Abstract
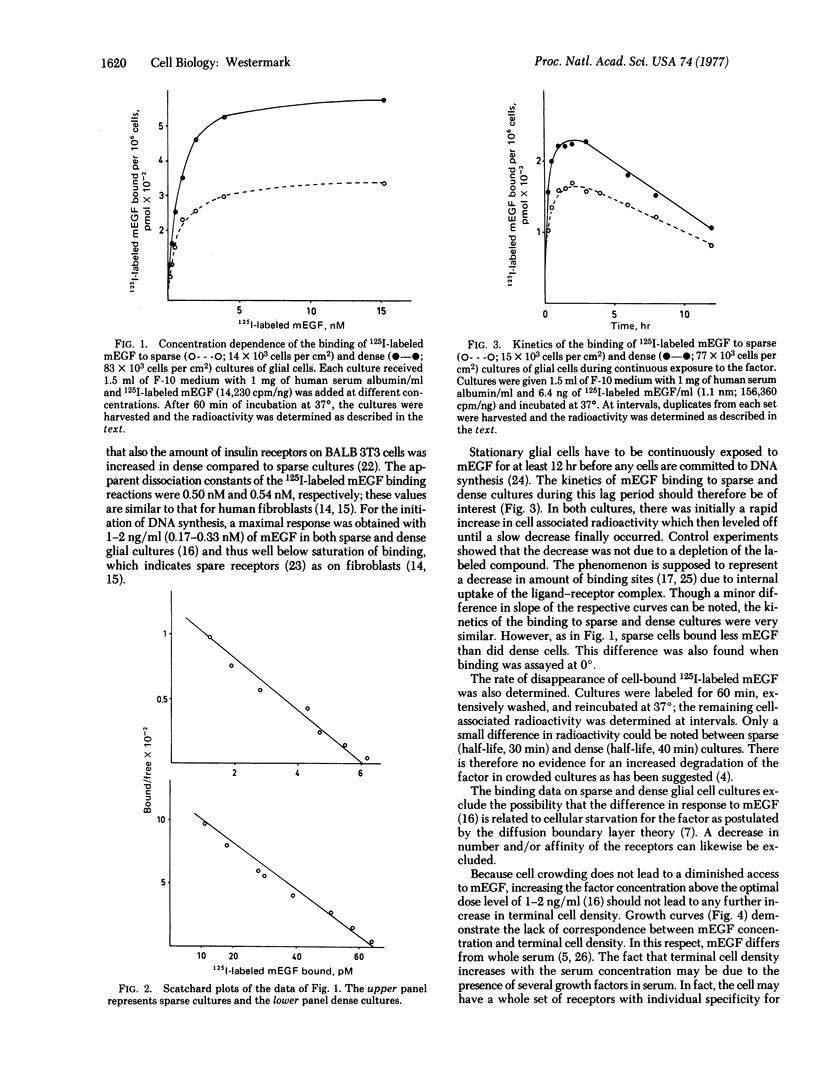
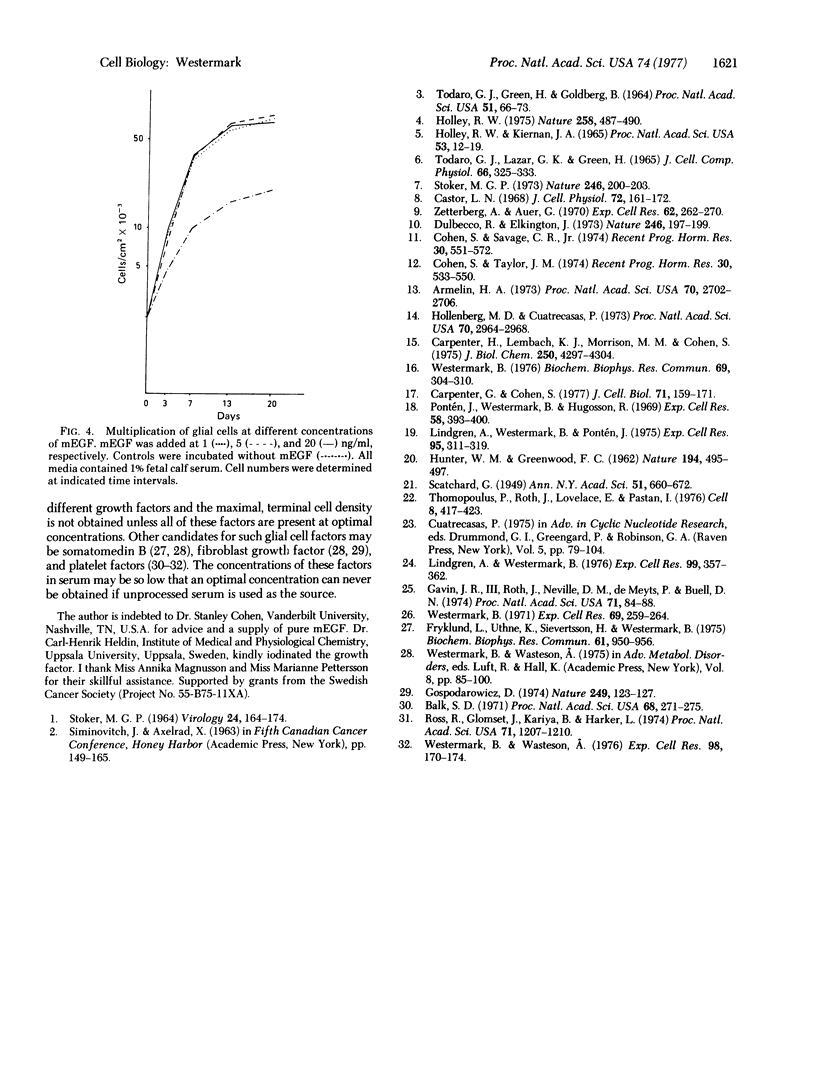
Mouse epidermal growth factor (mEGF) is a potent growth promoter of human glial cells in sparse cultures, whereas very little stimulation of growth in dense cultures is induced by the factor. In the present communication, the possibility that the density-dependent inhibition is caused by a reduced binding/uptake of the factor was scrutinized. It was found that the number of mEGF binding sites was 20,000 and 35,000 per cell in sparse and dense cultures, respectively. The dissociation constant of the binding reaction was not influenced by the cell density. It was concluded that crowded cells are not starved for the factor and that a decrease in number or affinity of the EGF receptors can be excluded as a cause of the inhibition.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2702–2706. doi: 10.1073/pnas.70.9.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D. Calcium as a regulator of the proliferation of normal, but not of transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci U S A. 1971 Feb;68(2):271–275. doi: 10.1073/pnas.68.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Lembach K. J., Morrison M. M., Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem. 1975 Jun 10;250(11):4297–4304. [PubMed] [Google Scholar]
- Castor L. N. Contact regulation of cell division in an epithelial-like cell line. J Cell Physiol. 1968 Dec;72(3):161–172. doi: 10.1002/jcp.1040720304. [DOI] [PubMed] [Google Scholar]
- Cohen S., Savage C. R., Jr Recent studies on the chemistry and biology of epidermal growth factor. Recent Prog Horm Res. 1974;30(0):551–574. doi: 10.1016/b978-0-12-571130-2.50018-3. [DOI] [PubMed] [Google Scholar]
- Cohen S., Taylor J. M. Epidermal growth factor: chemical and biological characterization. Recent Prog Horm Res. 1974;30(0):533–550. doi: 10.1016/b978-0-12-571130-2.50017-1. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Elkington J. Conditions limiting multiplication of fibroblastic and epithelial cells in dense cultures. Nature. 1973 Nov 23;246(5430):197–199. doi: 10.1038/246197a0. [DOI] [PubMed] [Google Scholar]
- Fryklund L., Uthne K., Sievertsson H. Isolation and characterization of polypeptides from human plasma enhancing the growth of human normal cells in culture. Biochem Biophys Res Commun. 1974 Dec 11;61(3):950–956. doi: 10.1016/0006-291x(74)90247-2. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROWE W. P., BLACK P. H., HUEBNER R. J. PRODUCTION OF "TUMOR-SPECIFIC" ANTIGENS BY ONCOGENIC VIRUSES DURING ACUTE CYTOLYTIC INFECTIONS. Proc Natl Acad Sci U S A. 1965 Jan;53:12–19. doi: 10.1073/pnas.53.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Epidermal growth factor: receptors in human fibroblasts and modulation of action by cholera toxin. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2964–2968. doi: 10.1073/pnas.70.10.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- Lindgren A., Westermark B., Pontén J. Serum stimulation of stationary human glia and glioma cells in culture. Exp Cell Res. 1975 Oct 15;95(2):311–319. doi: 10.1016/0014-4827(75)90556-x. [DOI] [PubMed] [Google Scholar]
- Lindgren A., Westermark B. Subdivision of the G1 phase of human glia cells in culture. Exp Cell Res. 1976 May;99(2):357–362. doi: 10.1016/0014-4827(76)90593-0. [DOI] [PubMed] [Google Scholar]
- Pontén J., Westermark B., Hugosson R. Regulation of proliferation and movement of human glialike cells in culture. Exp Cell Res. 1969 Dec;58(2):393–400. doi: 10.1016/0014-4827(69)90520-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M. REGULATION OF GROWTH AND ORIENTATION IN HAMSTER CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Oct;24:165–174. doi: 10.1016/0042-6822(64)90099-6. [DOI] [PubMed] [Google Scholar]
- Stoker M. G. Role of diffusion boundary layer in contact inhibition of growth. Nature. 1973 Nov 23;246(5430):200–203. doi: 10.1038/246200a0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H., GOLDBERG B. D. TRANSFORMATION OF PROPERTIES OF AN ESTABLISHED CELL LINE BY SV40 AND POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos P., Roth J., Lovelace E., Pastan I. Insulin receptors in normal and transformed fibroblasts: relationship to growth and transformation. Cell. 1976 Jul;8(3):417–423. doi: 10.1016/0092-8674(76)90154-9. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Westermark B. Density dependent proliferation of human glia cells stimulated by epidermal growth factor. Biochem Biophys Res Commun. 1976 Mar 22;69(2):304–310. doi: 10.1016/0006-291x(76)90522-2. [DOI] [PubMed] [Google Scholar]
- Westermark B. Proliferation control of cultivated human glia-like cells under "steady state" conditions. Exp Cell Res. 1971 Dec;69(2):259–264. doi: 10.1016/0014-4827(71)90222-9. [DOI] [PubMed] [Google Scholar]
- Westermark B., Wasteson A. A platelet factor stimulating human normal glial cells. Exp Cell Res. 1976 Mar 1;98(1):170–174. doi: 10.1016/0014-4827(76)90476-6. [DOI] [PubMed] [Google Scholar]
- Zetterberg A., Auer G. Proliferative activity and cytochemical properties of nuclear chromatin related to local cell density of epithelial cells. Exp Cell Res. 1970 Sep;62(1):262–270. doi: 10.1016/0014-4827(79)90527-5. [DOI] [PubMed] [Google Scholar]



