Abstract
Recent evidence suggests that HIV-induced pathogenesis is exacerbated by opioid abuse and that the synergistic toxicity may result from direct actions of opioids in immature glia or glial precursors. To assess whether opioids and HIV proteins are directly toxic to glial-restricted precursors (GRPs), we isolated neural stem cells from the incipient spinal cord of embryonic day 10.5 ICR mice. GRPs were characterized immunocytochemically and by RTPCR. At 1 day in vitro (DIV), GRPs failed to express μ (MOR or MOP) or κ-opioid receptors (KOR or KOP); however, at 5 DIV, most GRPs expressed MOR and KOR. The effects of morphine (500 nM) and/or Tat (100 nM) on GRP viability were assessed in GRPs at 5 DIV by examining the apoptotic effector caspase-3 and cell viability (ethidium monoazide exclusion) at 96 h following continuous exposure. Tat or morphine alone or in combination caused significant increases in GRP cell death at 96 h, but not at 24 h, following exposure. Although morphine or Tat caused increases in caspase-3 activity at 4 h, this was not accompanied with increased cleaved caspase-3 immunoreactive or ethidium monoazide-positive dying cells at 24 h. The results indicate that prolonged morphine or Tat exposure is intrinsically toxic to isolated GRPs and/or their progeny in vitro. Moreover, MOR and KOR are widely expressed by Sox2 and/or Nkx2.2-positive GRPs in vitro and the pattern of receptor expression appears to be developmentally regulated. The temporal requirement for prolonged morphine and HIV-1 Tat exposure to evoke toxicity in glia may coincide with the attainment of a particular stage of maturation and/or the development of particular apoptotic effector pathways and may be unique to spinal cord GRPs. Should similar patterns occur in vivo then we predict that immature astroglia and oligodendroglia may be preferentially vulnerable to HIV-1 infection or chronic opiate exposure.
Keywords: Human immunodeficiency virus (HIV), opiate drug abuse, glial progenitors, neural stem cells, gliogenesis, cell death
INTRODUCTION
Injection drug abuse is a major cause of the spread of HIV/AIDS. Heroin, morphine and other opioid drugs of abuse not only promote HIV infection and the progression of AIDS (Nath et al., 2002; Steele et al., 2003), but also appear to intrinsically exacerbate the frequency and severity of HIV encephalitis (HIVE) in the CNS (Bell et al., 1998; Gurwell et al., 2001; Anthony et al., 2005). Despite the prevalence of HIV infection among drug abusers and importance of the opioid system in the pathogenesis of HIV, it is uncertain how opioid abuse augments the neuropathology of HIV (Nath et al., 2000; Nath, 2002).
Developing neural cells can express μ (MOR or MOP), δ (DOR or DOP), and/or κ (KOR or KOP) opioid receptors, which can be important in regulating cell proliferation, differentiation, and survival (Hauser and Mangoura, 1998). The particular effects seen for each receptor type are contextual and differ among cell types and at different stages of development. Immature neurons (Hauser et al., 2000; Eisch and Harburg, 2006; Narita et al., 2006a), astrocytes (Stiene-Martin and Hauser, 1990; Eriksson et al., 1990; Eriksson et al., 1991; Stiene-Martin and Hauser, 1991; Hauser et al., 1996; Stiene-Martin et al., 1998; Belcheva et al., 2005), oligodendrocytes (Knapp et al., 1998; Stiene-Martin et al., 2001), and their precursors (Persson et al., 2003a; Persson et al., 2003b; Persson et al., 2006; Kim et al., 2006) can respond uniquely to opioids. For example, MOR receptor activation can inhibit proliferation in immature astroglia, while activation of the same receptor type in immature oligodendroglia increases proliferation (Knapp et al., 1998).
We previously found that μ opioid receptor (MOR or MOP) activation exacerbated HIV-1 Tat cytotoxicity in oligodendrocyte-type 2 astrocyte (O-2A) progenitors (Khurdayan et al., 2004). The interactive toxicity was highly selective for O-2A progenitors and was not evident in more differentiated type 2 astrocytes and oligodendrocytes. To determine whether opiates and HIV-1 proteins are intrinsically toxic to glial precursors, we examined the effects of morphine, a prototypic opioid drug of abuse and preferential MOR agonist, on glial restricted precursors (GRPs) isolated from neural stem cells from embryonic mouse spinal cord.
Moreover, because morphine can have subtle actions via κ opioid receptors (KOR or KOP), and because MOR and KOR are expressed by developing neural cells (Hauser and Mangoura, 1998), and can independently affect cellular response/responsiveness to HIV-1 (Peterson et al., 1993; Chao et al., 1996; Peterson et al., 1999; Peterson et al., 2001; Chao et al., 2001), the patterns of expression of MOR and KOR were assessed in detail. GRPs were characterized by nestin, Sox2, A2B5 and Nkx2.2 immunoreactivity. Our findings indicate that opioid receptors are widely expressed by Sox2-positive and Nkx2.2-positive GRPs. In addition, both Tat and morphine alone are intrinsically lethal to GRPs, while in combination they display no additional interactive toxicity. This differs from the situation with A2B5-positive, bipotential O-2A progenitors, in which toxicity was seen only with combined Tat and morphine exposure (Khurdayan et al., 2004), suggesting a developmentally or regionally specific response of glial progenitors to HIV and/or opioid toxicity.
EXPERIMENTAL PROCEDURES
Cell culture
Pregnant ICR mice were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA). All experimental protocols conformed to local Institutional Animal Care and Use Committee (IACUC) and national (PHS) guidelines on the care and ethical use of animals. Experiments were designed and conducted to minimize the number of mice used and their discomfort. On gestational day 10.5 ± 0.5 (E10.5), mice were euthanized with ether and the embryos removed aseptically and kept on ice in cell culture media. Spinal cords were isolated from each embryo and pooled together such that the neural cells derived from the spinal cords of an entire litter (8-12 embryos) constituted a single statistical observation. As reported previously (Kalyani et al., 1997), cells were dissociated by incubating spinal cords in 0.05% Trypsin-EDTA solution (15 min) on ice, followed by incubation in cell culture medium containing 1% bovine serum albumin (BSA; Roche Diagnostics, Indianapolis, IN, USA) for 15 min and then by triturating 18-times through a fire-polished, glass Pasteur pipette. GRPs were isolated by immunopanning in dishes precoated with A2B5 antibodies prepared from an A2B5-expressing hybridoma cell line (Clone 105[HB-29]; ATCC, Manassas, VA, USA) for 1 h at room temperature at pH 7.0 (Mayer-Proschel et al., 1997; Mujtaba et al., 1999; Cao et al., 2005). Non-adherent cells were removed by rinsing with Dulbecco's phosphate buffered saline (PBS) and discarded. A2B5-adherent cells were gently removed, using a cell scraper, before plating onto poly-L-ornithine (15 μl/ml; Sigma, St. Louis, MO, USA) and laminin (20 μl/ml; GIBCO-Invitrogen Corporation, Gaithersburg, MD, USA) coated cell culture plates (24 or 48 wells). Approximately 95% of cells isolated by panning were A2B5 immunoreactive. Cultures containing less than 95% A2B5 positive cells were discarded. GRPs were cultured for 1 or 5 DIV at 35.5 °C in 5% CO2/95% air and high humidity in Dulbecco's modified Eagle's medium/F12 (DMEM/F-12) containing N-2 supplement, recombinant murine fibroblast growth factor-2 (FGF-2, also known as basic FGF) (20 ng/ml; PeproTech Inc., Rocky Hill, NJ, USA) and platelet-derived growth factor-AA (PDGF-AA) (10 ng/ml; PeproTech Inc.) antibiotics (penicillin and streptomycin).
HIV-1 Tat protein
Recombinant Tat1-72 from HIVBRU was prepared as described previously (Ma and Nath, 1997) with slight modifications (Gurwell et al., 2001). A deletion mutant variant of Tat1-72 (TatΔ31-61) lacking the core and basic neurotoxic domains (Nath et al., 1996) or immunoneutralized Tat1-72 were used as controls as previously described (Gurwell et al., 2001; Khurdayan et al., 2004).
Experimental Treatments
GRPs were treated with morphine sulfate (500 nM), Tat1-72, immunoneutralized Tat1-72, or mutant TatΔ31-61 (100 nM). Some cultures were treated with the MOR antagonist β-funaltrexamine (β-FNA; Research Biochemical International, Natick, MA, USA) (1.5 μM).
PCR
Opioid receptor mRNA was assessed by reverse transcriptase-polymerase chain reaction (RTPCR). GRPs were grown for 1 or 5 DIV and total RNA isolated from GRPs using GenElute™ Mammalian total RNA kit (Sigma). The first strand of cDNA was synthesized using 0.2 μg total RNA and random decamers. The PCR cycles performed as 30 sec at 94 °C, 30 sec at 55 °C and 50 sec at 68 °C for 35 cycles using platinum Taq DNA polymerase (GIBCO-Invitrogen Corporation, Gaithersburg, MD, USA). Negative controls included all the reagents essential for amplification with the exception of template DNA. 15S ribosomal control primers (5’-TTCCGCAAGTTCACCTACC-3’ and 5’-CGGGCCATGCTTTACG-3’) (Ambion, Austin, TX, USA) were included as an internal standard. The sense primer used for MOR started from proximal initiation sites (Pm1:5’-CTCAGAGAGTGGCGCTTTGGGGATGC-3’) and the antisense primer was 5’-CGCTAAGGCGTCTGCCAGAGCAAG-3’. KOR primers consisted of 5’-ATCAGCGATCTGGAGCT-3’ and 5’-GCAAGGAGCATTCAATGAC-3’ (Wei et al., 2000). The final PCR products were analyzed on 2% agarose gels. The identities of the amplified PCR products were confirmed by sequencing (Interdisciplinary Center for Biotechnology Research, University of Florida, USA).
Immunocytochemistry
The presence of opioid receptors on GRPs was characterized immunocytochemically. After washing two times, GRPs grown for 1 or 5 DIV were fixed using 3% Zamboni's solution for 30 min, then permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature. Immunodetection was performed by incubating with 1% donkey serum in PBS with rabbit anti-MOR-1 antisera (1:1,000 dilution; AB1580, Chemicon International, Temecula, CA, USA), rabbit anti-δ (DOR-1 or OPRD1; AB1560 N-terminus; Chemicon) or rabbit anti-κ (KOR-1 or OPRK1; H-70; Santa Cruz Biotechnology, Santa Cruz, CA) opioid receptor antisera (both at 1:500 dilutions) overnight at 4°C; general procedures as previously described (Hauser et al., 2000). The above anti MOR-1 opioid receptor antibodies were generated against a synthetic peptide (CTNHQLENLEAETAPLP) corresponding to C-terminus of the cloned μ receptor (Chen et al., 1996). This synthetic peptide contains the amino acid sequence (NHQLENLEAETAPLP) originally used to generate anti-MOR-1 antibodies (Arvidsson et al., 1995) (with an N-terminal cysteine for conjugation), which we have extensively employed to characterize MOR expression in developing glia (Hauser et al., 1996; Stiene-Martin et al., 2001). Anti-opioid receptor primary antibodies were detected using donkey anti rabbit secondary antibodies conjugated to Cy3 (Invitrogen-Molecular probes, Eugene, OR, USA) (1:250 dilutions) for 1 h at room temperature in the dark. Controls lacking primary antibodies were used to assess antibody specificity and MOR and KOR expression was independently confirmed by RT-PCR.
Cells were incubated overnight at 4°C in goat anti-Sox2 or Nkx2.2 polyclonal antibodies (both at 1:100 dilutions; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Sox2 or Nkx2.2 primary antibodies were detected using secondary antibodies conjugated to Cy2 or Alexa Fluor 488 (Molecular Probes, Inc., Eugene, OR, USA) (1:250 dilution) by incubating for 1 h at room temperature in the dark. In some cases, mouse anti-Nkx2.2 IgG2b antibodies (1:200 dilution) obtained from the Developmental Studies Hybridoma Bank (DSHB, University of Iowa, Iowa City, USA) were used to co-localize Nkx2.2 with Sox2. Nestin was detected using anti-nestin monoclonal IgG1 (1:1 dilution; DSHB). Mouse anti-A2B5 IgM (1:200, Chemicon) was used to detect oligodendrocyte-type 2 astrocyte bipotent (O-2A) progenitors (Raff et al., 1983; Raff et al., 1984; Gard and Pfeiffer, 1990). In some instances, Hoechst 33342 (15 μg/ml in 0.1% BSA in PBS for 15 min at room temperature) was used to counterstain cell nuclei. To assess the proportion of cells possessing opioid receptor and/or developmental marker immunoreactivity, cell cultures at 1 to 6 DIV were double-labeled for Sox2 or Nkx2.2 and MOR, DOR, or KOR immunoreactivity. Nestin and A2B5 immunoreactivity was also assessed in the cell cultures as previously reported (Khurdayan et al., 2004). For each combination of opioid receptors and developmental markers, approximately 100 cells were counted in separate cell cultures within each experiment. The proportion of immature glia that were immunoreactive for an opioid receptor type or developmental marker are given as the mean ± SEM from 3-4 experiments.
The effects of opioids and HIV-1 Tat on individual GRPs were assessed through immunocytochemical detection of cleaved (activated) caspase-3. To detect cleaved caspase-3, cells were incubated in anti-active caspase-3 antibodies (1:4,000 dilution; R & D Systems, Inc., Minneapolis, MN, USA) overnight at 4°C followed by incubation in secondary antibodies tagged with Alexa 488 (1:250 dilution) for 1 h at room temperature in the dark. Coverslips containing GRPs were mounted on microscope slides using ProLong® Anti-fade mounting medium (Molecular Probes).
Cell Viability assay
Cells were incubated in calcein AM, 4 μM; ethidium homodimer-1, 3.5 μM for 40 min (Live/Dead assay kit, Molecular Probes). Gentle rinsing with PBS stopped the calcein AM and ethidium homodimer reactions. Cells were stored at 4°C in DMEM-F12 and counted within 24 h of the reaction. In some instances, the photoaffinity label, ethidium monoazide (EMA), was substituted for ethidium homodimer as previously described (Singh et al., 2004).
Caspase-3 activity
Caspase-3 enzyme activity was measured as previously described (Singh et al., 2004). Briefly, cell lysates were incubated at 37°C in a buffer (25 mM HEPES, pH 7.5; 10% sucrose; 0.1% CHAPS and 1 mM dithiothreitol supplemented with 50 μM Ac-DEVD-7-amino-4-methylcoumarin [AMC]) in 96 well Costar plates. Fluorescent activity due to cleavage of the fluorogenic AMC moiety was measured using a Victor3 microplate reader (Perkin-Elmer, Boston, MA) at 360 nm excitation and 460 nm emission wavelengths. Caspase-3 activity was expressed as fluorescence units (cleaved AMC) per milligram of total cytosolic protein.
Statistics
For measures where opioid receptors were co-localized with Sox2 and Nkx2.2, approximately 100 cells were counted per condition in each experiment and experiments were repeated at last 3 times. For assays of apoptosis, 400 cells were sampled per treatment group in each experiment. Experiments were repeated 4 to 11 times. Data were expressed as the mean ± the standard error of the mean (SEM). Differences among experimental groups were assessed statistically using ANOVA followed by Duncan's multiple comparisons test (Statistica version 6.1, StatSoft, Tulsa, OK).
RESULTS
Expression of glial markers
A majority of cells expressed Sox2 and Nkx2.2 (Table 1), as well as nestin (not shown) and A2B5, immunoreactivity at 1 DIV after immunopanning and generally retained this pattern of expression at 5 DIV, although there was a tendency for the proportion of Nkx2.2+ cells to decline with progressive maturation in vitro. The pattern of expression of these markers suggests that a majority of GRPs retain Sox2 expression, although subsets of cells are likely to be differentiating toward astroglial and/or oligodendroglial fates (Qi et al., 2001; Wei et al., 2005). In addition, cells appeared to retain their A2B5 antigenicity, since 94.3 ± 1.1% of the cells in our GRP cultures retained A2B5 immunoreactivity at 5 DIV. To assess whether opioid receptors are expressed in glial precursors, MOR, DOR, or KOR antigenicity were co-localized with Sox2 and/or Nkx2.2 immunoreactivity in the same cell.
Table 1.
Characterization of Sox2 and Nkx2.2 immunoreactivity in glial restricted precursors at 1 and 5 DIV.
| Days in vitro (DIV) | Percentage (%) of cells expressing Sox2+ | Percentage (%) of cells expressing Nkx2.2+* |
|---|---|---|
| 1 DIV | 75.5 ± 16.5 | 67.3 ± 4.5 |
| 5 DIV | 64.1 ± 7.5 | 41.9 ± 4.5 |
The proportion of glial precursors that are Sox2 or Nkx2.2 immunoreactive. Mean ± SEM from 3-4 experiments and approximately 100 cells per group were sampled in each experiment.
Opioid receptor expression
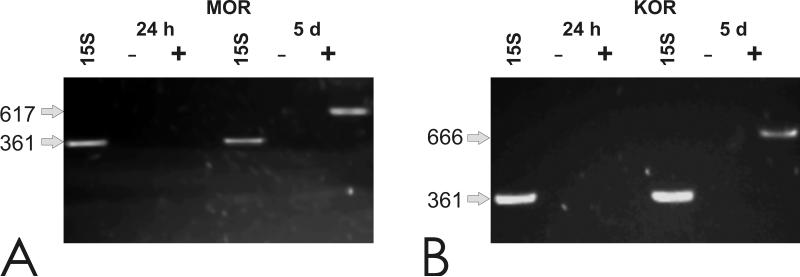
At exactly1 DIV after cells were isolated, GRPs did not express detectable levels of MOR or KOR mRNA (Fig. 1). By contrast, at 5 DIV, MOR and KOR transcripts were expressed by isolated GRPs, suggesting that the expression of these receptor types is developmentally regulated and increases with maturation (Fig. 1).
Fig. 1.
Detection of MOR and KOR opioid receptor transcripts by RT-PCR. MOR (A) or KOR (B) mRNA was not detected at 24 h, but was expressed at 5 DIV; GRPs (+) and blank lanes (−) lacking MOR or KOR mRNA, respectively.
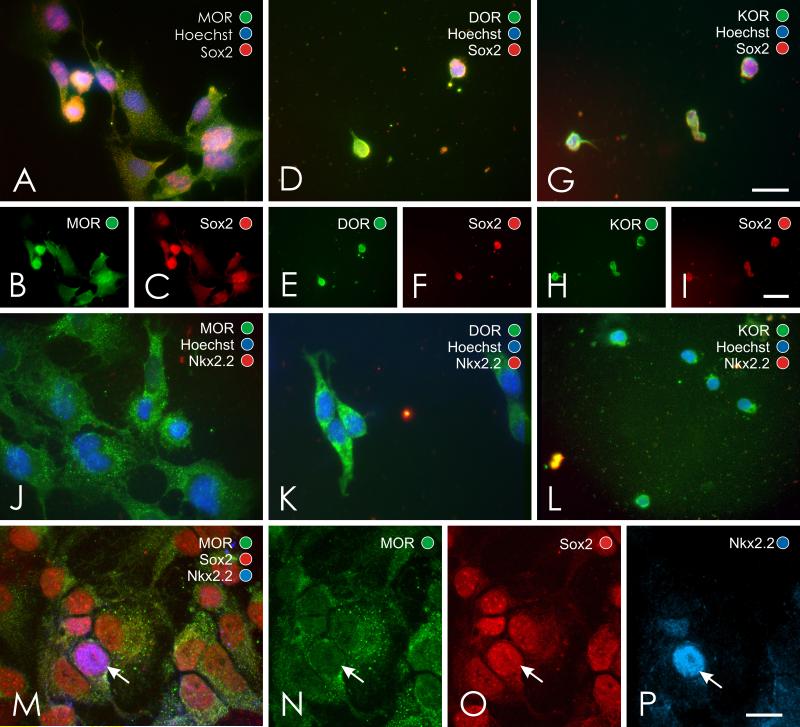
The proportion of glial precursors that expressed MOR or KOR immunoreactivity increased from 2-3 DIV compared to 5 DIV (Fig. 2). MOR, DOR, and KOR immunoreactivity was evident in some cells at 2-3 DIV, suggesting the onset of opioid receptor maturation occurs within a few days of plating. By contrast, by 5 DIV a vast majority of Sox2 or Nkx2.2 positive precursors possessed opioid receptor immunoreactivity (Table 2). In fact, approximately 90% of Sox2 positive cells possess MOR, DOR or KOR, while 69-88% of Nkx2.2 positive cells possess MOR, DOR or KOR immunoreactivity, suggesting that many cells co-expressing Sox2 and Nkx2.2 also possessed opioid receptors. Moreover, expression was not limited to a particular opioid receptor type; rather, MOR, DOR, and KOR immunoreactivity could be readily co-localized in Sox2 or Nkx2.2 positive cells.
Fig. 2.
Co-localization of μ (MOR), δ (DOR), and κ (KOR) opioid receptors with Sox2+ and Nkx2.2+ glial precursors at 2-3 DIV (A-I) and 5 DIV (J-P). Opioid receptors are widely expressed by subpopulations of Sox2+/Nkx2.2+ glial precursors (A-P). Note, MOR, Nkx2.2 and Sox2 immunoreactivity can be localized to the same cell (arrow in M-P) and overlapping Sox2 and Nkx2.2 immunoreactivity was evident in some DOR or KOR expressing cells (data not shown); scale bar = 20 μm (A,D,G); scale bar = 25 μm (B-C,E-F,H-I); scale bar = 10 μm (J-P).
Table 2.
Characterization of opioid receptor immunoreactivity in glial restricted precursors at 5 DIV.
| Developmental marker | Opioid receptor | Percentage (%) co-localized with opioid receptors* |
|---|---|---|
| Sox2 | MOR | 87.9 ± 4.4 |
| DOR | 90.5 ± 1.4 | |
| KOR | 93.9 ± 3.4 | |
| Nkx2.2 | MOR | 88.2 ± 4.0 |
| DOR | 77.8 ± 10.7 | |
| KOR | 69.0 ± 13.1 | |
The proportion of Sox2 or Nkx2.2 immunoreactive glial precursors that expressed μ (MOR), δ (DOR), or k (KOR) opioid receptors at 5 DIV. Mean ± SEM from 3-4 experiments and approximately 100 cells per group were sampled in each experiment.
Caspase-3 activation
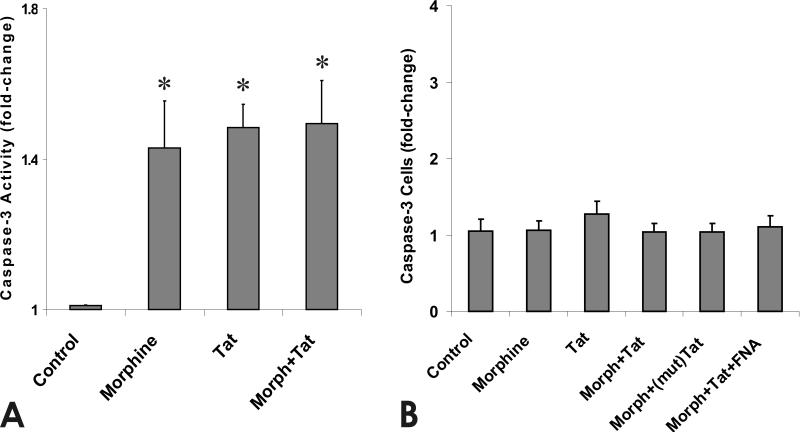
At 1 DIV, exposure to morphine or Tat1-72 for 4 h caused an approximate 1.6-fold increase in caspase-3 activity compared to control cultures (Fig. 3A), while combined exposure did not increase caspase-3 activity beyond levels seen with either substance alone. By contrast, caspase-3 activity was unaffected by morphine and/or Tat following 24 h of continuous exposure, suggesting that the response of immature glia is dependent on the duration of exposure (Fig. 3B).
Fig. 3.
Effects of morphine and/or Tat1-72 on caspase 3 activity at 4 h (A) and on the proportion of cleaved caspase-3 immunoreactive GRPs at 24 h (B). Although morphine or Tat1-72 increased caspase-3 activity at 4 h, the proportion of cleaved caspase-3 immunoreactive GRPs was unchanged at 24 h. Data represent (n=4) independent experiments performed on cells derived from separate litters cultured for 1 DIV. Values are mean fold-change from control ± SEM (*P < 0.05 vs. vehicle-treated control cultures).
Morphine and HIV-1 Tat-induced GRP cell death
Previous studies indicated that Tat and/or morphine, or gp120-induced increases in caspase-3 activity were accompanied by increased neuronal or O-2A progenitor death; however, 24-96 h were required before actual cell losses became evident (Singh et al., 2004; Khurdayan et al., 2004). For this reason, cleaved caspase-3 product was assessed at 24 h and viability was examined at 24 and 96 h in individual GRPs. Despite increases in caspase-3 activity at 4 h, the proportion of caspase-3 immunoreactive cells were not increased above numbers seen in untreated controls at 24 h (Fig. 3B).
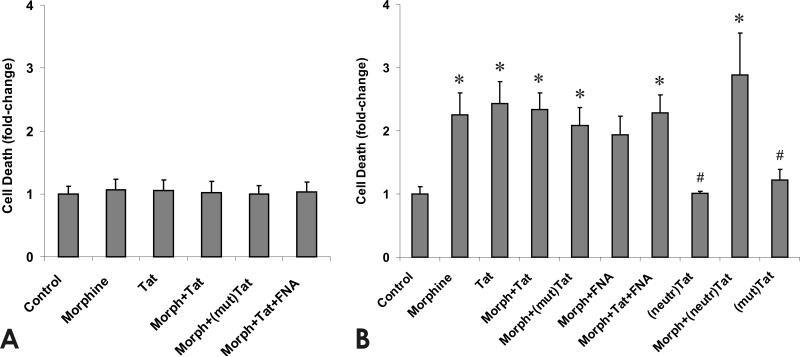
The effects of morphine, HIV-1 Tat1-72, inactive mutant TatΔ31-61 and/or the MOR-specific antagonist β-funaltrexamine (β-FNA) on GRP cell survival were assessed by EMA exclusion. At 24 h, there were no significant effects of morphine and/or Tat on GRP viability (Fig. 4A). Unlike the results at 24 h, continuous exposure for 96 h to morphine or HIV-1 Tat1-72 increased the number of EMA-labeled GRP cells (Fig. 4B). In combination, Tat and morphine displayed no additional cytotoxicity compared to either substance alone. Importantly, Tat toxicity was highly specific since GRP viability was unaffected by continuous exposure for 96 h to mutant TatΔ31-61 or immunoneutralized Tat1-72 (Fig. 3B). Lastly, when morphine was co-administered with the MOR selective antagonist β-FNA, morphine failed to cause significant increases in cell death. Approximately 12.3 ± 0.1% of control GRPs were EMA+ at 5 DIV.
Fig. 4.
Effects of opiates and/or Tat1-72 (Tat), TatΔ31-61 [(mut)Tat], or immunoneutralized Tat [(neutr)Tat] on the viability of glial precursors after 24 h (A) or 96 h (B) of continuous exposure. Both morphine (Morph) and Tat1-72 alone caused significant increases in the proportion of dying, ethidium monoxide-positive (EMA+) glial precursors (*P < 0.05 vs. vehicle-treated controls), while (neutr)Tat and (mut)Tat did not increase the percentage of EMA+ cells (#P < 0.05 vs. treatment with Tat1-72). In combination, morphine and Tat1-72 did not increase the proportion of EMA+ cells beyond the rate of death seen with either substance alone. In the presence of the MOR selective antagonist β-funaltrexamine (FNA), morphine did not cause significant increases in cell death. Values are the mean ± SEM fold-change in EMA+ cells compared to vehicle-treated controls from 4-11 experiments initiated at 1 DIV.
DISCUSSION
Our findings suggest that opioid receptor expression by GRPs isolated from the incipient spinal cord is developmentally regulated in vitro and suggest that opioid receptors are necessary, but not sufficient, in defining critical periods of vulnerability of GRPs and their progeny to opiates. At 1 DIV, GRPs were characteristically nestin, Sox2, and A2B5 immunoreactive (Liu and Rao, 2004), but failed to express MOR and KOR mRNA. However, at 1 DIV, cells exposed to morphine for 4 h showed significant increases in caspase-3 activity, suggesting that opioid receptor mRNA levels were below detection by PCR or that the onset of opioid receptor expression occurs very rapidly after 1 DIV. After 5 DIV, large proportions of glial precursors expressed MOR and KOR and this coincided with increases in the percentage of Nkx2.2 immunoreactive cells. The lack MOR and KOR expression by GRPs at 1 DIV was unexpected. Prior findings indicate that neural and glial precursors can express opioid receptors (Reznikov et al., 1999; Persson et al., 2003a; Persson et al., 2003b; Khurdayan et al., 2004; Kim et al., 2006; Hahn and Knapp, unpublished). The stress of isolating GRPs in culture and/or the loss of key factors available within the extracellular milieu in vivo may temporarily shut down opioid receptor expression. Alternatively, opioid receptor expression in developing glia may normally be regulated by multiple maturational factors and is extremely dynamic (Hauser and Mangoura, 1998).
MOR, KOR, and DOR were widely expressed by Sox2 and Nkx2.2 immunoreactive GRPs. Sox2 is a stem cell marker expressed by self-renewing, pluripotent neural cells within the ventricular zone of the developing CNS (Ellis et al., 2004; Wegner and Stolt, 2005), while Nkx2.2 is a glial specific transcription factor suggestive of an oligodendroglial fate. Although Nkx2.2 and Olig2 are required for oligodendrocyte maturation (Zhou et al., 2001), the expression of Nkx2.2 alone is not sufficient to indicate that a cell is an incipient oligodendrocyte (Qi et al., 2001). The patterns of Sox2 and Nkx2.2 expression suggest opioid receptors are extensively expressed by progenitors very early during gliogenesis. This is interesting because only a subpopulation of their mature astroglial or oligodendroglial progeny will subsequently express MOR, DOR, or KOR (Stiene-Martin et al., 1998; Knapp et al., 1998), although a very large percentage of young, O4+ oligodendrocytes are MOR-positive (Knapp et al., 1998; Tryoen-Toth et al., 2000; Stiene-Martin et al., 2001). Patterns of Sox2 and Nkx2.2 expression also suggest that some opioid-receptor expressing GRPs in the present study are likely to be differentiating into more mature O-2A progenitors, since the proportion of Sox2 and Nkx2.2 cells decline from 1 to 5 DIV. Thus, while opioid receptor expression is dynamic and highly coordinated with glial maturation, the presence of a particular receptor type does not uniquely delineate a particular stage of glial maturation.
Besides temporal differences, regional disparities in the pattern of opioid receptor expression by GRPs may also exist. Regional differences in MOR, DOR, and KOR are reported for astrocytes (Stiene-Martin et al., 1998). Few studies have examined opioid receptor expression in spinal cord glia (Cheng et al., 1997; Xu et al., 2004; Narita et al., 2006b). Prior studies report that MOR and KOR expression occurs prenatally in the developing rodent spinal cord, while the onset of DOR sites occurs approximately at birth (Attali et al., 1990; Leslie et al., 1998). In contrast, other investigators find MOR and KOR mRNA in embryonic day 12.5 (E12.5) mouse spinal cord and DOR transcripts by E15.5 (Zhu et al., 1998). Moreover, DOR selective radioligand binding is present in the gray matter of 14 week human fetal spinal cord (Sales et al., 1989), while the apparent absence of DOR transcripts in human adults suggests that DOR expression maybe transient (Peckys and Landwehrmeyer, 1999). The reported variability and late onset in DOR expression during maturation prompted us to focus on MOR and KOR. Importantly, the patterns of opioid receptor expression and susceptibility to opiates and HIV-1 Tat may be unique to GRPs from the spinal cord glia and may differ from GRPs from other brain regions.
Exposure to either morphine or HIV-1 Tat alone significantly increased GRP death. GRP death was preceded by increases in caspase-3 enzyme activity at 4 h. As noted, 1 day old GRPs showed elevations in caspase-3 activity, but no increases in cell death as measured by EMA exclusion, while after 96 h cells showed marked increases in cell death with longer exposure durations. The lack of significant cell losses when morphine was combined with β-FNA, suggests that morphine is acting through MOR; however, β-FNA only partially attenuated the cytotoxicity inferring that morphine might additionally modulate cell death via a second opioid receptor type. Whether KOR or potentially DOR might be involved awaits further study, but it is feasible that morphine's complex actions in glial precursors are mediated by multiple opioid receptor types. Cytotoxicity coincided with the onset of MOR and KOR expression and progressive glial differentiation in vitro. The present results in GRPs were interesting because they differed markedly from the response of more mature O-2A progenitors assessed in previous studies (Khurdayan et al., 2004). We previously found that MOR activation exacerbates HIV-1 Tat cytotoxicity in O-2A progenitors, while morphine or Tat alone were not cytotoxic and morphine actually reduced the proportion of O-2A progenitors displaying cleaved caspase-3 immunoreactivity (Khurdayan et al., 2004).
The reasons for the discrepancy between GRPs (present study) and more mature O-2A progenitors (Khurdayan et al., 2004) are uncertain, but may relate to maturational differences. Indeed, maturational differences were noticed between GRPs at 1 and 5 DIV in the present study. The protracted delay between caspase-3 activation (seen at 4 h) and onset of cell death (evident at 96 h) has been reported following viral protein ± morphine exposure in other cell types (Singh et al., 2004; Khurdayan et al., 2004). One explanation is there may be a critical threshold for caspase-3 activation before apoptosis is initiated, and the levels and/or duration of caspase-3 activation seen at 4 h were insufficient to trigger apoptosis. Others have shown large temporal discrepancies between caspase activation and cell losses (McEwen and Springer, 2005). Additionally, caspase-3 activation may not necessarily result in cell death (Bellizzi et al., 2006). Another explanation may be that components of Tat- or morphine-mediated apoptotic signaling cascades are not fully developed in GRPs at 1 DIV, although this seems unlikely since caspase-3 activation is one of the final steps in apoptosis (Yuan et al., 2003). Acute opioid agonist exposure can cause oxidative stress in astroglia; however, astroglia may be able to adapt (perhaps temporarily) to sustained exposure (Hauser et al., 1998). A speculative notion is that caspase-3 activation alone is insufficient and additional factors may be needed before cell death can proceed; perhaps the immature glia lack this factor. We previously found that Tat activates caspase-3 and causes endonuclease G release from mitochondria in striatal neurons (Singh et al., 2004). Interestingly, caspase inhibition does not prevent Tat-induced death of striatal neurons suggesting that an alternative, caspase-3-independent mechanism is operative (Singh et al., 2004).
Another consideration is the contribution of astrocytes. Unlike the present studies where cultured cells consisted of GRPs largely uncontaminated by other cell types, our past studies assayed O-2A cells in mixed cultures with astrocytes exposed to morphine and/or Tat. Morphine and Tat cause synergistic increases in the release of cytokines, including IL-6 by astrocytes (see El-Hage et al., 2005), as well as massive increases in oxyradical production (N El-Hage, unpublished), which may contribute to the cytotoxicity seen in O-2A progenitors (Khurdayan et al., 2004). IL-6 is toxic to neural stem cells in the subgranular layer of the dentate gyrus (Monje et al., 2003), and could mediate O-2A cytotoxicity in astrocyte co-cultures.
Morphine's effects during development are cell-type-specific and highly contextual (Hauser and Mangoura, 1998). For example, epidermal growth factor (EGF) ligands negate the antiproliferative effects of morphine in neuronal precursors (Opanashuk and Hauser, 1998). Coscia and coworkers have shown that FGF-2 and EGF signals can converge with MOR signals in astrocytes (Belcheva et al., 2002; Belcheva et al., 2003; Belcheva et al., 2005), and FGF-2 and EGF signals profoundly affect the survival of neural precursors. Importantly, opiates can modulate the response of cells to apoptotic signals (Singhal et al., 1998; Polakiewicz et al., 1998; Suzuki et al., 2003; Bhat et al., 2004) and have been paradoxically described as both neurotoxic and neuroprotective (Hauser and Mangoura, 1998; Tegeder and Geisslinger, 2004; Samways and Henderson, 2006). Thus, morphine's effects on survival are likely to be unique for GRPs and modulated by extracellular factors such as the FGF-2 supplement to the cell culture medium, which is necessary for GRP maintenance.
In summary, we find that GRPs isolated in vitro are intrinsically vulnerable to opiate drug and HIV-1 Tat exposure. Together with previous findings that glial precursors can be infected with HIV-1 (Lawrence et al., 2004), the present results indicate that glial precursors are important cellular targets of drug abuse and HIV-1 encephalitis. Assuming similar patterns of susceptibility occur in vivo, then our findings would suggest that the production and maintenance of astroglial and oligodendroglial populations is preferentially vulnerable to chronic opiate exposure and HIV-1 infection. Importantly, the vulnerability of glial progenitors appears to depend, in part, on stage-specific ontogenetic events and factors that remain to be defined.
Acknowledgements
This work was supported by NIDA grants P01 DA19398, R01 DA13287, and T32 DA016176. We thank Dr. Scott R. Whittemore for advice regarding the isolation of glial precursors. We also thank Dr. Anne Stiene-Martin for expert advice and Mr. Kevin Hascup and Mr. Kenneth Martin for valued technical assistance. Monoclonal nestin and Nkx2.2 antibodies developed, respectively, by Dr. Susan Hokfield and Dr. Thomas Jessell were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHHD and maintained by the University of Iowa, Department of Biological Sciences.
REFERENCES
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol. 2005;31:325–338. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee J-H, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B, Saya D, Vogel Z. Pre- and postnatal development of opiate receptor subtypes in rat spinal cord. Dev Brain Res. 1990;53:97–102. doi: 10.1016/0165-3806(90)90128-l. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Haas PD, Tan Y, Heaton VM, Coscia CJ. The fibroblast growth factor receptor is at the site of convergence between mu-opioid receptor and growth factor signaling pathways in rat C6 glioma cells. J Pharmacol Exp Ther. 2002;303:909–918. doi: 10.1124/jpet.102.038554. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Tan Y, Heaton VM, Clark AL, Coscia CJ. Mu opioid transactivation and down-regulation of the epidermal growth factor receptor in astrocytes: implications for mitogen-activated protein kinase signaling. Mol Pharmacol. 2003;64:1391–1401. doi: 10.1124/mol.64.6.1391. [DOI] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Bellizzi MJ, Lu S-M, Gelbard HA. Protecting the synaapse: evidence for a rational strategy to treat HIV-1 associated neurologic disease. J Neuroimmune Pharmacol. 2006;1:20–31. doi: 10.1007/s11481-005-9006-y. [DOI] [PubMed] [Google Scholar]
- Bhat RS, Bhaskaran M, Mongia A, Hitosugi N, Singhal PC. Morphine-induced macrophage apoptosis: oxidative stress and strategies for modulation. J Leukoc Biol. 2004;75:1131–1138. doi: 10.1189/jlb.1203639. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, Archer S, Bidlack JM, Peterson PK. kappa opioid receptors in human microglia downregulate human immunodeficiency virus 1 expression. Proc Natl Acad Sci U S A. 1996;93:8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Sheng WS, Hu S, Peterson PK. U50488 inhibits HIV-1 expression in acutely infected monocyte-derived macrophages. Drug Alcohol Depend. 2001;62:149–154. doi: 10.1016/s0376-8716(00)00185-x. [DOI] [PubMed] [Google Scholar]
- Chen C, Yin J, Riel JKd, DesJarlais RL, Raveglia LF, Zhu J, Liu-Chen LY. Determination of the Amino Acid Residue Involved in [3H]beta -Funaltrexamine Covalent Binding in the Cloned Rat¦Opioid Receptor. J Biol Chem. 1996;271:21422–21429. doi: 10.1074/jbc.271.35.21422. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Res. 1997;778:367–380. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Rönnbäck L. δ and kappa opiate receptors in primary astroglial cultures from rat cerebral cortex. Neurochem Res. 1990;15:1123–1126. doi: 10.1007/BF01101714. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Rönnbäck L. Mu and delta opiate receptors in neuronal and astroglial primary cultures from various regions of the brain-coupling with adenylate cyclase, localisation on the same neurones and association with dopamine (D1) receptor adenylate cyclase. Neuropharmacology. 1991;30:1233–1239. doi: 10.1016/0028-3908(91)90170-g. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Two proliferative stages of the oligodendrocyte lineage (A2B5+O4− and O4+GalC−) under different mitogenic control. Neuron. 1990;5:615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Harris-White ME, Jackson JA, Opanashuk LA, Carney JM. Opioids disrupt Ca2+ homeostasis and induce carbonyl oxyradical production in mouse astrocytes in vitro: transient increases and adaptation to sustained exposure. Exp Neurol. 1998;151:70–76. doi: 10.1006/exnr.1998.6788. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Houdi AA, Turbek CS, Elde RP, Maxson W., III Opioids intrinsically inhibit the genesis of mouse cerebellar granule cell precursors in vitro: Differential impact of μ and δ receptor activation on proliferation and neurite elongation. Eur J Neurosci. 2000;12:1281–1293. doi: 10.1046/j.1460-9568.2000.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1998;5:437–449. [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyani A, Hobson K, Rao MS. Neuroepithelial stem cells from the embryonic spinal cord: isolation, characterization, and clonal analysis. Dev Biol. 1997;186:202–223. doi: 10.1006/dbio.1997.8592. [DOI] [PubMed] [Google Scholar]
- Khurdayan VK, Buch S, El-Hage N, Lutz SE, Goebel SM, Singh IN, Knapp PE, Turchan-Cholewo J, Nath A, Hauser KF. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19:3171–3182. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Clark AL, Kiss A, Hahn JW, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu and kappa opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem. 2006 doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: μ and κ opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie FM, Chen Y, Winzer-Serhan UH. Opioid receptor and peptide mRNA expression in proliferative zones of fetal rat central nervous system. Can J Physiol Pharmacol. 1998;76:284–293. [PubMed] [Google Scholar]
- Liu Y, Rao MS. Olig genes are expressed in a heterogeneous population of precursor cells in the developing spinal cord. Glia. 2004;45:67–74. doi: 10.1002/glia.10303. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Springer JE. A mapping study of caspase-3 activation following acute spinal cord contusion in rats. J Histochem Cytochem. 2005;53:809–819. doi: 10.1369/jhc.4A6467.2005. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Piper DR, Kalyani A, Groves AK, Lucero MT, Rao MS. Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells. Dev Biol. 1999;214:113–127. doi: 10.1006/dbio.1999.9418. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Miyatake M, Sato F, Wachi H, Seyama Y, Suzuki T. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J Neurochem. 2006a;97:1494–1505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Narita M, Niikura K, Nakamura A, Miyatake M, Yajima Y, Suzuki T. mu-Opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: Comparison between etorphine and morphine. Neuroscience. 2006b;138:609–619. doi: 10.1016/j.neuroscience.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Nath A. Human Immunodeficiency Virus (HIV) Proteins in Neuropathogenesis of HIV Dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. S193-8. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr 31 Suppl. 2002;2:S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nath A, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopamine systems associated with AIDS dementia. Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opanashuk LA, Hauser KF. Opposing actions of the EGF family and opioids: Heparin binding-epidermal growth factor (HB-EGF) protects mouse cerebellar neuroblasts against the antiproliferative effect of morphine. Brain Res. 1998;804:87–94. doi: 10.1016/s0006-8993(98)00647-7. [DOI] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Persson AI, Bull C, Eriksson PS. Requirement for Id1 in opioid-induced oligodendrogenesis in cultured adult rat hippocampal progenitors. Eur J Neurosci. 2006;23:2277–2288. doi: 10.1111/j.1460-9568.2006.04764.x. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Eriksson PS. Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol Cell Neurosci. 2003a;23:360–372. doi: 10.1016/s1044-7431(03)00061-7. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003b;17:1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical mu-opioid receptor. Neuropharmacology. 1999;38:273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Lokensgard JR, Bidlack JM, Chang A, Fang X, Portoghese PS. kappa-Opioid receptor agonist suppression of HIV-1 expression in CD4(+) lymphocytes. Biochem Pharmacol. 2001;61:1145–1151. doi: 10.1016/s0006-2952(01)00574-3. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Schut R, Hu S, Balfour HH, Jr., Chao CC. Enhancement of HIV-1 replication by opiates and cocaine: The cytokine connection. Adv Exp Med Biol. 1993;335:181–188. doi: 10.1007/978-1-4615-2980-4_26. [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney EA, Miller RH. Two glial cell lineages diverge prenatally in rat optic nerve. Dev Biol. 1984;106:53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on the culture. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Reznikov K, Hauser KF, Nazarevskaja G, Trunova Y, Derjabin V, Bakalkin G. Opioids modulate cell division in the germinal zone of the late embryonic neocortex. Eur J Neurosci. 1999;11:2711–2719. doi: 10.1046/j.1460-9568.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- Sales N, Charnay Y, Zajac JM, Dubois PM, Roques BP. Ontogeny of mu and delta opioid receptors and of neutral endopeptidase in human spinal cord: an autoradiographic study. J Chem Neuroanat. 1989;2:179–188. [PubMed] [Google Scholar]
- Samways DS, Henderson G. Opioid elevation of intracellular free calcium: Possible mechanisms and physiological relevance. Cell Signal. 2006;18:151–161. doi: 10.1016/j.cellsig.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by HIV-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal PC, Sharma P, Kapasi AA, Reddy K, Franki N, Gibbons N. Morphine enhances macrophage apoptosis. J Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Opioid-dependent growth of glial cultures: Suppression of astrocyte DNA synthesis by Met-enkephalin. Life Sci. 1990;46:91–98. doi: 10.1016/0024-3205(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. J Neurosci Res. 1991;29:538–548. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Knapp PE, Martin KM, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in μ, δ, and κ–opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Doi RH, Chuang RY. Morphine suppresses lymphocyte apoptosis by blocking p53-mediated death signaling. Biochem Biophys Res Commun. 2003;308:802–808. doi: 10.1016/s0006-291x(03)01472-4. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival--unraveling mechanisms and revealing new indications. Pharmacol Rev. 2004;56:351–369. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- Tryoen-Toth P, Gaveriaux-Ruff C, Labourdette G. Down-regulation of mu-opioid receptor expression in rat oligodendrocytes during their development in vitro. J Neurosci Res. 2000;60:10–20. doi: 10.1002/(SICI)1097-4547(20000401)60:1<10::AID-JNR2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Wei LN, Hu X, Bi J, Loh H. Post-transcriptional regulation of mouse kappa-opioid receptor expression. Mol Pharmacol. 2000;57:401–408. [PubMed] [Google Scholar]
- Wei Q, Miskimins WK, Miskimins R. Stage-specific expression of myelin basic protein in oligodendrocytes involves Nkx2.2-mediated repression that is relieved by the Sp1 transcription factor. J Biol Chem. 2005;280:16284–16294. doi: 10.1074/jbc.M500491200. [DOI] [PubMed] [Google Scholar]
- Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Terman GW, Chavkin C. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J Neurosci. 2004;24:4576–4584. doi: 10.1523/JNEUROSCI.5552-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hsu MS, Pintar JE. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci. 1998;18:2538–2549. doi: 10.1523/JNEUROSCI.18-07-02538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]