described above. Briefly, a ~1.1-kb chloramphenicol-resistance cassette with 39-bp ends (italicized) homologous to the sequences flanking pIX in pAd-Red was PCR amplified from pKD3 with CATΔIX5′ (ATGTAGTTTTGTATCTGTTTTGCAGCAGCCGCCGCCGCCGTGTAGGCTGGAGCTGCTTC) and CATΔIX3′ (GATCCAAATCCAAACAAGAGTCTGGTTTTTTATTTATGTTCATATGAATATCCTCCTTAG). The PCR product was digested with DpnI, gel purified, and recombined with pAd-Red by cotransformation into BW25113 as described above. Selection with chloramphenicol and kanamycin resulted in isolation of pAd-ΔIX.
Cells
HeLa cells and murine C2C12 myoblasts were maintained in DMEM containing 10% fetal bovine serum (FBS) and antibiotic/antimycotic (Ab/Am). 293A human embryonic kidney cells were maintained in DMEM, 10% FBS, Ab/Am, supplemented with 30 µM d-biotin. K562 erythroid leukemia cells and CHO cells were maintained in RPMI medium containing 10% FBS and Ab/Am.
Viruses
Recombinant viruses were produced by PacI linearization of pAd-wt, pAd-IX-BAP, and pAd-ΔIX followed by Lipofectamine transfection of 293A cell monolayers overlaid with agarose-medium. Plaques of recombinant virus were visible within 10 – 14 days and viruses were amplified and CsCl purified as previously described [17]. The Ad-wt, Ad-IX-BAP, and Ad-ΔIX vectors express the dsRed2 fluorescent protein. Previously described Ad-Fiber-BAP expresses an EGFP – luciferase fusion reporter protein (EGFP-luc), and transduction can be visualized by either fluorescence or luciferase assay.
Viral Transduction
HeLa cells were plated at 100,000 cells per well in 48-well tissue culture plates. Prior to infection, complete medium was aspirated and cells were gently washed in serum-free DMEM. Infections were performed at 37°C for 90 min in 100 µl serum-free DMEM at the indicated m.o.i. After the 90-min incubation, virus was aspirated off and complete medium was added to the cells. For the capsid thermostability assay, virus diluted in serum-free DMEM was incubated at 47.5°C for various times before transduction of HeLa cells at an m.o.i. of 1000. Expression of fluorescent protein reporter genes was analyzed 48 h postinfection. Percentage positive cells and the mean fluorescence intensity (MFI) were measured by flow cytometry as previously described [17].
Transmission electron microscopy
CsCl-purified virus was adsorbed onto Formvar/carbon 300-mesh copper TEM grids. Samples were negatively stained with 1% phosphotungstic acid for 1 min and washed once in dH2O. Samples were visualized with a Hitachi 7500 transmission electron microscope at 100 kV and 40,000× magnification.
SDS–PAGE and Western blot analysis
CsCl-purified samples of virus were boiled in SDS–PAGE loading buffer and electrophoresed on a 7.5% polyacrylamide gel. Separated proteins were then transferred to a PVDF membrane in a Bio-Rad Trans-Blot semidry transfer cell (Hercules, CA, USA). Blots were blocked in 5% milk –TBST overnight at 4°C and probed with neutravidin–HRP (diluted 1:2500) in TBST or rabbit anti-[Ad5] polyclonal antibody (1:5000) in TBST. Bound rabbit IgG’s were detected with donkey anti-[rabbit] –HRP conjugate (1:10,000). HRP conjugates were detected with SuperSignal chemiluminescence reagent on Kodak BioMax film.
Direct ELISA
CsCl-purified virus was diluted in PBS and immobilized on Corning high-binding flat-bottom 96-well microtiter plates. Wells were blocked with 5% milk –TBST for 3 h and washed with TBST. Neutravidin – HRP (1:5000) in TBST was bound to immobilized virions and wells were washed extensively in TBST. Bound HRP was detected by adding 100 µl of TMB substrate, incubating for 30 min, and stopping the reaction with 50 µl of 1.8 M H2SO4. Absorbance at 450 nm was measured on an automated microplate reader and values were plotted as mean absorbances of triplicate samples ± standard deviation.
MACS streptavidin µbead depletion assay
Vector (1 × 109 VP) was diluted in 250 µl of serum-free DMEM containing 25 µl of MACS streptavidin-coated µbeads (avg diameter 50 nm). Streptavidin µbeads were complexed to the vector for 15 min at 4°C and half of all samples were loaded on a MACS magnetic µcolumn. Flowthrough fraction was collected for each sample. Aliquots of each sample (input and flowthrough) were assayed for gene transfer on HeLa cells as described above. Percentage transduction was calculated by dividing the MFI of the column flowthrough fractions by the MFI of the input fractions and multiplying by 100.
Magnetofection
CHO cells were grown to confluence in 12-well plates (approximately 570,000 cells per well). Rare-earth magnets (5 mm diameter) were taped underneath half of the wells prior to plating. Each vector (6 × 108 VP) was diluted into 500 µl HBSS to which 2 µl of streptavidin-coated superparamagnetic µbeads (1 µm diameter) or 2 µl control buffer was added. Samples were mixed by pipetting and incubated for 15 min at 4°C. CHO cell monolayers were washed twice in HBSS and overlaid with 500 µl of each vector complex (m.o.i. c 1000 VP/cell). Plates were placed on an orbital shaker for 20 min at room temperature to allow the magnets to trap the paramagnetic µbeads. Complete RPMI (1.5 ml) was added to each well and the plates were incubated at 37°C. Cells were photographed 24 h posttransduction under a fluorescence microscope to show expression of dsRed and EGFP-luc. Groups with magnets were photographed in the location where the magnet was affixed. To illustrate the phenomenon better, the samples containing the Ad-Fiber-BAP vector were also assayed for luciferase expression with the Night Owl CCD camera. Samples were washed once with HBSS, and 1 ml of d-luciferin (1 mg/ml in HBSS) was added to each well. Samples were photographed with the Night Owl camera to visualize luciferase expression.
Avidin-based Targeted Transduction
K562 cells (106) were washed in HBSS-BSA (HBSS with 1% bovine serum albumin, Ab/Am) and resuspended in 100 µl of biotinylated antibodies (1:10 dilution in HBSS-BSA), NHS-LC-biotin (0.5 ng/cell in HBSS), or control HBSS and incubated for 30 min at 4°C. Cells were then washed and pelleted three times before the addition of 1 ml of HBSS-BSA plus neutravidin (0.01 ng/cell for antibody targeting or 0.1 ng/cell for NHS-LC-biotin targeting). Incubation and washes were repeated as described above and finally Ad5 vectors were added at an m.o.i. of 5000 VP/cell in 1 ml of HBSS-BSA. Cells were incubated for 30 min at 4°C and washed three times before final resuspension in 2 ml of complete RPMI medium. Transduction was analyzed by flow cytometry 48 h later for antibody-mediated transduction and 96 h later for chemically biotinylated cells, as previously described [17]. C2C12 myoblasts (2.5 × 105) were plated in 24-well plates and maintained with complete DMEM. Once a confluency of ~60–70% was reached, the cells were tubed by culturing them in DMEM with 2% horse serum plus Ab/Am for 2 days. Once a confluent myotube phenotype was present the cells were washed with HBSS-BSA and incubated with 250 µl biotinylated transferrin (1:10 dilution in HBSS-BSA) or control HBSS for 30 min at 4°C. Cells were then washed with HBSS-BSA three times before the addition of 250 µl of HBSS-BSA plus neutravidin (0.01 ng/cell). Cells were incubated as before and washed three more times and incubated with biotinylated vector at an m.o.i. of 5000 in 250 µl. Cells were incubated as before and rinsed three times prior to the addition of 1 ml DMEM plus 2% horse serum plus Ab/Am. Transduction was visualized 36 h later with fluorescence microscopy.
Purification on Monomeric Avidin Resin
293A cells were plated on 150-mm dishes and grown to ~95% confluence in biotin-supplemented medium. Medium was aspirated off and cells were infected with virus at an m.o.i. of 20. Infections were performed in 7 ml DMEM plus Ab/Am, 30 µM biotin for 3 h at 37°C. Cells were then supplemented with 18 ml complete medium and grown for 72 h. Once all cells were rounded and detached, medium and cells were collected in 50-ml conical tubes and spun down at 300g for 10 min, and the supernatant was dumped. The cell pellets were gently washed twice in 15 ml stabilization buffer (SB; 50 mM Tris, pH 8, 150 mM NaCl, 5% glycerol) and transferred to 15-ml conical tubes. The final cell pellet was resuspended in 1 ml SB, transferred to µcentrifuge tubes, and put through three freeze – thaw cycles with vortexing to lyse the cells. SoftLink monomeric avidin resin (2.5 ml) was preadsorbed with biotin and regenerated according to the manufacturer’s instructions. Refolded resin was washed once in 10 ml SB and resuspended in a final volume of 2.5 ml SB. Cell lysates were spun down at 10,000g and 500 µl of cleared lysate was added to 500 µl resuspended resin and incubated for 4 h with gentle shaking at 4°C. After the initial incubation, the resin was pelleted and washed 5× with 12 ml SB. All unbound and wash fractions were collected and stored at 4°C. Virus was eluted by adding 500 µl SB + 5 mM biotin and gently shaking overnight at 4°C. Aliquots of each fraction were assayed for bioactivity on HeLa cells and for protein content with SDS–PAGE and Western blot analysis.
Acknowledgments
We thank Mary E. Barry, Jared Abramian, and Torey Batts for their excellent technical assistance. This work was supported by a grant to M.A.B. from the Muscular Dystrophy Association (3073). S.K.C. was supported by the NIH Biotechnology Training Grant at Rice University (5 T32 GMO 08362). M.B.P. was supported by the BCM Department of Immunology Training Grant (AI07495) from NIH-NIAIDS and the Medical Scientist Training Program at BCM.
References
- 1.Akalu A, Liebermann H, Bauer U, Granzow H, Seidel W. The subgenus-specific C-terminal region of protein IX is located on the surface of the adenovirus capsid. J. Virol. 1999;73:6182–6187. doi: 10.1128/jvi.73.7.6182-6187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry MA, et al. Biotinylated gene therapy vectors. Expert Opin. Biol. Ther. 2003;3:926–940. doi: 10.1517/14712598.3.6.925. [DOI] [PubMed] [Google Scholar]
- 3.Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J. Virol. 2002;76:8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson JM, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dmitriev I, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dmitriev IP, Kashentseva EA, Curiel DT. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J. Virol. 2002;76:6893–6899. doi: 10.1128/JVI.76.14.6893-6899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furcinitti PS, van Oostrum J, Burnett RM. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J. 1989;8:3563–3570. doi: 10.1002/j.1460-2075.1989.tb08528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh-Choudhury G, Haj-Ahmad Y, Graham FL. Protein IX, a minor component of the human adenovirus capsid, is essential for the packaging of full length genomes. EMBO J. 1987;6:1733–1739. doi: 10.1002/j.1460-2075.1987.tb02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greber UF. Virus assembly and disassembly: the adenovirus cysteine protease as a trigger factor. Rev. Med. Virol. 1998;8:213–222. doi: 10.1002/(sici)1099-1654(1998100)8:4<213::aid-rmv225>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 12.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Qian ZM. Transferrin/transferrin receptor-mediated drug delivery. Med. Res. Rev. 2002;22:225–250. doi: 10.1002/med.10008. [DOI] [PubMed] [Google Scholar]
- 14.Medina-Kauwe LK. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Delivery Rev. 2003;55:1485–1496. doi: 10.1016/j.addr.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J. Virol. 2000;74:7085–7095. doi: 10.1128/jvi.74.15.7085-7095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandori MW, Hobson DA, Sano T. Adenovirus-microbead conjugates possess enhanced infectivity: a new strategy for localized gene delivery. Virology. 2002;299:204–212. doi: 10.1006/viro.2002.1510. [DOI] [PubMed] [Google Scholar]
- 17.Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK, Barry MA. Metabolically biotinylated adenovirus for cell-targeting, ligand screening, and vector purification. Mol. Ther. 2003;8:689–702. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 18.Parrott MB, Barry MA. Metabolic biotinylation of recombinant proteins in mammalian cells and in mice. Mol. Ther. 2000;1:96–104. doi: 10.1006/mthe.1999.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrott MB, Barry MA. Metabolic biotinylation of secreted and cell surface proteins from mammalian cells. Biochem. Biophys. Res. Commun. 2001;281:993–1000. doi: 10.1006/bbrc.2001.4437. [DOI] [PubMed] [Google Scholar]
- 20.Poteete AR. What makes the bacteriophage λ Red system useful for genetic engineering: molecular mechanism and biological function. FEMS. 2001;201:9–14. doi: 10.1111/j.1574-6968.2001.tb10725.x. [DOI] [PubMed] [Google Scholar]
- 21.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 22.Reddy DV, Shenoy BC, Carey PR, Sönnichsen FD. High resolution structure of the 1.3S subunit of transcarboxylase from Propionibacterium shermanii. Biochemistry. 2000;39:2509–2516. doi: 10.1021/bi9925367. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds PN, Dmitriev I, Curiel DT. Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Ther. 1999;6:1336–1339. doi: 10.1038/sj.gt.3300941. [DOI] [PubMed] [Google Scholar]
- 24.Rosa-Calatrava M, Grave L, Puvion-Dutilleul F, Chatton B, Kedinger C. Functional analysis of adenovirus protein IX identifies domains involved in capsid stability, transcriptional activity, and nuclear reorganization. J. Virol. 2001;75:7131–7141. doi: 10.1128/JVI.75.15.7131-7141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherer F, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 26.Shenk TE. Adenoviridae: The Viruses and Their Replication. 4th ed. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 27.Smith JS, et al. Redirected infection of directly biotinylated recombinant adenovirus vectors through cell surface receptors and antigens. Proc. Natl. Acad. Sci. USA. 1999;96:8855–8860. doi: 10.1073/pnas.96.16.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith TAG, et al. In vivo hepatic adenoviral gene delivery occurs independent of the coxsackievirus – adenovirus receptor. Mol. Ther. 2002;5:770–779. doi: 10.1006/mthe.2002.0613. [DOI] [PubMed] [Google Scholar]
- 29.Smith TAG, et al. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 2003;14:777–787. doi: 10.1089/104303403765255165. [DOI] [PubMed] [Google Scholar]
- 30.Stewart PL, Burnett RM, Cyrklaff M, Fuller SD. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67:145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- 31.Trotman LC, Achermann DP, Keller S, Straub M, Greber UF. Non-classical export of an adenovirus structural protein. Traffic. 2003;4:390–402. doi: 10.1034/j.1600-0854.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 32.van Oostrum J, Burnett RM. Molecular composition of the adenovirus type 2 virion. J. Virol. 1985;56:439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigne E, Mahfouz I, Dedieu JF, Brie A, Perricaudet M, Yeh P. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J. Virol. 1999;73:5156–5161. doi: 10.1128/jvi.73.6.5156-5161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- 35.Wickham TJ, Mathias P, Cheresh DA, Nemerow GL. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not adenovirus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 36.Wickham TJ, et al. Targeted adenovirus gene transfer to endothelial and smooth muscle cells using bispecific antibodies. J. Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wickham TJ, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv. Drug Delivery Rev. 2003;55:1439–1466. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]