Abstract
The activity of a Ca2+-adenosinetriphosphatase (ATP phosphohydrolase, EC 3.6.1.3) increases during the mitotic phase of synchronized mouse mastocytoma P-815X2 cells. The enzyme is synchronized mouse mastocytoma P-815X2 cells. The enzyme is synthesized mainly during a distinct period of the interphase and is activated at mitosis. It is thought to regulate the formation of the mitotic apparatus by controlling the concentration of Ca2+ ions at the site of formation of the mitotic spindle.
Full text
PDF
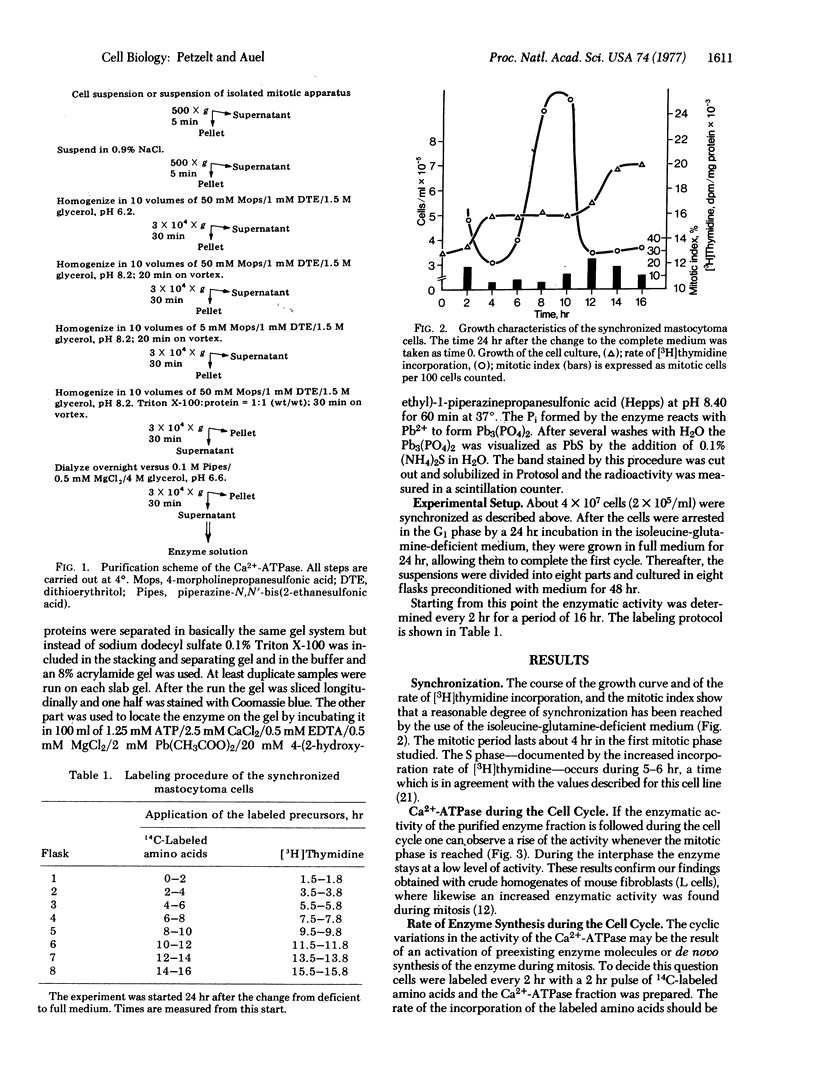
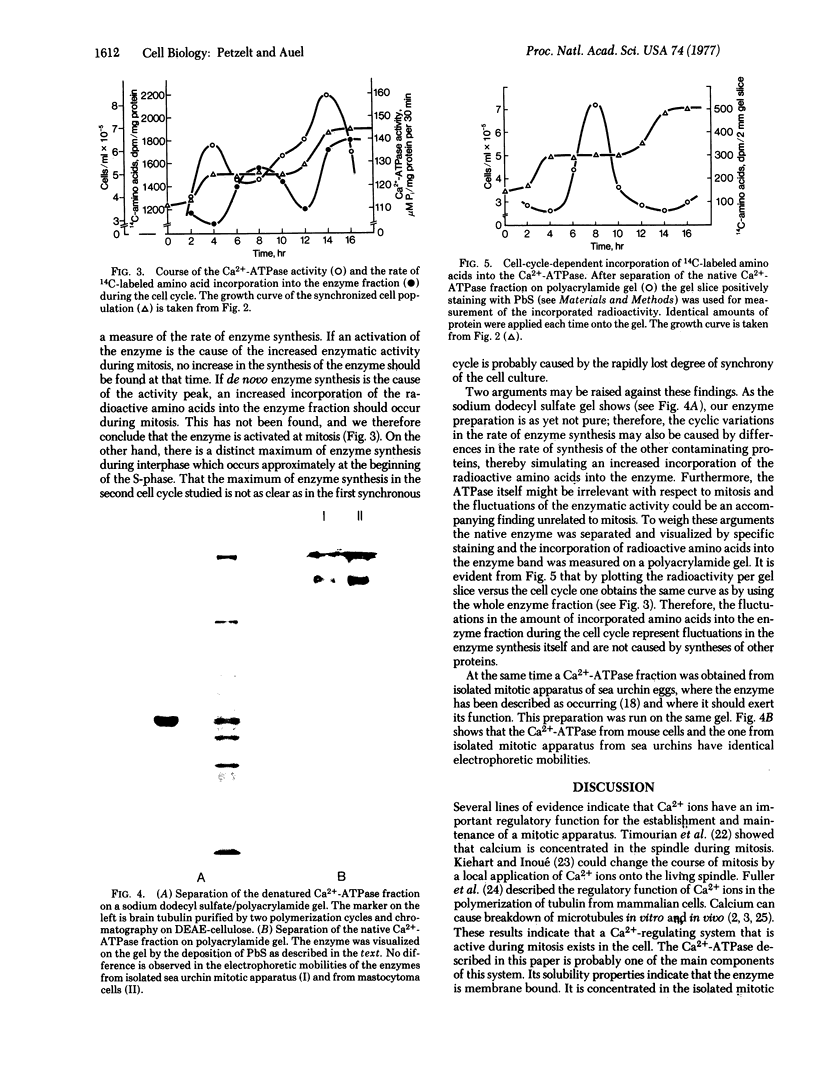

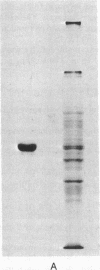
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIBRING T., COUSINEAU G. H. PERCENTAGE INCORPORATION OF LEUCINE LABELLED WITH CARBON-14 INTO ISOLATED MITOTIC APPARATUS DURING EARLY DEVELOPMENT OF SEA URCHIN EGGS. Nature. 1964 Nov 21;204:805–807. doi: 10.1038/204805a0. [DOI] [PubMed] [Google Scholar]
- Byfield J. E., Scherbaum O. H. Temperature effect on protein synthesis in a heat-synchronized protozoan treated with actinomycin D. Science. 1967 Jun 16;156(3781):1504–1505. doi: 10.1126/science.156.3781.1504. [DOI] [PubMed] [Google Scholar]
- Eibl H., Lands W. E. A new, sensitive determination of phosphate. Anal Biochem. 1969 Jul;30(1):51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- GROSS P. R., COUSINEAU G. H. MACROMOLECULE SYNTHESIS AND THE INFLUENCE OF ACTINOMYCIN ON EARLY DEVELOPMENT. Exp Cell Res. 1964 Feb;33:368–395. doi: 10.1016/0014-4827(64)90002-3. [DOI] [PubMed] [Google Scholar]
- Harris P. The role of membranes in the ogranization of the mitotic apparatus. Exp Cell Res. 1975 Sep;94(2):409–425. doi: 10.1016/0014-4827(75)90507-8. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Matsumura F. Calcium binding to bovine tubulin. FEBS Lett. 1975 Oct 15;58(1):222–225. doi: 10.1016/0014-5793(75)80264-x. [DOI] [PubMed] [Google Scholar]
- JACOBSON B. S., SALMON R. J., LANSKY L. L. ANTIMITOTIC EFFECTS OF CHLORAMPHENICOL AND OTHER INHIBITORY AGENTS IN CHLAMYDOMONAS. Exp Cell Res. 1964 Oct;36:1–13. doi: 10.1016/0014-4827(64)90154-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luykx P. Cellular mechanisms of chromosome distribution. Int Rev Cytol. 1970;(Suppl):1–173. [PubMed] [Google Scholar]
- Mazia D. Chromosome cycles turned on in unfertilized sea urchin eggs exposed to NH4OH. Proc Natl Acad Sci U S A. 1974 Mar;71(3):690–693. doi: 10.1073/pnas.71.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. Microtubule research in perspective. Ann N Y Acad Sci. 1975 Jun 30;253:7–13. doi: 10.1111/j.1749-6632.1975.tb19188.x. [DOI] [PubMed] [Google Scholar]
- Mazia D., Petzelt C., Williams R. O., Meza I. A Ca-activated ATPase in the mitotic apparatus of the sea urchin egg (isolated by a new method). Exp Cell Res. 1972 Feb;70(2):325–332. doi: 10.1016/0014-4827(72)90143-7. [DOI] [PubMed] [Google Scholar]
- Nath J., Rebhun J. I. Effects of caffeine and other methylxanthines on the development and metabolism of sea urchin eggs. Involvement of NADP and glutathione. J Cell Biol. 1976 Mar;68(3):440–450. doi: 10.1083/jcb.68.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchman L. G., Stern H. The inhibition of protein synthesis in meiotic cells and its effect on chromosome behavior. Chromosoma. 1969;26(3):298–311. doi: 10.1007/BF00326524. [DOI] [PubMed] [Google Scholar]
- Petzelt C. Ca 2+ -activated APTase during the cell cycle of the sea urchin Strongylocentrotus purpuratus. Exp Cell Res. 1972 Feb;70(2):333–339. doi: 10.1016/0014-4827(72)90144-9. [DOI] [PubMed] [Google Scholar]
- Petzelt C. Further evidence that a Ca 2+ -activated ATPase is connected with the cell cycle. Exp Cell Res. 1972 Sep;74(1):156–162. doi: 10.1016/0014-4827(72)90491-0. [DOI] [PubMed] [Google Scholar]
- Petzelt C. NH3-treatment of unfertilized sea urchin eggs turns on the Ca2+-ATPase cycle. Exp Cell Res. 1976 Oct 1;102(1):200–204. doi: 10.1016/0014-4827(76)90315-3. [DOI] [PubMed] [Google Scholar]
- Petzelt C. UV-irradiation of sea urchin eggs changes the course of the Ca2+-activated ATPase activity. Exp Cell Res. 1974 Jun;86(2):404–408. doi: 10.1016/0014-4827(74)90731-9. [DOI] [PubMed] [Google Scholar]
- Petzelt C., von Ledebur-Villiger M. Ca2+-stimulated ATPase during the early development of parthenogenetically activated eggs of the sea urchin Paracentrotus lividus. Exp Cell Res. 1973 Sep;81(1):87–94. doi: 10.1016/0014-4827(73)90114-6. [DOI] [PubMed] [Google Scholar]
- RUSTAD R. C. Changes in the sensitivity to ultraviolet-induced mitotic delay during the cell division cycle of the sea urchin egg. Exp Cell Res. 1960 Dec;21:596–602. doi: 10.1016/0014-4827(60)90294-9. [DOI] [PubMed] [Google Scholar]
- Raff R. A., Brandis J. W., Green L. H., Kaumeyer J. F., Raff E. C. Microtubule protein pools in early development. Ann N Y Acad Sci. 1975 Jun 30;253:304–317. doi: 10.1111/j.1749-6632.1975.tb19209.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld A. C., Zackroff R. V., Weisenberg R. C. Magnesium stimulation of calcium binding to tubulin and calcium induced depolymerization of microtubules. FEBS Lett. 1976 Jun 1;65(2):144–147. doi: 10.1016/0014-5793(76)80466-8. [DOI] [PubMed] [Google Scholar]
- SCHINDLER R., DAY M., FISCHER G. A. Culture of neoplastic mast cells and their synthesis of 5-hydroxytryptamine and histamine in vitro. Cancer Res. 1959 Jan;19(1):47–51. [PubMed] [Google Scholar]
- Sachsenmaier W., Bohnert E., Clausnizer B., Nygaard O. F. Cycle dependent variation of X-ray effects on synchronous mitosis and thymidine kinase induction in Physarum polycephalum. FEBS Lett. 1970 Oct 5;10(3):185–189. doi: 10.1016/0014-5793(70)80449-5. [DOI] [PubMed] [Google Scholar]
- Schaer J. C., Schindler R. The requirement of mammalian cell cultures for serum proteins. Growth-promoting activity of pepsin-digested serum albumin in different media. Biochim Biophys Acta. 1967 Sep 19;147(1):154–161. doi: 10.1016/0005-2795(67)90098-0. [DOI] [PubMed] [Google Scholar]
- Schindler R., Schaer J. C. Preparation of synchronous cell cultures from early interphase cells obtained by sucrose gradient centrifugation. Methods Cell Biol. 1973;6:43–65. doi: 10.1016/s0091-679x(08)60047-3. [DOI] [PubMed] [Google Scholar]
- Timourian H., Jotz M. M., Clothier G. E. Intracellular distribution of calcium and phosphorus during the first cell division of the sea urchin egg. Exp Cell Res. 1974 Feb;83(2):380–386. doi: 10.1016/0014-4827(74)90352-8. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Wilt F. H., Sakai H., Mazia D. Old and new protein in the formation of the mitotic apparatus in cleaving sea urchin eggs. J Mol Biol. 1967 Jul 14;27(1):1–7. doi: 10.1016/0022-2836(67)90346-4. [DOI] [PubMed] [Google Scholar]
- Zeuthen E. Independent synchronization of DNA synthesis and of cell division in same culture of Tetrahymena cells. Demonstration that heat-shock induced division-synchrony is by differential extension of the G2 phase. Exp Cell Res. 1970 Aug;61(2):311–325. doi: 10.1016/0014-4827(70)90453-2. [DOI] [PubMed] [Google Scholar]