Abstract
Calcitonin gene-related peptide (CGRP) has been viewed as a neuropeptide and vasodilator. However, CGRP is more appropriately thought of as a pleiotropic signaling molecule. Indeed, CGRP has key regulatory functions on immune and inflammatory processes within the skin. CGRP-containing nerves are intimately associated with epidermal LCs and CGRP has profound regulatory effects on Langerhans cell antigen-presenting capability. When LCs are exposed to CGRP in vitro, their ability to present antigen for in vivo priming of naïve mice or elicitation of delayed-type hypersensitivity is inhibited in at least some situations. Administration of CGRP intradermally inhibits acquisition of immunity to Th1-dominant haptens applied to the injected site while augmenting immunity to Th2-dominant haptens, although the cellular targets of activity in these experiments remains unclear. Although CGRP can be a pro-inflammatory agent, several studies have demonstrated that administration of CGRP can inhibit the elicitation of inflammation by inflammatory stimuli in vivo. In this regard, CGRP inhibits the release of certain chemokines by stimulated endothelial cells. This is likely to be physiologically relevant since cutaneous blood vessels are innervated by sensory nerves. Exciting new studies suggest a significant role for CGRP in the pathogenesis of psoriasis and, most strikingly, that CGRP inhibit the ability of LCs to transmit the human immunodeficiency virus 1 to T lymphocytes. A more complete understanding of the role of CGRP in the skin immune system may lead to new and novel approaches for the therapy of immune mediated skin disorders.
Keywords: calcitonin gene-related peptide, LCs, immunity, inflammation
Introduction
CGRP is a sensory neuropeptide, frequently co-expressed with substance P or somatostatin in sensory neurons (Molander et al. 1987). It is a 37 amino acid neuropeptide produced by an alternative splicing of the calcitonin gene (Wimalawansa, 1997). CGRP-containing nerves are distributed throughout various tissues and organs (Franco-Cereceda et al. 1987) and CGRP is expressed in both the central and peripheral nervous systems (Franco-Cereceda et al. 1987, Kresse et al. 1995). However, many other cell types, including monocytes/macrophages (Linscheid et al. 2004), Langerhans cells (LCs, dendritic antigen-presenting cells that reside within the epidermis) (He et al. 2000) and keratinocytes (Hou et al. 2011), amongst others, can produce CGRP. There are two isoforms of CGRP, αCGRP and βCGRP that differ by 1 amino acid in the rat and 3 amino acids in humans (Breimer et al. 1988). βCGRP is produced by a separate gene located in the vicinity of the αCGRP gene on the same chromosome (Lips et al. 1989). It is believed that αCGRP and βCGRP genes were generated from an ancestor gene by gene duplication (Lips et al. 1989). The biological activities of the two isoforms are overlapping (Juaneda et al. 2000). Although this review will cover many of the regulatory and anti-inflammatory effects of CGRP, it has long been known to be a mediator of inflammation. It is a potent vasodilator and plays a role in the recruitment of inflammatory cells at sites of inflammation (Huang et al. 2011, Li et al. 2006, Hartung et al. 1989, Merhi et al. 1998, Benrath et al. 1995). In this regard, CGRP enhances neutrophil adherence to endothelium (Huang et al. 2011, Zimmerman et al. 1992). This review will summarize the evidence that CGRP is an important regulator of immunity within the skin with implications for the pathophysiology of inflammatory skin disorders.
Calcitonin gene-related peptide and Langerhans cells
Within the skin, epidermal LCs are anatomically associated with CGRP-containing nerves (Hosoi et al. 1993). LCs are dendritic antigen-presenting cells that reside in the suprabasalar portion of the epidermis. Classically, LCs were believed to be potent antigen-presenting cells important for the initiation of immune responses in the skin (Inaba et al. 1986, Grabbe et al. 1991). However, many of the experiments examining LC function used cells that have been cultured ex vivo where they would mature during the culture process (Schuler et al. 1985). In the maturation process, LCs upregulate many signaling molecules including CD80, CD86, CD54, CD40, CD83, DC-LAMP, IL-12p40 and CCR7 while downregulating langerin (Madva et al. 2013, Nakagawa et al. 1999, Berthier-Vergnes et al. 2005). Macropinocytosis also is downregulated with maturity (Madva et al. 2013, Sparber et al. 2010). More recent evidence, however, suggests that in the steady state, LCs may serve to downregulate or limit immunity and, perhaps, induce immunologic tolerance (Kaplan et al. 2005, Igyártó et al. 2010). Teleologically, this activity may serve to prevent unwanted immune reactivity against beneficial or commensal organisms. We speculate that chronic/repetitive exposure to CGRP from the associated nerves may play a role in maintaining LCs in an immature state in situ in the absence of a danger signal (see below).
CGRP has long been known to be present in epidermal nerves and has been reported to be associated with Merkel cells [mechanosensitive cells that function in touch sensation (Woo et al. 2014)] within the epidermis (Berthier-Vergnes et al. 2005, Sparber et al. 2010, Kaplan et al. 2005, Igyártó et al. 2010, Cheng-Chew et al. 1996, Vaalasti et al. 1988). With regard to a possible association with LCs, Singaram and colleagues reported that LCs in the esophagus showed CGRP immunoreactivity and staining for CGRP was markedly increased in the setting of esophagitis (Singaram et al. 1991). Within the skin, it was determined that LCs are very closely associated anatomically with CGRP containing epidermal nerves (Hosoi et al. 1993). Of particular importance, it was found that CGRP could inhibit murine LC antigen-presenting function by several criteria (Hosoi et al. 1993). Exposure of mouse epidermal cells to CGRP inhibited their ability to present alloantigen in the mixed epidermal cell-lymphocyte reaction and dose-dependently suppressed their ability to present antigen to a responsive Th1 T-T hybridoma (Hosoi et al. 1993). When epidermal cells (containing LCs) were exposed to CGRP in vitro followed by pulsing with antigen and use for eliciting delayed-type hypersensitivity to that antigen in previously immunized mice, pretreatment with CGRP dose-dependently inhibited the ability to elicit the immune response (Hosoi et al. 1993). Additionally, CGRP treatment of epidermal cells pulsed with antigen inhibited their ability to prime naïve mice to the antigen by subcutaneous injection (Asahina et al. 1995a). Subsequent experiments confirmed that CGRP treatment of highly-enriched populations of LCs (up to ∼95%) inhibited the ability to present antigen for Th1-type responses (Asahina et al. 1995b, Ding et al. 2008). Furthermore, and surprisingly, it was found that CGRP treatment of murine LCs enhanced their ability to present antigen to a Th2 clone and, upon presentation of a fragment of chicken ovalbumin to T cells from DO11.10 chicken ovalbumin T cell receptor transgenic mice, pretreatment with CGRP resulted in increased IL-4 production accompanied by decreased interferon-γ production (Ding et al. 2008). CGRP also inhibited stimulated production of the Th1 chemokines CXCL9 and CXCL10 but induced production of the Th2 chemokines CCL17 and CCL22 (Ding et al. 2008). Thus, CGRP appears to shift LC antigen function away from the Th1 pole toward the Th2 pole.
In accordance with these findings, intradermal administration of CGRP to naïve mice followed by immunization at the injected site by topical application of a hapten leads to an inhibited contact hypersensitivity response to Th1-dominant haptens but an enhanced contact hypersensitive response to Th2-dominant haptens (Asahina et al. 1995a, Mikami et al. 2011).
The molecular and cell biologic changes responsible for these effects are only partly understood. CGRP appears to inhibit stimulated NFκB signaling in murine LCs and, indeed, an inhibitor of NFκB signaling inhibits the ability of LCs to present antigen to a Th1 clone (Ding et al. 2007). CGRP also inhibited the expression of IL-12p40 and IL-1β by murine macrophages and a murine-LC-like cell line induced by treatment with lipopolysaccharide while augmenting the expression of interleukin-10 (Torii et al. 1997). It also inhibits the induction of CD86 expression (Torii et al. 1997).
Consistent with these findings, CGRP has also been found to inhibit antigen presentation by human peripheral blood mononuclear cells, by human blood-derived dendritic cells (Fox et al. 1997, Carucci et al. 2000) and by murine bone marrow-derived dendritic cells (Mikami et al. 2014). To our knowledge experiments examining CGRP effects on dermal dendritic cells or mucosal LCs other than an effect of CGRP on increasing LC langerin expression in human adult inner foreskin explants (discussed below) (Ganor et al. 2013) have not been reported.
Calcitonin gene-related peptide and human immunodeficiency virus (HIV)
Infection with HIV most commonly occurs through sexual activity. In this regard, there is substantial evidence that dendritic cells (DCs) in mucosa transmit HIV-1 to T cells, thus establishing infection within the T cell compartment (Harman et al. 2013). It is believed that HIV-1 entry onto DCs is facilitated by interactions with C-type lectins (Harman et al. 2013, de Witte et al. 2007). Since LCs reside in the epidermis of genital mucosa, they are believed to be the first dendritic cell-type to encounter HIV-1 (Harman et al. 2013). Abundant evidence demonstraes that human LCs can become infected with HIV-1 and transmit the virus to T cells in vitro (Harman et al. 2013). Interestingly, data has been reported that langerin may play a role in preventing HIV-1 transmission by LCs (de Witte et al. 2007, Ganor et al. 2013). Langerin, a C-type lectin, is believed to recognize mannose, fucose and N-acetylglucosamine structures on a number of microorganisms (Lee et al. 2011). It appears to bind to HIV-1 with the virus internalized into Birbeck granules and then degraded (de Witte et al. 2007). Inhibition of langerin binding to HIV-1 through the use of a blocking antibody or mannan (that binds to C-type lectins), resulted in enhanced transmission of HIV-1 to T cells (de Witte et al. 2007). The authors hypothesize that langerin protects LCs from infection with the virus and, thus, subsequent transmission to T cells (de Witte et al. 2007). de Witte et al concluded that LCs actually function as a protective mechanism in intact mucosa working to prevent HIV-1 infection (de Witte et al. 2007). However, this protective effect was observed only with relatively low concentrations of HIV-1; at high doses the protective effect is lost.
Most interestingly, a recent paper reports a possible role for CGRP in inhibiting LC-mediated HIV-1 transmission (Ganor et al. 2013). In this study, monocyte-derived human LCs (MDLCs) were utilized. When MDLCS were pre-treated with CGRP, a dose and time-dependent inhibition of HIV-1 transfer to T cells in vitro was observed (Ganor et al. 2013). Maximal inhibition was seen with treatment for 24 hours with 100 nM CGRP; this resulted in an inhibition of approximately 73% compared to controls not treated with CGRP (Ganor et al. 2013). Interestingly, pre-treatment of T cells with CGRP, instead of MDLCs, had no effect on transmission. CGRP was found to exert its ability to inhibit HIV-1 transfer via its receptor as the antagonist CGRP8–37 prevented the CGRP effect while blockade of the amylin receptor had no activity (Ganor et al. 2013). Of interest, CGRP treatment significantly increased langerin expression on MDLCs and decreased expression of certain integrins (Ganor et al. 2013). Furthermore, inhibitors of the NF-κB pathway abrogated the inhibition of HIV-1 transfer, suggesting that activation of NF-κB is involved in this CGRP effect (Ganor et al. 2013). CGRP treatment of human adult inner foreskin explants also increased langerin expression on resident LCs (Ganor et al. 2013). These authors also found that CGRP treatment decreased viral replication within MDLCs (Ganor et al. 2013). CGRP pre-treatment of MDLCs decreased expression of CD29, CD49e and CD50 (Ganor et al. 2013) and, in accordance with this finding, CGRP treatment of MDLCs inhibited the proportion of cells adhering to fibronectin-coated plates and also decreased the percentage of conjugates formed between MDLCs and T cells in vitro. In a subsequent study, the same group found that LCs in the basal state secreted low basal levels of endogenous CGRP, which increased markedly following CGRP treatment (Ganor et al. 2014). CGRP exposure also enhanced expression of its cognate receptor on LCs (Ganor et al. 2014).
Also of interest, these investigators found that CGRP levels in blood were significantly decreased in a group of HIV-1 infected individuals compared with healthy controls and in a group of HIV-1 infected persons receiving highly active anti-retroviral therapy, CGRP levels normalized (Ganor et al. 2013). These observations may relate to the earlier finding that infection is associated with loss of cutaneous innervation and reduced epidermal nerve fiber density (Ganor et al. 2013, McCarthy et al. 1995, Zhou et al. 2007).
These quite interesting findings, of course, suggest that CGRP may play a protective role in situ against LC-mediated HIV-1 infection and suggest an important new area of investigation with obvious clinical implications.
Calcitonin gene-related peptide inhibits inflammation
Many studies have reported that systemic or local administration of CGRP to animals inhibits the magnitude of an induced inflammatory stimulus in the animal. For example, Gomes and collaborators reported that intraperitoneal administration of CGRP inhibited by approximately 50% the number of neutrophils found in mouse blood and in the peritoneal cavity 4 hours after injection of the peritoneal cavity with lipopolysaccharide (Gomes et al. 2005); lipopolysacchride (also known as endotoxin) is an inflammatory component of the membrane of gram-negative bacteria (Rhee 2014). Strikingly, pretreatment of mice with CGRP protected against a lethal dose of lipopolysaccharide (Gomes et al. 2005). The protective effect could be inhibited by the CGRP receptor antagonist CGRP8–37 and a protective effect of CGRP correlated with inhibition of tumor necrosis factor alpha (TNFα) while inducing serum levels of IL-6 and IL-10 (Gomes et al. 2005). Furthermore, CGRP enhanced IL-10 production production by peritoneal macrophages in vitro while inhibiting TNFα secretion (Gomes et al. 2005).
In another study, hamster cheek pouches were treated topically with CGRP or were not treated followed by application of histamine [a mast cell mediator that, amongst other physiologic functions, causes vasodilitation and increases permeability capillaries; it is involved in urticaria and some other inflammatory skin disorders (Greaves et al. 205)] and assessment of capillary leakage (Raud et al. 1991). Pretreatment with CGRP reduced the total leakage significantly. CGRP treatment had to be before histamine; in co-administration experiments CGRP had no effect. In another experiment, CGRP was administered by sub-plantar injection into a rat paw while the other paw was injected with the vehicle only (Raud et al. 1991). Twenty minutes later, both paws were challenged with subplantar injections of 5-hydroxytryptamine and paw swelling assessed. Paw swelling was markedly suppressed on the CGRP-treated side compared to the control paw (Raud et al. 1991). Most interestingly, human volunteers received an intradermal injection of CGRP in one arm and dilulent alone in the other arm (Raud et al. 1991). Subsequently, each site was injected intradermally with histamine and the wheal and flare responses quantified. CGRP significantly suppressed the histamine-induced wheal but had no effect on the flare reaction (Raud et al. 1991). Injection of CGRP by itself produced a flare but no wheal.
CGRP demonstrated anti-inflammatory activities in two additional in vivo models. When croton oil was applied to the ears of mice (producing irritant contact dermatitis) followed by application of CGRP topically, CGRP significantly inhibited the inflammatory response induced (Clementi et al. 1994). Systemic administration of CGRP also inhibited peritoneal exudation induced by intraperitoneal administration of acetic acid (Clementi et al. 1995). This inhibition was observed when the CGRP was given 5 minutes before administration of acetic acid but no effect was seen when it was given 30 or 60 minutes after acetic acid. Topical treatment of mouse ears with CGRP also inhibited inflammation induced by subsequent treatment of the ears with topical croton oil, arachidonic acid or tetradecanoylphorbol acetate (Clementi et al. 1995).
Calcitonin gene-related peptide and endothelial cells
Recent experiments suggest a novel mechanism by which CGRP produces its anti-inflammatory effects. When the human microvascular endothelial cell line HMEC-1 or primary human dermal microvascular endothelial cells (pHDMECs) were treated with CGRP in vitro, it was found that CGRP treatment inhibited lipopolysaccharide-induced production of the chemokines growth-related oncogene-1 (GROα ,CXCL1), monocyte chemotactic protein-1 (MCP1, CCL2) and IL-8 (CXCL8) (Huang et al. 2011). This inhibition could be blocked by antagonists of the CGRP receptor and both cell types were found to express components of the CGRP and adrenomedullin receptors (Huang et al. 2011). Furthermore, CGRP was found to inhibit lipopolysaccharide-induced activation of NFκB and Bay 11–7085, an inhibitor of NFκB activation and the phosphorylation of IκBα, also inhibited lipopolysaccharide-induced release of these chemokines (Huang et al. 2011). These results strongly indicate that the NFκB pathway is involved in CGRP-mediated suppression of chemokine production. In accord with these findings, pre-treatment of HMEC-1 cells with CGRP prior to stimulation with lipopolysaccharide significantly suppressed the ability of these cells, or of supernatants conditioned by these cells, to chemoattract human mononuclear cells or neutrophils (Huang et al. 2011). Presumably this is due to inhibition of release of chemokines by pretreatment with CGRP. Thus, some of the in-vivo effects of CGRP may be mediated by binding to receptors on endothelial cells and, thereby, decreasing production of certain chemokines.
Calcitonin gene-related peptide and inflammatory skin diseases
Psoriasis
Psoriasis is a common papulosquamous disease of the skin characterized by scaly, red papules and plaques (Di Meglio et al. 2014). Recent evidence suggests a link to the metabolic syndrome (Di Meglio et al. 2014). A growing body of evidence supports the idea that nerves play an important role in the pathogenesis of this disorder. It has long been known that the denervation of skin bearing psoriasis leads to its improvement or resolution (Dewing 1971, Raychaudhuri et al. 1993). Additionally, intralesional injection of local anesthetics improves psoriatic lesions (Perlman 1972). Recently two animal models have supported the involvement of nerves in psoriasis. In one model, mice are engineered to overexpress the receptor tyrosine kinase Tie2 in keratinocytes (Wolfram et al. 2009, Ostrowski et al. 2011). These animals develop a psoriasiform dermatitis characterized by the presence of Th17 cells, involvement of the IL-17 family of cytokines, and improvement in the dermatitis with many of the therapies effective in human psoriasis (Wolfram et al. 2009). Of particular interest, denervation of the skin leads to improvement in the psoriatic phenotype (Ostrowski et al. 2011). It has been reported, however, that after denervation if CGRP is administered systemically the improvement in the psoriatic phenotype, particularly acanthosis, is blunted (Ostrowski et al. 2011). Similarly, administration of substance P systemically after denervation inhibits the resolution of the inflammatory cell infiltrate associated with the psoriasiform dermatitis (Ostrowski et al. 2011). Of course, this suggests that CGRP and substance P may be products of nerves relevant to maintenance of the psoriasiform dermatitis. In a second model, application of imiquimod [a TLR7 agonist used clinically to enhance innate immunity for the treatment of viral neoplasms of the skin (e.g. warts) and certain skin malignancies and pre-malignancies (Hemmi et al. 2002)] to mouse skin daily for 5–6 days also induces psoriasiform hyperplasia (Van Belle et al. 2012). In this model also, innervation is required for expression of the rash (Baerveldt et al. 2012).
Recent preliminary work from out laboratory suggests that CGRP may play a role in biasing immune responses towards the Th17 pole through actions on endothelial cells which, in turn, influence the outcome of Langerhans antigen presentation to T cells (Granstein et al. 2014). If confirmed, this may suggest a mechanism by which CGRP may contribute to the psoriasis phenotype as Th17 cells and the IL-17 family of cytokines appear to be important in psoriasis pathogenesis. Additional circumstantial evidence that CGRP may be involved in psoriasis through actions on endothelial cells comes from the observation that endothelial cells in dermal blood vessels in psoriatic lesions can be found to have CGRP on their surface (He et al. 2000) and that CGRP containing nerves are increased in the epidermis of lesions of psoriasis (Jiang et al. 1998). Furthermore, plasma concentrations of CGRP are significantly elevated in psoriatic individuals compared with healthy controls (Reich et al. 2007).
Atopic dermatitis
Atopic dermatitis (“eczema”) is a common disorder characterized by itching of the skin with dryness, erythema and excoriations (Thomsen, 2014). The pathogenesis of this disorder involves aberrant immunity in a manner only partially understood. The disease is associated, in at least some patients, with a mutation affecting a skin protein important to normal skin barrier function (Thomsen, 2014). Circumstantial evidence exists linking CGRP to atopic dermatitis. As mentioned above, CGRP biases antigen presentation towards the Th2 pole (Asahina et al. 1995b, Ding et al. 2008) and atopic dermatitis, in part, is a Th2-mediated disease. In this regard, CGRP-bearing nerve fibers are increased in lesions of atopic dermatitis (Järvikallio et al, 2003) and, additionally, circulating levels of CGRP are elevated in these patients (Hodeib et al. 2010). Investigators have shown, using an in vitro innervated skin model, that neurons induce the proliferation of keratinocytes through release of CGRP and CGRP enhanced keratinocyte proliferation and epidermal thickness in these models (Roggenkamp et al. 2013). Keratinocytes from atopic individuals exhibited higher expression levels of CGRP receptor components in innervated skin models employing atopic keratinocytes and had a thicker epidermis and a higher neurite density than those with keratinocytes from healthy controls (Roggenkamp et al. 2013). Furthermore, suction blister roofs from patients with atopic dermatitis had higher levels of mRNAs for CGRP receptor components ramp1 and rcp compared with healthy skin (Roggenkamp et al. 2013). Suction blister fluids obtained from atopic skin also contained more CGRP than healthy controls (Roggenkamp et al. 2013).
With regard to other inflammatory mechanisms, CGRP has been reported to induce mucosal mast cell degranulation (De Jonge et al. 2004). On the other hand, in a mouse model where a spontaneous mutation results in induction of an atopic dermatitis-like rash in mice housed under conventional conditions but not when housed in specific pathogen-free conditions, the CGRP concentration in the skin lesions was found to be lower than in non-affected skin in these mice (Katsuno et al. 2003). Of course, it remains unknown how closely this model represents human atopic dermatitis.
While a role for CGRP in the pathogenesis in atopic dermatitis is still speculative at this time, the data collected suggests that additional, detailed studies are warranted to further determine whether CGRP indeed is involved in this disease.
Perspectives
The immune and nervous systems cannot be considered as separate entities. An enormous amount of data demonstrate the regulatory and effector interactions between these homeostatic and protective systems (21). In this regard, the neuropeptide, vasodilator, signaling molecule and immunologic signal CGRP is a key actor (Table 1 and Figure 1). The data summarized herein demonstrate its important functions in regulating immune and inflammatory processes within the skin. Although much of the data is derived from in vitro experiments, it is clear that CGRP has a number of important regulatory effects including inhibitory effects on LC (and at least some other dendritic cell) antigen presentation for Th1 responses and augmentation of antigen presentation for Th2 responses. Through effects on endothelial cells, CGRP also inhibits the release of at least some proinflammatory chemokines. The recent report from Ganor et al that CGRP may inhibit LC-mediated HIV-1 transmission suggests another potentially important role for this neuropeptide. Of course key questions remain: Are the findings reported with mouse cells indicative of what happens with human cells? Do effects seen in vitro faithfully reflect in vivo activities? With regard to the possible role of langerin in LC-HIV-1 interactions, can the results of Ganor et al be reproduced with LCs derived from mucosa? Perhaps the most important question to be answered is what are the signals that regulate CGRP release or non-release by peripheral nerves? Do such signals arise from psychological factors? If so, CGRP may play a role in psychological stress-induced modulation of inflammatory skin disorders.
Table 1.
CGRP Effects Relevant to Cutaneous Immunity (refs)
|
|
|
|
|
|
|
|
|
|
|
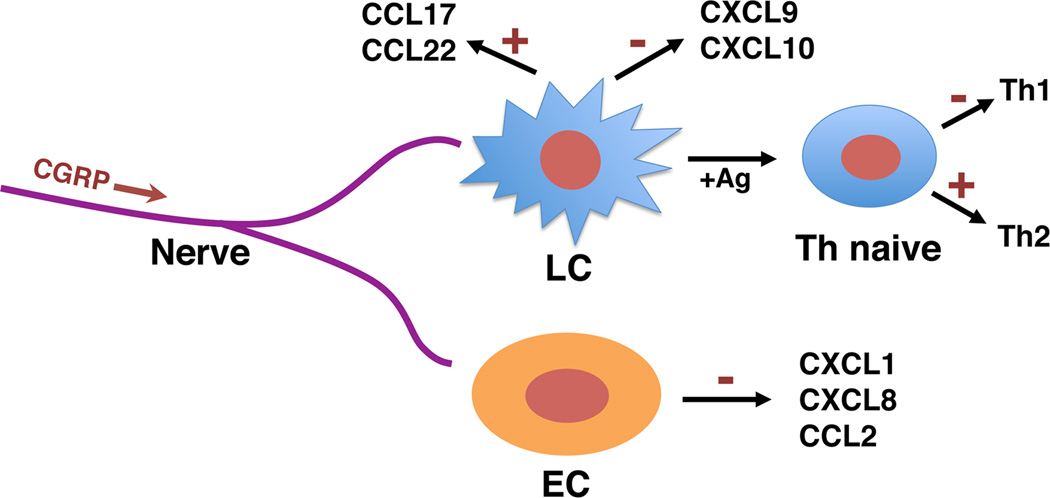
Figure 1.
Effects of CGRP in vitro. Pre-exposure of murine LC to CGRP inhibits antigen presentation for Th1 cell generation while enhancing antigen presentation for Th2 cell generation. The simultaneous presence of CGRP inhibits interferon-γ-induced production of the Th1 chemokines CXCL9 and CXCL10 by a LC-like cell line derived form BALB/c epidermis while treatment with CGRP alone induced production of the Th2 chemokines CCL17 and CCL22 (Ding et al. 2008). Additionally, CGRP inhibits lipopolysaccharide (LPS)-induced production of CXCL1, CXCL8 and CCL2 by human dermal microvascular endothelial cells (cells were cultured in CGRP and 1 hour later LPS was added without removing the CGRP) (Huang et al. 2011).
Although circumstantial, some of the evidence discussed above indeed suggests that CGRP may be important in the pathogenesis of inflammatory skin disorders and relevant cellular and molecular mechanisms are being delineated. If this proves to be the case, CGRP signaling pathways may prove to be druggable targets. Figure 1 shows several relevant activities of CGRP that have been determined in in vitro experiments. A greater understanding of the role of CGRP in immune and inflammatory processes in the skin may lead to new and novel approaches to prevent or treat skin disorders for the benefit of our patients.
Acknowledgements
Funded in part by 1 R21 AR064907 from the US National Institutes of Health.
Footnotes
Conflict of interest
None
References
- Asahina A, Hosoi J, Beissert S, Stratigos A, Granstein RD. Inhibition of the induction of delayed-type and contact hypersensitivity by calcitonin gene-related peptide. J Immunol. 1995a;154:3056–3061. [PubMed] [Google Scholar]
- Asahina A, Moro O, Hosoi J, Lerner E, Xu S, Takashima A, Granstein RD. Specific induction of cAMP in Langerhans cells by calcitonin gene-related peptide: relevance to functional effects. Proc Natl Acad Sci. 1995b;92:8323–8327. doi: 10.1073/pnas.92.18.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerveldt EM, Onderdijk AJ, Wohn C, Kant M, Florencia EF, Hekking-Weijma IJ, Walbeehm ET, Swindell WR, Gudjonsson JE, Prens EP. Denervation impairs clinical development of imiquimod-induced psoriasiform skin inflammation in mice. J Invest Dermatol. 2012;132(Suppl 2s):S17. abstract. [Google Scholar]
- Benrath J, Eschenfelder C, Zimmerman M, Gillardon F. Calcitonin gene-related peptide, substance P and nitric oxide are involved in cutaneous inflammation following ultraviolet irradiation. Eur J Pharmacol. 1995;293:87–96. doi: 10.1016/0926-6917(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Berthier-Vergnes O, Bermond F, Flacher V, Massacrier C, Schmitt D, Péguet-Navarro J. TNF-alpha enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 2005;579:3660–3668. doi: 10.1016/j.febslet.2005.04.087. [DOI] [PubMed] [Google Scholar]
- Breimer LH, MacIntyre I, Zaidi M. Peptides from the calcitonin genes: molecular genetics, structure and function. Biochem J. 1988;255:377–390. doi: 10.1042/bj2550377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carucci JA, Ignatius R, Wei Y, Cypess AM, Schaer DA, Pope M, Steinman RM, Mojsov S. Calcitonin gene-related peptide decreases expression of HLA-DR and CD86 by human dendritic cells and dampens dendritic cell-driven T cell-proliferative responses via the type I calcitonin gene-related peptide receptor. J Immunol. 2000;164:3494–3499. doi: 10.4049/jimmunol.164.7.3494. [DOI] [PubMed] [Google Scholar]
- Cheng-Chew SB, Leung PY. Localisation of VIP-and CGRP-like substances in the skin and sinus hair follicles of various mammalian species. Histochem Cell Biol. 1996;105:443–452. doi: 10.1007/BF01457657. [DOI] [PubMed] [Google Scholar]
- Clementi G, Caruso A, Cutuli VM, Prato A, de Bernardis E, Fiore CE, Amico-Roxas M. Anti-inflammatory activity of amylin and CGRP in different experimental models of inflammation. Life Sci. 1995;57:PL193–PL197. doi: 10.1016/0024-3205(95)02100-w. [DOI] [PubMed] [Google Scholar]
- Clementi G, Amico-Roxas M, Caruso A, Catena Cutuli VM, Prato A, Maugeri S, de Bernardis E, Scapagnini U. Effects of CGRP in different models of mouse ear inflammation. Life Sci. 1994;54:PL119–PL124. doi: 10.1016/0024-3205(94)90011-6. [DOI] [PubMed] [Google Scholar]
- De Jonge F, De Laet A, Van Nassauw L, Brown JK, Miller HR, van Bogaert PP, Timmermans JP, Kroese AB. In vitro activation of murine DRG neurons by CGRP-mediated mucosal mast cell degranulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G178–G191. doi: 10.1152/ajpgi.00528.2003. [DOI] [PubMed] [Google Scholar]
- de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220–221. [PubMed] [Google Scholar]
- Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a015354. pii: a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol. 2008;181:6020–6026. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Wagner JA, Granstein RD. CGRP, PACAP, and VIP modulate Langerhans cell function by inhibiting NF-kappaB activation. J Invest Dermatol. 2007;127:2357–2367. doi: 10.1038/sj.jid.5700858. [DOI] [PubMed] [Google Scholar]
- Fox FE, Kubin M, Cassin M, Niu Z, Hosoi J, Torii H, Granstein RD, Trinchieri G, Rook AH. Calcitonin gene-related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: effects on B7, interleukin 10, and interleukin 12. J Invest Dermatol. 1997;108:43–48. doi: 10.1111/1523-1747.ep12285627. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A, Henke H, Lundberg JM, Petermann JB, Hökfelt T, Fischer JA. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987;8:399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- Ganor Y, Drillet-Dangeard AS, Lopalco L, Tudor D, Tambussi G, Delongchamps NB, Zerbib M, Bomsel M. Calcitonin gene-related peptide inhibits Langerhans cell-mediated HIV-1 transmission. J Exp Med. 2013;210:2161–2170. doi: 10.1084/jem.20122349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganor Y, Drillet-Dangeard AS, Bomsel M. Calcitonin gene-related peptide inhibits human immunodeficiency type 1 transmission by Langerhans cells via an autocrine feedback mechanism. Acta Physiol (Oxf) 2014 doi: 10.1111/apha.12366. in press. [DOI] [PubMed] [Google Scholar]
- Gomes RN, Castro-Faria-Neto HC, Bozza PT, Soares MB, Shoemaker CB, David JR, Bozza MT. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock. 2005;24:590–594. doi: 10.1097/01.shk.0000183395.29014.7c. [DOI] [PubMed] [Google Scholar]
- Grabbe S, Bruvers S, Gallo RL, Knisely TL, Nazareno R, Granstein RD. Tumor antigen presentation by murine epidermal cells. J Immunol. 1991;146:3656–3661. [PubMed] [Google Scholar]
- Granstein RD, Stohl LL, Manni M, Wagner JA, Ding W. Calcitonin gene-related peptide (CGRP) biases Langerhans cell (LC) antigen presentation towards a Th17 response through actions on endothelial cells. J Invest Dermatol. 2014;134:S1. abstract. [Google Scholar]
- Greaves MW. Antihistamines in dermatology. Skin Pharmacol Physiol. 2005;18:220–229. doi: 10.1159/000086667. [DOI] [PubMed] [Google Scholar]
- Harman AN, Kim M, Nasr N, Sandgren KJ, Cameron PU. Tissue dendritic cells as portals for HIV entry. Rev Med Virol. 2013;23:319–333. doi: 10.1002/rmv.1753. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Toyka KV. Substance P, the immune system and inflammation. Int Rev Immunol. 1989;4:229–249. doi: 10.3109/08830188909054420. [DOI] [PubMed] [Google Scholar]
- He Y, Ding G, Wang X, Zhu T, Fan S. Calcitonin gene-related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl) 2000;113:747–751. [PubMed] [Google Scholar]
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- Hodeib A, El-Samad ZA, Hanafy H, El-Latief AA, El-Bendary A, Abu-Raya A. Nerve growth factor, neuropeptides and cutaneous nerves in atopic dermatitis. Indian J Dermatol. 2010;55:135–139. doi: 10.4103/0019-5154.62735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- Hou Q, Barr T, Gee L, Vickers J, Wymer J, Borsani E, Rodella L, Getsios S, Burdo T, Eisenberg E, Guha U, Lavker R, Kessler J, Chittur S, Fiorino D, Rice F, et al. Keratinocyte expression of calcitonin gene-related peptide β: implications for neuropathic and inflammatory pain mechanisms. Pain. 2011;152:2036–2051. doi: 10.1016/j.pain.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Stohl l.L, Zhou X, Ding W, Granstein RD. Calcitonin gene-related peptide inhibits chemokine production by human dermal microvascular endothelial cells. Brain Behav Immun. 2011;25:787–799. doi: 10.1016/j.bbi.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyártó BZ, Kaplan DH. The evolving function of Langerhans cells in adaptive skin immunity. Immunol Cell Biol. 2010;88:361–365. doi: 10.1038/icb.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Schuler G, Witmer MD, Valinsky J, Atassi B, Steinman RM. Immunologic properties of purified epidermal LC: distinct requirements for the stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986;164:605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvikallio A, Harvima IT, Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res. 2003;295:2–7. doi: 10.1007/s00403-002-0378-z. [DOI] [PubMed] [Google Scholar]
- Jiang WY, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. Int J Dermatol. 1998;37:572–574. doi: 10.1046/j.1365-4362.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- Juaneda C, Dumont Y, Quirion R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol Sci. 2000;21:432–438. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Aihara M, Kojima M, Osuna H, Hosoi J, Nakamura M, Toyoda M, Matsuda H, Ikezawa Z. Neuropeptides concentraitons in the skin of a murine (NCNga mice) model of atopic dermatitis. J Dermatol Sci. 2003;33:55–65. doi: 10.1016/s0923-1811(03)00155-5. [DOI] [PubMed] [Google Scholar]
- Kresse A, Jacobowitz DM, Skofitsch G. Detailed mapping of CGRP mRNA expression in the rat central nervous system: comparison with previous immunocytochemical findings. Brain Res Bull. 1995;36:261–274. doi: 10.1016/0361-9230(94)00201-b. [DOI] [PubMed] [Google Scholar]
- Lee RT, Hsu TL, Huang SK, Hsieh SL, Wong CH, Lee YC. Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology. 2011;21:512–520. doi: 10.1093/glycob/cwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang T, Ma C, Xiong T, Zhu Y, Wang X. Calcitonin gene-related peptide inibits interleukin-1beta-induced endogenous monocyte chemoattractant protein-1 secretion in type II alveolar epithelial cells. Am J Physiol Cell Physiol. 2006;291:C456–C465. doi: 10.1152/ajpcell.00538.2005. [DOI] [PubMed] [Google Scholar]
- Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Müller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32:1715–1721. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- Lips CJ, Geerdink RA, Nieuwenhuis MG, van der Sluys Veer J. Evolutionary pathways of the calcitonin (CALC) genes. Henry Ford Hosp Med J. 1989;37:201–203. [PubMed] [Google Scholar]
- Madva EN, Granstein RD. Nerve-derived transmitters including peptides influence cutaneous immunology. Brain Behav Immun. 2013;34:1–10. doi: 10.1016/j.bbi.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: Evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- Merhi M, Dusting GJ, Khalil Z. CGRP and nitric oxide of neuronal origin and their involvement in neurogenic vasodilatation in rat skin microvasculature. Br J Pharmacol. 1998;123:863–868. doi: 10.1038/sj.bjp.0701696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami N, Matsushita H, Kato T, Kawasaki R, Sawazaki T, Kishimoto T, Ogitani Y, Watanabe K, Miyagi Y, Sueda K, Fukada S, Yamamoto H, Tsujikawa K. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J Immunol. 2011;186:6886–6893. doi: 10.4049/jimmunol.1100028. [DOI] [PubMed] [Google Scholar]
- Mikami N, Sueda K, Ogitani Y, Otani I, Takatsuji M, Wada Y, Watanabe K, Yoshikawa R, Nishioka S, Hashimoto N, Miyagi Y, Fukada S, Yamamoto H, Tsujikawa K. Calcitonin gene-related peptide regulates type IV hypersensitivity through dendritic cell functions. PLoS One. 2014;9:e86367. doi: 10.1371/journal.pone.0086367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander C, Ygge J, Dalsgaard C. Substance P-, somatostatin- and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci Lett. 1987;74:37–42. doi: 10.1016/0304-3940(87)90047-4. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Koomen CW, Bos JD, Teunissen MB. Differential modulation of human epidermal Langerhans cell maturation by ultraviolet B radiation. J Immunol. 1999;163:5192–5200. [PubMed] [Google Scholar]
- Ostrowski SM, Belkadi A, Loyd CM, Diaconu D, Ward NL. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530–1538. doi: 10.1038/jid.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128–129. doi: 10.1001/archderm.1972.01620040088028. [DOI] [PubMed] [Google Scholar]
- Raud J, Lundeberg T, Brodda-Jansen G, Theodorsson E, Hedqvist P. Potent anti-inflammatory action of calcitonin gene-related peptide. Biochem Biophys Res Commun. 1991;180:1429–1435. doi: 10.1016/s0006-291x(05)81356-7. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Farber EM. Are sensory nerves essential for the development of psoriatic lesions? J Am Acad Dermatol. 1993;28:488–489. doi: 10.1016/s0190-9622(08)81760-4. [DOI] [PubMed] [Google Scholar]
- Reich A, Orda A, Wiśnicka B, Szepietowski JC. Plasma concentration of selected neuropeptides in patients suffering from psoriasis. Exp Dermatol. 2007;16:421–428. doi: 10.1111/j.1600-0625.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Rhee SH. Lipopolysaccharide: basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest Res. 2014;12:90–95. doi: 10.5217/ir.2014.12.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenkamp D, Köpnick S, Stäb F, Wenck H, Schmelz M, Neufang G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol. 2013;133:1620–1628. doi: 10.1038/jid.2012.464. [DOI] [PubMed] [Google Scholar]
- Schuler G, Romani N, Steinman RM. A comparison of murine epidermal Langerhans cells with spleen dendritic cells. J Invest Dermatol. 1985;85(Suppl 1):99s–106s. doi: 10.1111/1523-1747.ep12275566. [DOI] [PubMed] [Google Scholar]
- Singaram C, Sengupta A, Stevens C, Spechler SJ, Goyal RK. Localization of calcitonin gene-related peptide in human esophageal Langerhans cells. Gastroenterology. 1991;100:560–563. doi: 10.1016/0016-5085(91)90231-9. [DOI] [PubMed] [Google Scholar]
- Sparber F, Tripp CH, Hermann M, Romani N, Stoitzner P. Langerhans cells and dermal dendritic cells capture protein antigens in the skin: possible targets for vaccination through the skin. Immunobiology. 2010;215:770–779. doi: 10.1016/j.imbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014:354250. doi: 10.1155/2014/354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii H, Hosoi J, Beissert S, Xu S, Fox FE, Asahina A, Takashima A, Rook AH, Granstein RD. Regulation of cytokine expression in macrophages and the Langerhans cell-like line XS52 by calcitonin gene-related peptide. J Leukoc Biol. 1997;61:216–223. doi: 10.1002/jlb.61.2.216. [DOI] [PubMed] [Google Scholar]
- Vaalasti A, Tainio H, Johansson O, Rechardt L. Light and electron microscopic immunocytochemical demonstration of intraepidermal CGRP-containing nerves in human skin. Skin Pharmacol. 1988;1:225–229. doi: 10.1159/000210779. [DOI] [PubMed] [Google Scholar]
- Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, Fouser LA, Renauld J, Dumoutier L. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188:462–469. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, Askew D, Gilliam AC, McCormick TS, Ward NL. Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–1458. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalawansa SJ. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol. 1997;11:167–239. doi: 10.1615/critrevneurobiol.v11.i2-3.40. [DOI] [PubMed] [Google Scholar]
- Zhou L, Kitch DW, Evans SR, Hauer P, Raman S, Ebeneezer GJ, Gerschenson M, Marra CM, Valcour V, Diaz-Arrastia R, Goodkin K, Millar L, Shriver S, Asmuth DM, Clifford DB, Simpson DM, et al. Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology. 2007;68:2113–2119. doi: 10.1212/01.wnl.0000264888.87918.a1. [DOI] [PubMed] [Google Scholar]
- Woo SH, Lumpkin EA, Patapoutian A. Merkel cells and neurons keep in touch. Trends Cell Biol. 2014 doi: 10.1016/j.tcb.2014.10.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman BJ, Anderson DC, Granger DN. Neuropeptides promote neutrophil adherence to endothelial cell monolayers. Am J Physiol. 1992;263:G678–G682. doi: 10.1152/ajpgi.1992.263.5.G678. [DOI] [PubMed] [Google Scholar]